Abstract
Microcirculatory dysfunction plays a crucial role in renal ischemia/reperfusion (IR)-induced injury. Dexmedetomidine was reported to ameliorate IR-induced acute kidney injury. This study investigated the effects of dexmedetomidine on renal microcirculation after IR-induced acute kidney injury in rats. In total, 50 rats were randomly allocated to the following five groups (10 in each group): Sham, Control‒IR, Dex (dexmedetomidine) ‒Sham, Dex‒IR, and IR‒Dex group. The microcirculation parameters included total small vessel density, perfused small vessel density (PSVD), proportion of perfused small vessels, microvascular flow index, and tissue oxygen saturation (StO2) were recorded. The repeated measures analysis showed that PSVD on renal surface was higher in the Dex‒IR group than in the Control‒IR group (3.5 mm/mm2, 95% confidence interval [CI] 0.6 to 6.4 mm/mm2, P = 0.01). At 240 min, StO2 on renal surface was lower in the Control‒IR group than in the Sham group (– 7%, 95% CI − 13 to − 1%, P = 0.021), but StO2 did not differ significantly among the Sham, Dex‒IR, and IR‒Dex groups. Our results showed that pretreatment with dexmedetomidine improved renal microcirculation in rats with IR-induced acute kidney injury. However, the adverse effects of low mean arterial pressure and heart rate might offset the protective effect of dexmedetomidine on organ injury.
Similar content being viewed by others
Introduction
Acute kidney injury that resulted from shock and ischemia/reperfusion (IR)-induced injury is a major challenge in critical care medicine. Microcirculatory dysfunction plays a crucial role in IR-induced injury1,2. Many treatments were investigated to improve microcirculation3,4,5. Dexmedetomidine is a potent selective alpha-2-adrenergic agonist with sedative and analgesic effects6,7. Dexmedetomidine was proven to improve microcirculation in a septic rat model8, in a surgical stress and pain rat model9, and in patients after cardiac surgery10. In addition, dexmedetomidine ameliorates renal IR-induced injury11, reduces oxidative stress, and decreases kidney-injury-related biomarker levels11,12,13; however, some of these treatment effects remain controversial. To our knowledge, few studies were conducted on animals to investigate the effects of dexmedetomidine in terms of renal microcirculation on rats with renal IR-induced injury. This study investigated the effects of dexmedetomidine in terms of renal microcirculation on rats with renal IR-induced injury. Our primary outcome was the investigation of renal microcirculation at 240 min. For secondary outcome measurement, at 240 min, a blood test and enzyme-linked immunosorbent assay (ELISA) were performed to investigate biochemical laboratory data and kidney-injury-related molecules.
Results
Hemodynamic parameters
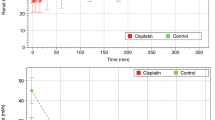
Repeated measures analysis showed that mean arterial pressure (MAP) was lower in the Dex‒Sham, Dex‒IR, and IR‒Dex groups than in the Sham (– 17 mm Hg, 95% confidence interval [CI] − 24 to − 9 mm Hg, P < 0.001; – 18 mm Hg, 95% CI − 25 to − 10 mm Hg, P < 0.001; – 11 mm Hg, 95% CI − 18 to − 3 mm Hg, P = 0.001; respectively; Fig. 1A) and Control‒IR groups (– 15 mm Hg, 95% CI − 23 to − 8 mm Hg, P < 0.001; – 16 mm Hg, 95% CI − 24 to − 9 mm Hg, P < 0.001; – 9 mm Hg, 95% CI − 16 to − 2 mm Hg, P = 0.008; respectively; Fig. 1A). Repeated measures analysis showed that heart rates were lower in the Dex‒Sham and Dex‒IR groups than in the Sham (– 51 beats per minute [bpm], 95% CI − 86 to − 16 bpm, P = 0.001; – 46 bpm, 95% CI − 81 to − 12 bpm, P = 0.004; Fig. 1B) and Control‒IR groups (– 50 bpm, 95% CI − 85 to − 15 bpm, P = 0.002; – 45 bpm, 95% CI − 80 to − 10 bpm, P = 0.006; respectively; Fig. 1B).
Mean arterial pressure and heart rate. *P < 0.05 vs. the Sham group determined using repeated measures analysis. #P < 0.05 vs. the Control‒IR group determined using repeated measures analysis. X indicates the mean of each group. Sham indicates sham operation; Control‒IR (ischemia/reperfusion), left kidney ischemia for 60 min and reperfusion; Dex (dexmedetomidine)‒Sham, sham operation and infusion of dexmedetomidine; Dex‒IR, pre-ischemia infusion of dexmedetomidine 30 min before left kidney ischemia; IR‒Dex, post-ischemia infusion of dexmedetomidine 30 min after reperfusion of the left ischemic kidney.
Microcirculatory dysfunction and lower tissue oxygen saturation (StO2) after ischemia/reperfusion injury
The microcirculation images of the renal surface from the SDF video microscope at baseline and 240 min after ischemic kidney reperfusion are shown in Fig. 2. In Fig. 3, repeated measures analysis show that perfused small vessel density (PSVD) on renal surface was lower in in the Control-IR group than the Sham group (– 7.7 mm/mm2, 95% CI − 10.7 to − 5.0 mm/mm2, P < 0.001). In Fig. 3C, repeated measures analysis indicated that tissue oxygen saturation (StO2) on renal surface was lower in the Control‒IR group than in the Sham group (– 12%, 95% CI − 17 to − 8%, P < 0.001). At 240 min, StO2 was lower in the Control‒IR group than in the Sham group (– 7%, 95% CI − 13 to − 1%, P = 0.021). PSVD and StO2 on renal surface did not differ significantly between the Sham and Dex‒Sham groups.
Microcirculation images of the renal surface from a sidestream dark field video microscope at baseline and 240 min after ischemic kidney reperfusion. (A, C, E, G, I) baseline of the five groups. (B, D, F, H, J) At 240 min after ischemic kidney reperfusion. At 240 min, perfused small vessel density was lower in the Control‒IR and IR‒Dex groups than in the Sham group. Sham indicates sham operation; Control‒IR (ischemia/reperfusion), left kidney ischemia for 60 min and reperfusion; Dex (dexmedetomidine)‒Sham, sham operation and infusion of dexmedetomidine; Dex‒IR, pre-ischemia infusion of dexmedetomidine 30 min before left kidney ischemia; IR‒Dex, post-ischemia infusion of dexmedetomidine 30 min after reperfusion of the left ischemic kidney. PSVD, perfused small vessel density.
Total small vessel density, perfused small vessel density, and tissue oxygenation saturation. *P < 0.05 vs. the Sham group and #P < 0.05 vs. the Control‒IR group determined using repeated measures analysis. §P < 0.05 vs. the Sham group at 240 min determined using one-way analysis of variance with Tukey’s test. X indicates the mean of each group. Sham indicates sham operation; Control‒IR (ischemia/reperfusion), left kidney ischemia for 60 min and reperfusion; Dex (dexmedetomidine)‒Sham, sham operation and infusion of dexmedetomidine; Dex‒IR, pre-ischemia infusion of dexmedetomidine 30 min before left kidney ischemia; IR‒Dex, post-ischemia infusion of dexmedetomidine 30 min after reperfusion of the left ischemic kidney.
Treatment effect of dexmedetomidine on ischemia/reperfusion microcirculatory dysfunction
Repeated measures analysis for our primary outcome indicated that PSVD was higher in the Dex‒IR group than in the Control‒IR group (3.5 mm/mm2, 95% CI 0.6 to 6.4 mm/mm2, P = 0.01; Fig. 3B), but PSVD did not differ significantly between the IR‒Dex and Control‒IR groups (1.5 mm/mm2, 95% CI − 1.3 to 4.4 mm/mm2, P = 0.564; Fig. 3B). At 240 min, PSVD on renal surface was higher in the Dex‒IR group than in the Control‒IR group (6.5 mm/mm2, 95% CI 1.9 to 11.0 mm/mm2, P = 0.002), but PSVD on renal surface did not differ significantly between the IR‒Dex and Control‒IR groups (3.4 mm/mm2, 95% CI − 1.2 to 8.0 mm/mm2, P = 0.237). At 240 min, microvascular flow index (MFI), and proportion of perfused small vessels (PPSV) on renal surface were higher in the Dex‒IR group than in the Control‒IR group [3.0 (3.0–3.0) vs. 2.6 (2.3–2.9), P < 0.001; 100 (100–100) vs 89 (78–94)%, P < 0.001; respectively], but MFI and PPSV did not differ significantly between the IR‒Dex and Control‒IR groups [2.7 (2.5–2.9) vs. 2.6 (2.3–2.9), P = 0.353; 93 (88–98) vs. 89 (78–94)%, P = 0.190; respectively]. The microcirculation parameters of terminal ileal mucosa did not differ significantly among the five groups.
Treatment effect of dexmedetomidine on StO2 after ischemia/reperfusion injury
In Fig. 3C, repeated measures analysis indicated that StO2 on renal surface did not differ significantly among the Control‒IR, Dex‒IR, and IR‒Dex groups, and StO2 was lower in the Dex‒IR and IR‒Dex groups than in the Sham group (− 9%, 95% CI − 13 to − 5%, P < 0.001; − 10%, 95% CI − 14 to − 6%, P < 0.001; respectively). At 240 min, StO2 on renal surface did not differ significantly among the Sham, Dex‒IR, and IR‒Dex groups. StO2 of terminal ileal mucosa did not differ significantly among the five groups.
Histologic score and laboratory data
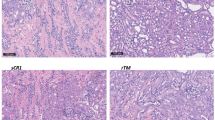
In Fig. 4, the histologic scores of renal tubular necrosis, renal tubular dilation, and peritubular capillary congestion in outer medulla did not differ significantly among the Control‒IR, Dex‒IR, and IR‒Dex groups. The blood test results for biochemical laboratory data are presented in Table 1. Although aspartate aminotransferase (AST) was statistically significant by an one-way ANOVA, further post hoc analysis showed that AST level did not differ significantly among the Dex‒IR, IR‒Dex, and Control‒IR groups. No significant difference was observed in creatinine, alanine aminotransferase (ALT), neutrophil gelatinase-associated lipocalin (NGAL), monocyte chemoattractant protein-1 (MCP-1), and kidney injury molecule-1 (KIM-1) levels among the five groups (Table 1).
Histologic changes of ischemic/reperfusion injury in kidney. (A) Sham group; (B) Control-IR group; (C) Dex-IR group; (D) arrow a represents renal tubular necrosis; (E) arrow b represents renal tubular dilation; (F), arrow c, represents peritubular capillary congestion in outer medulla; (G), histologic scores of renal tubular necrosis; (H) histologic score of renal tubular dilation; (I) histologic scores of peritubular capillary congestion in outer medulla, * Two rats scores 1 in the IR-Dex group. Sham indicates sham operation; Control‒IR (ischemia/reperfusion), left kidney ischemia for 60 min and reperfusion; Dex (dexmedetomidine)‒Sham, sham operation and infusion of dexmedetomidine; Dex‒IR, pre-ischemia infusion of dexmedetomidine 30 min before left kidney ischemia; IR‒Dex, post-ischemia infusion of dexmedetomidine 30 min after reperfusion of the left ischemic kidney.
Discussion
Our result indicated that pretreatment with dexmedetomidine preserved renal microcirculation after IR-induced acute kidney injury, whereas post-treatment with dexmedetomidine did not. However, neither pretreatment nor post-treatment significantly decreased the injury biomarker levels. Additionally, dexmedetomidine lowered the MAP and heart rate of rats in this study.
Several possible mechanisms might explain why dexmedetomidine could preserve renal microcirculation. First, dexmedetomidine is an alpha-2 agonist, which can cause vasodilation of small vessels, reduce sympathetic activity, and maintain renal microvascular blood flow14. This protective effect of dexmedetomidine on renal microcirculation in rabbits with renal IR injury was reported in the study of Si et al.15, dexmedetomidine can increase the tissue level of superoxide dismutase and increase the antioxidant effect against the oxidative stress resulting from IR injury13,16,17. Moreover, dexmedetomidine has an anti-inflammatory effect through alpha-2 adrenergic receptors, which can reduce the level of inflammatory mediators resulting from IR-induced injury18,19. Antioxidant and anti-inflammatory effects of dexmedetomidine protect the endothelium of small vessels and attenuate the severity of microcirculatory dysfunction. Third, dexmedetomidine has anticoagulation properties, which can prevent microthrombosis of small vessels and thus maintain microvascular blood flow20.
Whether dexmedetomidine can decrease kidney-injury-related biomarkers remains debatable. Several studies revealed that dexmedetomidine decreases kidney-injury-related biomarkers such as NGAL21, KIM-122, and MCP-123. Conversely, Bayram et al.24 and de Carvalho et al.12 found that dexmedetomidine did not reduce NGAL levels. Moreover, our study did not identify a significant decrease in NGAL, KIM-1 and MCP-1 levels. The lower dose of dexmedetomidine administered (3 mcg/kg/h) in our study is a possible reason. Other postulations relate to different doses of dexmedetomidine, different methods of dexmedetomidine administration, and the use of unilateral or bilateral renal artery clamping. For example, Lempiainen et al. proved that preconditioning bolus with a high dexmedetomidine dose (10 mcg/kg) ameliorates renal IR-induced injury11. In the study of Si et al., a high dose of dexmedetomidine (25 mcg/kg) was aministered through intraperitoneal injection23.
Bradycardia and hypotension are the most common side effects of dexmedetomidine7. Low MAP and slow heart rate in the three groups with dexmedetomidine treatment may cause some detrimental effects to the rat kidneys. First, dexmedetomidine might blunt the compensated tachycardia as a result of shock. In our previous endotoxemic rat model, the anti-sympathetic effect of dexmedetomidine reduced excessive tachycardia. In addition, dexmedetomidine can preserve intestinal microvascular blood flow and reduce intestine injury8. However, in this study, we found that although renal microvascular blood flow was preserved, the kidney injuries were not reduced. Thus, the kidney may require high microvascular blood flow and heterogeneity was observed in the blood flow among different organs. Second, renal perfusion is highly pressure dependent25, a dexmedetomidine‒related low MAP level may result in inadequate perfusion pressure while microvascular blood flow is preserved. Inadequate perfusion pressure may cause further kidney injury. It is suggested that researchers of further studies consider combining the administration of dexmedetomidine with vasopressor infusion or fluid supplement to maintain adequate perfusion pressure.
This study has several limitations. First, the sample size was too small to determine the differences of secondary outcomes among the five groups. Each secondary outcome may require different sample sizes to obtain adequate power. Second, observation of the renal surface microcirculation was limited by the instrument used. Advanced instruments or contrast-enhanced ultrasound may help us investigate microcirculation in the deeper kidney layers. Third, the severity of distant organ damage after renal IR-induced injury in this study was mild. Increasing the clamping time of the left renal artery or clamping of the bilateral renal arteries might be considered for investigating severer distant organ damage after renal IR-induced injury. Fourth, only male rats were used in this study. Gender difference of the effect of dexmedetomidine was reported between male and female patients26. Further studies are required to investigate the influence of gender on the microcirculation protective effect of dexmedetomidine. Fifth, circulatory levels of KIM-1, MCP-1, and NGAL did not differ significantly after treatment with dexmedetomidine. Immunohistochemistry for these markers were not available in this study, and further studies are required to investigate whether the tissue levels of these injury biomarkers differ significantly after treatment with dexmedetomidine.
In conclusion, this study demonstrates that pretreatment with dexmedetomidine before IR-induced injury could preserve renal surface microcirculation. However, the adverse effects of hypotension and bradycardia might offset the protective effect of dexmedetomidine on organ injury. Further studies of investigating the protective effects of dexmedetomidine are suggested to consider treating the adverse effects of hypotension and bradycardia.
Methods
Rats and ethics statement
In total, 50 male Wistar rats (body weight 250 ± 50 g; Biolasco Taiwan, Taipei, Taiwan) were used in this study. This study was approved by the Institute Animal Care and Use Committee of National Taiwan University (No. 20170071, College of Medicine, National Taiwan University, Taipei, Taiwan). The rats were handled according to the Ethical Guidelines for the Treatment of Laboratory Animals of the Institute Animal Care and Use Committee of National Taiwan University and kept on a 12-h light/dark cycle and had free access to water and food.
Grouping
These 50 rats were randomly allocated to the following five groups (with 10 rats in each group) (Fig. 5.): (1) Sham: sham operation, (2) Control‒IR: left kidney ischemia for 60 min and reperfusion, (3) Dex (dexmedetomidine)‒Sham: sham operation and infusion of dexmedetomidine (3 mcg/kg/h), (4) Dex‒IR: pre-ischemia infusion of dexmedetomidine (3 mcg/kg/h) 30 min before left kidney ischemia, and (5) IR‒Dex: post‒ischemia infusion of dexmedetomidine 30 min after reperfusion of the left ischemic kidney.
Time course of interventions for different groups. Ten rats were allocated to each group. Microcirculation were examined at baseline and 0, 30, 120, and 240 min. *Time point of the blood test. Sham indicates sham operation; Control‒IR (ischemia/reperfusion), left kidney ischemia for 60 min and reperfusion; Dex (dexmedetomidine)‒Sham, sham operation and infusion of dexmedetomidine; Dex‒IR, pre-ischemia infusion of dexmedetomidine 30 min before left kidney ischemia; IR‒Dex, post-ischemia infusion of dexmedetomidine 30 min after reperfusion of the left ischemic kidney.
Anesthesia, surgical procedure, and ischemic kidney model
The anesthetic procedure was similar to that of our previous related study9. Anesthesia was induced with 4% isoflurane by using an induction chamber connected to an animal anesthesia machine (Midmark, Orchard Park, NY, USA). After a rat was anaesthetized, it was placed in a supine position on an animal warming pad. Then, isoflurane was adjusted to 2% to maintain the anesthetic state. Intramuscular injection of 0.05 mg/kg atropine sulfate was administered to reduce respiratory tract secretion and block vagal reflexes elicited through intestinal viscera manipulation. Tracheostomy was performed, and a 14-G catheter (Surflo; Terumo Corporation, Laguna, Philippines) was inserted into the trachea. Subsequent anesthesia was maintained using 1.0% isoflurane. Polyethylene catheters (PE-50; Intramedic 7411, Clay Adams, Parsippany, NJ, USA) were inserted into the right common carotid artery and external jugular vein. The right common carotid artery catheter was used to continuously monitor arterial blood pressure and heart rate, and the external jugular vein catheter was used for 0.9% saline infusion at a rate of 2 mL/kg/h. Body temperature was continuously monitored. A 3-cm-long midline laparotomy was performed to exteriorize the left kidney for baseline microcirculation examination with a sidestream dark-field (SDF) video microscope and a tissue oxygen monitor (moorVMS‒OXY). Thirty minutes after baseline microcirculation was examined, the left renal artery was clamped for 60 min in the Control‒IR, Dex‒IR, and IR‒Dex groups. The arterial clamp was removed after 60 min, and left kidney reperfusion was observed for 240 min. MAP, heart rate, and subsequent microcirculation examinations were performed at 0, 30, 120, and 240 min after arterial clamp removal. At 240 min, a 2-cm-long sectioning was performed on the antimesenteric aspect of a segment of the terminal ileum, 6‒10 cm from the ileocecal valve, and the exposed mucosa was used for microcirculation examination. After the final examination, blood samples were collected for further laboratory and ELISA tests. Both kidneys were extracted for histologic examinations. Afterwards, the rats was euthanized through exsanguination cardiac arrest under anesthesia.
Protocol of dexmedetomidine treatment
In the Dex‒Sham and Dex‒IR groups, dexmedetomidine was infused at a rate of 3 mcg/kg/h after baseline microcirculation examination, and the infusion fluid rate was controlled at 2 mL/kg/h. The inspired concentration of isoflurane was adjusted from 1.0% to 0.7%. In the IR‒Dex group, dexmedetomidine was infused at rate of 3 mcg/kg/h 30 min after the arterial clamp was removed, and the infusion fluid rate was controlled at 2 mL/kg/h. The inspired concentration of isoflurane was adjusted from 1.0% to 0.7%.
Measurement of microcirculation and tissue oxygen saturation
SDF video microscope (MicroScan, Microvision Medical, Amsterdam, The Netherlands) was used to record microcirculation images of the kidney surface. At each time point, three continuous image sequences (10 s) were digitally stored for each measured site, and data for the three images were averaged for statistical calculations. The images were analyzed using semi-automated analysis software (AVA 3.0, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands)27. TSVD, PSVD, PPSV, and MFI score were obtained according to suggestion from a microcirculation round table conference27. The analyses were conducted by a single investigator who was blinded to grouping. StO2 of the left kidney surface was measured using white light reflectance spectroscopy (tissue oxygen monitor, moorVMS‒OXY, Moor Instruments Ltd, United Kingdom) at the same time points(baseline, and 0, 30, 120, and 240 min). At each time point, three random sites of the measured area on the left kidney surface were examined, and data for these sites were averaged for statistical calculations. StO2 of the terminal ileal mucosa was measured 240 min after the arterial clamp was removed.
Laboratory test and histologic scores
The blood samples obtained at 240 min were used to measure the serum level of ALT, AST, lactate, and creatinine. NGAL, MCP-1, and KIM-1 assays were performed using ELISA kits according to manufacturers’ instructions. The kidneys were sent to the Laboratory Animal Center, College of Medicine, National Taiwan University, for histologic analysis. In the pathology report, the severity of renal tubular necrosis, renal tubular dilation, and congestion of peritubular capillary in outer medulla were defined using histologic scores as follows: 1 = minimal (< 1%); 2 = slight (1‒25%); 3 = moderate (26‒50%); 4 = moderate/severe (51‒75%); and 5 = severe/high (76‒100%)28.
Statistical analysis
Data analysis was conducted using statistical software (SPSS 20; IBM SPSS, USA). Continuous variables with a normal distribution were presented as mean ± standard deviation. Differences in means over time among the five groups were analyzed through repeated measures analysis followed by a post hoc analysis using Tukey’s test. Furthermore, differences in means at 240 min among the five groups were analyzed through an one-way analysis of variance (ANOVA) followed by a post hoc analysis using Tukey’s test. The primary outcome of this study was to compare PSVD among the five groups. This study was powered (10 rats for each group) to detect a 9% difference in PSVD on kidney surface at 240 min after reperfusion of ischemic kidney, with a α level of 0.05 and a β level of 0.2, assuming a control PSVD of 40 ± 2.2 mm/mm2 based on the result of our pilot study. A P value of < 0.05 was considered to indicate statistical significance. Non-normally distributed data, including creatinine and histologic scores were presented as median (interquartile range) values among the five groups and analyzed using the Kruskal‒Wallis test followed by a post hoc comparison using the Mann‒Whitney U test. A P value < 0.005 was considered significant with a Bonferroni correction. Because non-normally distributed data, including MFI scores and PPSV, were constants in the Sham and Dex-sham groups, these data were presented as median (interquartile range) and compared among the Control-IR, Dex-IR, and IR-Dex groups using the Kruskal‒Wallis test followed by a post hoc comparison using the Mann‒Whitney U test. A P value < 0.017 was considered significant with a Bonferroni correction.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hartmann, P. et al. Limb ischemia-reperfusion differentially affects the periosteal and synovial microcirculation. J. Surg. Res. 178, 216–222. https://doi.org/10.1016/j.jss.2012.03.001 (2012).
Cicco, G., Panzera, P. C., Catalano, G. & Memeo, V. Microcirculation and reperfusion injury in organ transplantation. Adv. Exp. Med. Biol. 566, 363–373 (2005).
Tang, H. et al. Role of NADPH oxidase in total salvianolic acid injection attenuating ischemia-reperfusion impaired cerebral microcirculation and neurons: implication of AMPK/Akt/PKC. Microcirculation 21, 615–627. https://doi.org/10.1111/micc.12140 (2014).
Chen, W. X. et al. Effect of notoginsenoside R1 on hepatic microcirculation disturbance induced by gut ischemia and reperfusion. World J. Gastroenterol. 14, 29–37 (2008).
Cabrales, P., Tsai, A. G. & Intaglietta, M. Perfluorocarbon in microcirculation during ischemia reperfusion. J. Am. Coll. Surg. 204, 225–235. https://doi.org/10.1016/j.jamcollsurg.2006.11.007 (2007).
Keating, G. M. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs 75, 1119–1130. https://doi.org/10.1007/s40265-015-0419-5 (2015).
Coursin, D. B. & Maccioli, G. A. Dexmedetomidine. Curr. Opin. Crit. Care 7, 221–226 (2001).
Yeh, Y. C. et al. Effects of dexmedetomidine on intestinal microcirculation and intestinal epithelial barrier in endotoxemic rats. Anesthesiology 125, 355–367. https://doi.org/10.1097/ALN.0000000000001135 (2016).
Yeh, Y. C. et al. Dexmedetomidine prevents alterations of intestinal microcirculation that are induced by surgical stress and pain in a novel rat model. Anesth. Analg. 115, 46–53. https://doi.org/10.1213/ANE.0b013e318253631c (2012).
Liu, X. et al. Dexmedetomidine versus propofol sedation improves sublingual microcirculation after cardiac surgery: a randomized controlled trial. J. Cardiothorac. Vasc Anesth. 30, 1509–1515. https://doi.org/10.1053/j.jvca.2016.05.038 (2016).
Lempiainen, J. et al. Dexmedetomidine preconditioning ameliorates kidney ischemia-reperfusion injury. Pharmacol. Res. Perspect. 2, e00045. https://doi.org/10.1002/prp2.45 (2014).
de Carvalho, A. L. et al. Dexmedetomidine on renal ischemia-reperfusion injury in rats: assessment by means of NGAL and histology. Ren. Fail. 37, 526–530. https://doi.org/10.3109/0886022X.2015.1006118 (2015).
Cakir, M. et al. The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren. Fail. 37, 704–708. https://doi.org/10.3109/0886022X.2015.1011550 (2015).
Soliman, R. & Hussien, M. Comparison of the renoprotective effect of dexmedetomidine and dopamine in high-risk renal patients undergoing cardiac surgery: a double-blind randomized study. Ann. Card Anaesth. 20, 408–415. https://doi.org/10.4103/aca.ACA_57_17 (2017).
Si, Y. N. et al. Effects of dexmedetomidine on microcirculatory perfusion in rabbits with renal ischemia/reperfusion injury: quantitative evaluation with contrast-enhanced ultrasound. Nan Fang Yi Ke Da Xue Xue Bao 36, 628–632 (2016).
Sahin, T. et al. The effects of dexmedetomidine on liver ischemia-reperfusion injury in rats. J. Surg. Res. 183, 385–390. https://doi.org/10.1016/j.jss.2012.11.034 (2013).
Ammar, A. S., Mahmoud, K. M., Kasemy, Z. A. & Helwa, M. A. Cardiac and renal protective effects of dexmedetomidine in cardiac surgeries: a randomized controlled trial. Saudi J. Anaesth. 10, 395–401. https://doi.org/10.4103/1658-354X.177340 (2016).
Sugita, S., Okabe, T. & Sakamoto, A. Continuous infusion of dexmedetomidine improves renal ischemia-reperfusion injury in rat kidney. J. Nippon Med. Sch. 80, 131–139. https://doi.org/10.1272/jnms.80.131 (2013).
Si, Y. et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J. Transl. Med. 11, 141. https://doi.org/10.1186/1479-5876-11-141 (2013).
Chen, Z. et al. Effect of dexmedetomidine on blood coagulation in patients undergoing radical gastrectomy under general anesthesia: a prospective, randomized controlled clinical trial. Medicine 97, e11444. https://doi.org/10.1097/MD.0000000000011444 (2018).
Balkanay, O. O., Goksedef, D., Omeroglu, S. N. & Ipek, G. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact. Cardiovasc. Thorac. Surg. 20, 209–214. https://doi.org/10.1093/icvts/ivu367 (2015).
Tan, F. et al. Dexmedetomidine protects against acute kidney injury through downregulating inflammatory reactions in endotoxemia rats. Biomed. Rep. 3, 365–370. https://doi.org/10.3892/br.2015.427 (2015).
Si, Y. N. et al. Dexmedetomidine protects against ischemia/reperfusion injury in rat kidney. Eur. Rev. Med. Pharmacol. Sci. 18, 1843–1851 (2014).
Bayram, A. et al. The effects of intraoperative infusion of dexmedetomidine on early renal function after percutaneous nephrolithotomy. Acta Anaesthesiol. Scand 55, 539–544. https://doi.org/10.1111/j.1399-6576.2011.02427.x (2011).
Badin, J. et al. Relation between mean arterial pressure and renal function in the early phase of shock: a prospective, explorative cohort study. Crit. Care 15, R135. https://doi.org/10.1186/cc10253 (2011).
Xiong, M. et al. Propofol-sparing effect of different concentrations of dexmedetomidine : comparison of gender differences. Anaesthesist 68, 15–21. https://doi.org/10.1007/s00101-018-0506-6 (2019).
De Backer, D. et al. How to evaluate the microcirculation: report of a round table conference. Crit. Care 11, R101, https://doi.org/10.1186/cc6118 (2007).
Shackelford, C., Long, G., Wolf, J., Okerberg, C. & Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 30, 93–96. https://doi.org/10.1080/01926230252824761 (2002).
Acknowledgements
We thank Roger Lien (Technician, MicroStar Instruments CO., Ltd., Taipei, Taiwan) for their technical assistances in microcirculation recording. This manuscript was edited by Wallace Academic Editing.
Funding
This work was supported, in part, by research grants from National Taiwan University Hospital (NTUH 105-A125 and NTUH 107-003739).
Author information
Authors and Affiliations
Author notes
A comprehensive list of consortium members appears at the end of the paper.
Consortia
Contributions
J.C.T., W.S.C., and Y.C.Y. designed the study. W.S.C. and Y.C.Y. conducted the study. S.J. Y., C.N.F, M.J.W., and Y.C.Y. analyzed the data. S.J. Y., C.N.F, and Y.C.Y. wrote the main manuscript. S.J. Y. and Y.C.Y. prepared the Figs. 3–5 and Table 1. S.Z.F., W.Z.S., W.S.C., and Y.C.Y. revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, SJ., Fan, CN., Wang, MJ. et al. Effects of dexmedetomidine on renal microcirculation in ischemia/reperfusion-induced acute kidney injury in rats. Sci Rep 11, 2026 (2021). https://doi.org/10.1038/s41598-021-81288-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81288-3
This article is cited by
-
Hexarelin alleviates apoptosis on ischemic acute kidney injury via MDM2/p53 pathway
European Journal of Medical Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.