Abstract
This study aimed to compare the accuracy of novel lipid indices, including the visceral adiposity index (VAI), lipid accumulation product (LAP), triglycerides and glucose (TyG) index, TyG-body mass index (TyG-BMI), and TyG-waist circumference (TyG-WC), in identifying insulin resistance and establish valid cutoff values. This cross-sectional study used the data of 11,378 adults, derived from the United States National Health and Nutrition Examination Survey (1999–2016). Insulin resistance was defined as a homeostasis model assessment-insulin resistance value above the 75th percentile for each sex and race/ethnicities. The area under the curves (AUCs) were as follows: VAI, 0.735; LAP, 0.796; TyG index, 0.723; TyG-BMI, 0.823, and; TyG-WC, 0.822. The AUCs for TyG-BMI and TyG-WC were significantly higher than those for VAI, LAP, and TyG index (vs. TyG-BMI, p < 0.001; vs. TyG-WC, p < 0.001). The cutoff values were as follows: VAI: men 1.65, women 1.65; LAP: men 42.5, women 42.5; TyG index: men 4.665, women 4.575; TyG-BMI: men 135.5, women 135.5; and TyG-WC: men 461.5, women 440.5. Given that lipid indices can be easily calculated with routine laboratory tests, these values may be useful markers for insulin resistance risk assessments in clinical settings.
Similar content being viewed by others
Introduction
Insulin resistance (IR) is a pathological situation, in which there is a lack of physiological response to insulin acting on peripheral tissues1,2. Insulin resistance reduces glucose utilization in the muscles and fats and increases gluconeogenesis in the liver, leading to metabolic and hemodynamic disturbances known as metabolic syndrome, which is a major risk factor for coronary heart disease and cerebrovascular disease1,2,3,4,5,6,7. Considering the prevalence of insulin resistance and metabolic syndromes, it would be necessary to detect insulin resistance early even in healthy individuals8.
Insulin resistance was initially evaluated using the pancreatic suppression test, hyperinsulinemic euglycemic clamp technique (HIEG clamp), or minimal model approximation of the metabolism of glucose (MMAMG)9,10,11. However, these methods are invasive, complicated, expensive, and difficult to use clinically12. For these reasons, indices that measure insulin resistance indirectly have been developed. The homeostasis model for IR (HOMA-IR), which uses fasting blood glucose levels and insulin concentration as variables, was developed in 1985 and has been widely used to estimate IR13. However, a significant drawback of HOMA-IR is the lack of a standard assay for the measurement of fasting insulin concentration14. Therefore, considering these concerns regarding standardization, the HOMA-IR has a significant limitation in establishing an overall acceptable reference value. Furthermore, while several studies have defined IR as a value greater than the 75th percentile value of the HOMA-IR in individuals without diabetes mellitus, the reported cutoff values vary widely, ranging from 2.0 to 3.812,15,16,17,18,19. Given that the measurement of fasting insulin concentration is cumbersome and expensive, the HOMA-IR is not routinely measured in the clinical setting20.
Therefore, insulin-free equations for estimating IR, such as lipid indices, were developed. Lipid indices include visceral adiposity index (VAI), lipid accumulation product (LAP), and triglycerides and glucose (TyG) index21,22,23,24,25. These parameters were proposed as a useful surrogate measure of insulin resistance25,26,27,28. In addition, several studies have evaluated modified indices that combine TyG index and obesity indices such as body mass index (BMI) and waist circumference (WC)29,30,31. However, limited evidence is available regarding the discriminatory accuracy and cutoff values of these novel lipid indices for detecting insulin resistance.
Therefore, this study aimed to compare the accuracy of novel lipid indices in identifying insulin resistance using a representative sample of the US population and establish valid cutoff values for IR.
Results
The study included 11,378 adults (men 5478, women 7900; mean age, 40 years) from the National Health and Nutrition Examination Survey (NHANES) 1999–2016 (Fig. 1). Participants’ demographic and clinical characteristics were compared based on the presence or absence of IR, and the results are shown in Table 1. Age, BMI, WC, and blood pressure were higher in participants with insulin resistance. In addition, blood tests demonstrated high values of fasting glucose, hemoglobin A1C, fasting insulin, total cholesterol, and triglycerides, and low value of high-density lipoprotein (HDL) cholesterol, in participants with IR. Data (median with interquartile range) of each parameter according to race/ethnicity and sex are summarized in Table 2.
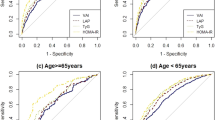
The receiver operating characteristic (ROC) curve for IR is presented in Fig. 2. The AUC was 0.723 for TyG index and 0.735 for VAI (Table 3). The AUC of LAP (0.796) was significantly higher than that of TyG index (p < 0.001). However, the AUCs of TyG-BMI (0.823) and TyG-WC (0.822) were significantly higher than that of LAP (vs. TyG-BMI, p < 0.001; vs. TyG-WC, p < 0.001). Subgroup analysis according to sex and race/ethnicities showed that TyG-BMI and TyG-WC had the highest AUC in every subgroup. Further analysis using 1:1 propensity score matching (PSM) data with age, sex, and race/ethnicities showed similar results (Table 3). The cutoff values of each lipid index were as follows: VAI: men 1.65, women 1.65; LAP: men 42.5, women 42.5; TyG index: men 4.665, women 4.575; TyG-BMI: men 135.5, women 135.5; and TyG-WC: men 461.5, women 440.5. The cutoff values with their corresponding sensitivity, specificity, and odds ratio (OR) of insulin resistance according to sex and race/ethnicities are summarized in Table 4.
Discussion
In this study, we investigated the discriminatory accuracy of novel lipid indices for IR and confirmed that LAP showed significantly higher AUC than TyG index and VAI. There was a significant increase in AUC when BMI or WC was combined with TyG index, exhibiting an even higher discriminatory accuracy than that of LAP. Another important aspect of this study is that the cutoff value of each parameter for IR was presented using large-scale data, facilitating the clinical application of each parameter.
Although the mechanism through which lipid indices cause IR remains unclear, numerous studies have reported that glucolipotoxicity is a key mechanism in the modulation of IR32,33. Ectopic lipid accumulation in the liver and skeletal muscle tissue activates pathways that are associated with IR, leading to a decrease in the glucose uptake in muscle tissue and glycogen synthesis in the liver34,35,36,37. Insulin resistance in muscle tissue due to ectopic lipid accumulation increases hepatic lipogenesis and leads to IR in the liver and hyperlipidemia38,39,40. In addition, macrophage infiltration into white adipose tissue increases lipolysis, which stimulates hepatic triglyceride synthesis, thereby, promoting hyperlipidemia33. Macrophage-induced lipolysis in white adipose tissue also leads to increased hepatic gluconeogenesis and results in hyperglycemia through increased fatty acid delivery to the liver, which results in increased glycerol conversion to glucose41,42,43.
VAI uses BMI, WC, and triglyceride and HDL cholesterol levels to evaluate IR and was proposed by Amato et al. in 201021. First proposed by Kahn et al. using the NHANES data22,23, LAP is calculated using WC and fasting triglyceride levels. In a previous study conducted by Amato et al., VAI showed a significant inverse correlation with insulin sensitivity measured using a HIEG clamp, providing evidence that VAI can be a surrogate marker for IR21. In addition, VAI was reported to be associated with the glucose distribution rate evaluated through the HIEG clamp test in a study on patients with type 1 diabetes mellitus (DM)26, and it was shown to be inversely correlated with HIEG clamp tested insulin sensitivity in studies conducted on women with polycystic ovary syndrome (PCOS) in South Korea and China27,44. In the case of LAP, a small-scaled study with PCOS patients demonstrated a modest inverse correlation with insulin sensitivity measured through HIEG clamp test27. However, most of these studies using the HIEG clamp test had a small sample, and the clinical application of VAI and LAP requires an investigation of appropriate cutoff values through large scale population-based studies. In addition to studies measuring insulin resistance directly, there are numerous studies on the accuracy of VAI and LAP in assessing HOMA-IR defined insulin resistance. However, studies on cutoff values were mainly conducted on a small number of PCOS patients45,46,47. Thus, a study with a larger population with healthy adults is necessary. The current study is significant as it presents the cutoff values of VAI and LAP by sex, using a large and healthy population. Interestingly, the VAI cutoff value identified in this study is similar to the VAI cutoff value of 1.6–1.8 reported in the previous studies with PCOS patients27,45,46. However, in the case of LAP, previous studies have reported diverse cutoff values ranging from 18.5 to 33.8, and this study shows a higher cutoff value than that reported in the preceding studies27,45,46.
TyG index was proposed as a useful surrogate measure of IR by Guerrero-Romero et al. in 200824. Despite the small scale of previous studies, TyG index displayed an inverse correlation with insulin sensitivity measured through HIEG clamp and MMAMG25,28. Furthermore, various epidemiological studies have reported that TyG index is associated with the incidences of cardiovascular disease (CVD) and DM, indicating that TyG index is able to predict diseases that result from IR48,49,50. However, there are a few studies on the estimation of the cutoff value of TyG index for IR. Guerrero-Romero et al. suggested that the best value of the TyG index for the diagnosis of IR was 4.68 using the HIEG clamp test with a small sample size25. In a study with Korean NHANES, the cutoff values for metabolic syndrome, which is a pathological condition associate with IR, were 4.76 in men and 4.71 in women19. In a prospective cohort study with Korean population, the cutoff value to predict DM was 4.6949. In our study, the cutoff value of TyG index was 4.66 in men and 4.57 in women, which is similar to those of previous studies.
Recently, there have been studies on indices that combine adiposity status with the TyG index29,30,31. Considering that adipose tissues secrete inflammatory cytokines, adipokines, and reactive oxygen species, contributing to a variety of metabolic problems51,52,53, compound indices with TyG index and obesity parameters such as BMI and WC might be better indicators of IR than TyG index alone. Several studies reported that the compound indices were significantly associated with metabolic abnormalities such as high blood pressure, nonalcoholic fatty liver disease, prediabetes, DM, and hyperuricemia29,30,31,54,55,56. In addition, recent studies have indicated that TyG-BMI or TyG-WC is more effective in the identification of IR than VAI, LAP, and TyG index29,31. Er et al. reported the AUCs for IR were 0.734 for VAI, 0.761 for LAP, 0.708 for TyG index, 0.801 for TyG-BMI, and 0.772 for TyG-WC and proposed TyG-BMI as a clinically useful surrogate marker for the early identification of IR29. Lim et al. reported that the AUCs for TyG-BMI and TyG-WC (0.748 and 0.731, respectively) were larger than that for TyG index (0.690)31. However, such studies were conducted only in Asian populationss, and no studies have been conducted on other ethnic populations. Therefore, the current study is meaningful as it confirms using large-scale data that TyG-BMI or TyG-WC can be an effective surrogate marker for IR in the US population with various races/ethnicities. Nonetheless, to accurately assess the correlation of IR with TyG-BMI and TyG-WC, verification through the HIEG clamp test is required as has been performed for HOMA-IR and TyG index.
The present study has several strengths. This study is the largest to evaluate the performance of the novel lipid indices to identify insulin resistance in the general US population. In addition, this study conducted various subgroup analyses of IR, with age, sex, and PSM data, to minimize the bias caused by heterogeneity due to demographic characteristics. Moreover, it is important to propose valid cutoff values for each lipid index so that they can be used as a reference in clinical settings for identifying groups at risk for IR. To the best of our knowledge, this is the first study that evaluated the performance and cutoff values of TyG-BMI and TyG-WC in a non-Asian population. However, considering that this is a cross-sectional study, further prospective studies are required to validate the relationship between each surrogate measure and cardiovascular risk factors.
Conclusion
The present study supports the clinical relevance of novel lipid indices in identifying IR in the general US population. Considering that lipid indices can be easily calculated with routine laboratory tests, they can be useful markers of insulin resistance risk assessments in clinical settings. Moreover, the cutoff values presented in our study may be useful in interpreting the results of lipid indices for IR.
Methods
Study population
The NHANES is a cross-sectional study that uses a representative sample of the population living in the United States. The NHANES, administered by the Centers for Disease Control and Prevention every two years, consists of health and nutrition surveys, physical examinations, and laboratory tests. Of the 92,062 individuals who participated in the NHANES between 1999 and 2016, 11,378 adult participants were included in this study after excluding those who were aged < 20 years (n = 42,550), those with missing or incomplete anthropometric and fasting laboratory data (n = 28,694), those on medication for dyslipidemia and hypertension (n = 6598), those with DM, CVD, stroke, cancer, chronic liver disease, and estimated glomerular filtration rate < 60 ml/min/1.73 m2 (n = 2842) (Fig. 1).
Anthropometric and laboratory measurements
Waist circumference was measured using a flexible tape between the uppermost lateral border of the right ilium and that of the left ilium. BMI was defined as the weight in kilograms divided by the height in meters squared (kg/m2). Blood pressure was measured 3 times in the sitting position, with at least 5 min of rest in between each reading. The mean value of the three recorded blood pressure readings was used in this study. Fasting blood glucose and lipid levels were measured using the enzymatic method, and fasting insulin was measured using an immune-enzymometric assay. Detailed sample collection and processing instructions are described in the NHANES Laboratory Procedures Manual57.
Calculation of parameters for insulin resistance
Parameters for insulin resistance were calculated as follows21,22,23,24,25,29,58:
We define IR as a HOMA-IR value above the 75th percentile for each race/ethnicity and sex (Hispanics: Men > 3.62, Women > 3.44; Non-Hispanic Whites: Men > 3.07, Women > 2.53; Non-Hispanic Blacks: Men > 3.18, Women > 3.59; other race: Men > 2.96, Women > 2.53)12,15.
Statistical analysis
Data were presented as mean with standard deviation, or number with prevalence (%) of IR status. Between groups, the differences were determined using t-tests and a Pearson chi-square test. The values of each lipid index for IR were presented as median and interquartile range. To compare the relative diagnostic strength of each lipid index for insulin resistance, AUC was compared using the ROC curve; de Long’s test was used to identify the surrogate measures that were significantly superior for insulin resistance. The cutoff value of each lipid index was determined as the value with the highest Youden index score. Considering the heterogeneity of demographic characteristics such as sex and race/ethnicities, subgroup analyses for IR were performed. Further analysis was performed by 1:1 PSM with age, sex, and race/ethnicities. This was performed using the “MatchIt” package with nearest-neighbor 1-to-1 matching59. Furthermore, OR of HOMA-IR defined IR was checked using the multivariate logistic regression models based on the estimated cutoff values. Statistical analysis was performed using IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA) and R ver. 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). The results were considered statistically significant if the p-value was less than 0.05.
Ethics statement
This study was approved by the institutional review board of Kangnam Sacred Heart Hospital (IRB No. HKS 2017-07-007) and the NHANES was approved by the Research Ethics Review Board of the National Center for Health Statistics, US Centers for Disease Control and Prevention (NHANES 1999–2004, Protocol #98-12; NHANES 2005–2010, Protocol #2005–06; NHANES 2011–2016, Protocol #2011-17). All participants volunteered and provided written informed consent before enrolment. All participants’ records were anonymized before being accessed by the authors. All methods were carried out in accordance with the principles contained in the Declaration of Helsinki.
References
Ascaso, J. F. et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26, 3320–3325 (2003).
Hanefeld, M. The metabolic syndrome: Roots, myths, and facts. In The Metabolic Syndrome (eds Hanefeld, M. & Leonhardt, W.) 13–24 (Gustav Fischer, Portland, 1997).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: Common threads and missing links. Cell 148, 852–871 (2012).
Després, J. P. et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 334, 952–957 (1996).
Kuusisto, J., Mykkanen, L., Pyorala, K. & Laakso, M. Hyperinsulinemic microalbuminuria. A new risk indicator for coronary heart disease. Circulation 91, 831–837 (1995).
Shinozaki, K. et al. Role of insulin resistance associated with compensatory hyperinsulinemia in ischemic stroke. Stroke 27, 37–43 (1996).
Goodarzi, M. O. et al. Relative impact of insulin resistance and obesity on cardiovascular risk factors in polycystic ovary syndrome. Metabolism 52, 713–719 (2003).
Ford, E. S., Giles, W. H. & Dietz, W. H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and nutrition examination survey. JAMA 287, 356–359 (2002).
DeFronzo, R. A., Tobin, J. D. & Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 237, E214-223 (1979).
Bergman, R. N., Prager, R., Volund, A. & Olefsky, J. M. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J. Clin. Investig. 79, 790–800 (1987).
Greenfield, M. S. et al. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes 30, 387–392 (1981).
Du, T. et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 13, 146 (2014).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Miller, W. G. et al. Toward standardization of insulin immunoassays. Clin. Chem. 55, 1011–1018 (2009).
Radikova, Z. et al. Insulin sensitivity indices: A proposal of cut-off points for simple identification of insulin-resistant subjects. Exp. Clin. Endocrinol. Diabetes 114, 249–256 (2006).
Hedblad, B., Nilsson, P., Janzon, L. & Berglund, G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet. Med. 17, 299–307 (2000).
Marques-Vidal, P. et al. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care 25, 1371–1377 (2002).
Gayoso-Diz, P. et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 13, 47 (2013).
Moon, S. et al. The cut-off values of surrogate measures for insulin sensitivity in a healthy population in Korea according to the Korean National Health and nutrition examination survey (KNHANES) 2007–2010. J. Korean Med. Sci. 33, e197 (2018).
Yeh, W. C. et al. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: A cross-sectional study. Lipids Health Dis. 18, 176 (2019).
Amato, M. C. et al. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33, 920–922 (2010).
Kahn, H. S. & Valdez, R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am. J. Clin. Nutr. 78, 928–934 (2003).
Kahn, H. S. The lipid accumulation product is better than BMI for identifying diabetes: A population-based comparison. Diabetes Care 29, 151–153 (2006).
Simental-Mendia, L. E., Rodriguez-Moran, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 6, 299–304 (2008).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351 (2010).
Uruska, A. et al. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J. Clin. Lipidol. 12, 734–740 (2018).
Long, J. et al. Screening for a simple and effective indicator of insulin resistance in Chinese reproductive-aged women, with the insulin clamp technique as a reference. Metab. Syndr. Relat. Disord. 17, 423–429 (2019).
Vasques, A. C. et al. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 93, e98–e100 (2011).
Er, L. K. et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PLoS ONE 11, e0149731 (2016).
Zheng, S. et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Transl. Med. 14, 260 (2016).
Lim, J., Kim, J., Koo, S. H. & Kwon, G. C. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and nutrition examination survey. PLoS ONE 14, e0212963 (2019).
Bickerton, A. S. et al. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56, 168–176 (2007).
Samuel, V. T. & Shulman, G. I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 126, 12–22 (2016).
Dresner, A. et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Investig. 103, 253–259 (1999).
Yu, C. et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidyli nositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236 (2002).
Itani, S. I., Ruderman, N. B., Schmieder, F. & Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51, 2005–2011 (2002).
Szendroedi, J. et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl. Acad. Sci. U.S.A. 111, 9597–9602 (2014).
Kim, J. K. et al. Glucose toxicity and the development of diabetes in mice with muscle specific inactivation of GLUT4. J. Clin. Investig. 108, 153–160 (2001).
Petersen, K. F. et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 104, 12587–12594 (2007).
Petersen, K. F. et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Investig. 109, 1345–1350 (2002).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Perry, R. J. et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Med. 20, 759–763 (2014).
Previs, S. F., Cline, G. W. & Shulman, G. I. A critical evaluation of mass isotopomer distribution analysis of gluconeogenesis in vivo. Am. J. Physiol. 277, E154–E160 (1999).
Oh, J. Y., Sung, Y. A. & Lee, H. J. The visceral adiposity index as a predictor of insulin resistance in young women with polycystic ovary syndrome. Obesity (Silver Spring) 21, 1690–1694 (2013).
Ramezani Tehrani, F., Minooee, S. & Azizi, F. Comparison of various adiposity indexes in women with polycystic ovary syndrome and normo-ovulatory non-hirsute women: A population-based study. Eur. J. Endocrinol. 171, 199–207 (2014).
Huang, X. et al. Body fat indices as effective predictors of insulin resistance in obese/non-obese polycystic ovary syndrome women in the Southwest of China. Endocrine 65, 81–85 (2019).
Abruzzese, G. A. et al. Lipid accumulation product (LAP) and visceral adiposity index (VAI) as markers of insulin resistance and metabolic associated disturbances in young argentine women with polycystic ovary syndrome. Horm. Metab. Res. 49, 23–29 (2017).
Li, S. et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: A retrospective cohort analysis. Sci. Rep. 9, 7320 (2019).
Kim, B. et al. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean genome and epidemiology study (KOGES). PLoS ONE 13, e0206994 (2018).
Lee, S. H. et al. Predicting the development of diabetes using the product of triglycerides and glucose: The Chungju metabolic disease cohort (CMC) study. PLoS ONE 9, e90430 (2014).
Kershaw, E. E. & Flier, J. S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89, 2548–2556 (2004).
Matsuda, M. & Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 7, e330-341 (2013).
Kahn, S. E., Hull, R. L. & Utzschneider, K. M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Zhang, S. et al. Triglyceride glucose-body mass index is effective in identifying nonalcoholic fatty liver disease in nonobese subjects. Medicine (Baltimore) 96, e7041 (2017).
Gu, Q. et al. Associations of triglyceride-glucose index and its derivatives with hyperuricemia risk: A cohort study in Chinese general population. Int. J. Endocrinol. 2020, 3214716 (2020).
Ramdas Nayak, V. K., Nayak, K. R., Vidyasagar, S. & Rekha, P. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab. Syndr. 14, 1265–1272 (2020).
Centers for Disease Control and Prevention. The National Health and Nutrition Examination Survey (NHANES) MEC Laboratory Procedures Manual (2016). https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_mec_laboratory_procedures_manual.pdf. Accessed 18 January 2021.
Morales-Gurrola, G. et al. The triglycerides and glucose index is associated with cardiovascular risk factors in metabolically obese normal-weight subjects. J. Endocrinol. Investig. 43, 995–1000 (2020).
Ho, D. E., Imai, K., King, G. & Stuart, E. A. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 42, 1–28 (2011).
Author information
Authors and Affiliations
Contributions
S.M. and J.G.K. contributed to the research design; W.K., C.A., H.Y.C., J.G.K., J.K. and H.S. participated in the design and performance of the research and data analysis; S.M., B.K., J.G.K. and J.L. wrote the main manuscript text and S.M. prepared Figs. 1 and 2. All authors reviewed the manuscript. Correspondence and requests for materials should be addressed to J.G.K. or S.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J., Kim, B., Kim, W. et al. Lipid indices as simple and clinically useful surrogate markers for insulin resistance in the U.S. population. Sci Rep 11, 2366 (2021). https://doi.org/10.1038/s41598-021-82053-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82053-2
This article is cited by
-
The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018
Cardiovascular Diabetology (2024)
-
Association between the cumulative average triglyceride glucose-body mass index and cardiovascular disease incidence among the middle-aged and older population: a prospective nationwide cohort study in China
Cardiovascular Diabetology (2024)
-
Triglyceride glucose-body mass index and cardiovascular mortality in patients undergoing peritoneal dialysis: a retrospective cohort study
Lipids in Health and Disease (2023)
-
Association of cardiometabolic factors and insulin resistance surrogates with mortality in participants from the Korean Genome and Epidemiology Study
Lipids in Health and Disease (2023)
-
Combined effect of pancreatic lipid content and gene variants (TCF7L2, WFS1 and 11BHSD1) on B-cell function in Middle Aged Women in a Post Hoc Analysis
Diabetology & Metabolic Syndrome (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.