Abstract
Continuous multi-channel monitoring of biopotential signals is vital in understanding the body as a whole, facilitating accurate models and predictions in neural research. The current state of the art in wireless technologies for untethered biopotential recordings rely on radiative electromagnetic (EM) fields. In such transmissions, only a small fraction of this energy is received since the EM fields are widely radiated resulting in lossy inefficient systems. Using the body as a communication medium (similar to a ’wire’) allows for the containment of the energy within the body, yielding order(s) of magnitude lower energy than radiative EM communication. In this work, we introduce Animal Body Communication (ABC), which utilizes the concept of using the body as a medium into the domain of untethered animal biopotential recording. This work, for the first time, develops the theory and models for animal body communication circuitry and channel loss. Using this theoretical model, a sub-inch\(^3\) [1″ × 1″ × 0.4″], custom-designed sensor node is built using off the shelf components which is capable of sensing and transmitting biopotential signals, through the body of the rat at significantly lower powers compared to traditional wireless transmissions. In-vivo experimental analysis proves that ABC successfully transmits acquired electrocardiogram (EKG) signals through the body with correlation \(>99\%\) when compared to traditional wireless communication modalities, with a 50\(\times\) reduction in power consumption.
Similar content being viewed by others
Introduction
Chronic monitoring of biopotential signals has paved the way for a better understanding of neural pathways along with improved therapeutic treatments. Recent proliferation in small form-factor wearables has enabled a new domain of continuous health monitoring. This coupled with miniaturized biological sensors, both in the wearable and the implantable domain has resulted in gathering valuable information regarding the body. Surface biopotential signals such as EKG, sEMG (Surface Electromyography), EEG (Electroencephalogram) have been studied as a means of understanding the behavior of the body. Neural recording systems interfaced with the peripheral nervous system have been extensively explored as a method to acquire meaningful data that is used to predict and understand the motor, sensory, proprioceptive, and feedback functions of the brain.
The use of animals in biological research and medicine has been a longstanding practice given the similarity between the animal and human anatomy and physiology. The current state of the art in animal signal recording includes miniaturized wearable or implantable devices with stimulation and recording capabilities. These devices placed on the body of the animal or implanted inside the animal body, transmit data to an external unit capable of receiving and processing this information. A vast majority of animal recording systems still rely on tethered units especially in cases when continuous long-term information is a priority. Tethered systems are not limited by the data rates and are a gold standard for reliable comprehensive information, this, however, does come with a few caveats. Tethered systems are limited by the bias and irritation introduced by these devices on the subject. Long experimental duration is possible, however, the experimental arena is hindered by the need for long wires which in turn results in restricted movement of animals. Noisy systems result from the animals tucking and biting at the wires. Tethered systems require signal conditioning electronics to be placed external to the body, long wires also act as a site for infection and require buffers to prevent signal attenuation1. To reduce these effects, wireless telemetry of signal information is needed. Wireless recording systems have since evolved from discrete modules to system on chip devices. These small form-factor wireless devices eliminate the bias introduced by the tethered systems; however, it is limited by the high-power consumption due to the need for up-conversion of the baseband signal to higher radio frequencies2 and loss due to radiative communication. Animal studies, in particular, require the animal to wear a heavy battery pack to meet this high-power requirement. The need for constant replacement of batteries limits the experimental duration and causes undue stress on the animals which in turn corrupts the data3. The advancement in the field of wireless power transfer allowed for a longer experimental duration without the need for heavy battery packs or constant replacement of batteries4. However, due to the inherent high-power consumption of the sensing node with electromagnetic communication, high-power needs to be harvested, increasing the on-device harvester size significantly.
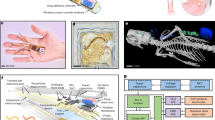
Animal Body Communication: Overview of Animal Body Communication on a Rodent Model. Custom designed sensor node is placed on the back of the rat. This sensor node is capable of sensing and transmitting the surface biopotential signals via Bluetooth and Animal Body Communication. The sensed signal is transmitted through the body to the conductive surface in the form of OOK (On–Off Keying) sequences. The specially designed rat cage is isolated from the ground surface. A conductive surface is placed on the base of the rat cage which is then connected to a Data Acquisition System (DAQ) which receives the transmitted signals. The Bluetooth receiver and DAQ are connected to a PC for processing, with the DAQ and PC ground referenced. In this model Bluetooth communication acts as a validity check for ABC. This figure was created using the software Paint 3D by Microsoft Corporation (Version 6.2009.30067.0).
To overcome these constraints there is a need for a low power communication modality capable of withstanding the continuance of the experiment, along with having an unbiased model in which the animals are free to move in their natural environments. In this work, we introduce a novel communication modality that uses the animal body as a medium to transmit information. This system eliminates the bias introduced by the tethered systems and has a significant size, weight, area, and power benefits compared to electromagnetic communication systems. We demonstrate this Electro-Quasistatic Animal Body Communication (EQS-ABC) as a low loss, efficient channel which addresses the aforementioned drawbacks of both tethered and wireless EM communication systems. Using the body of the animal as a ‘wire’ensures signal confinement resulting in significantly lower losses when compared to traditional wireless technologies. Here, we demonstrate ABC using a rodent model and explain the theory and biophysical models of ABC, followed by ABC demonstration with a sub-inch\(^3\) [1″ × 1″ × 0.4″], custom-designed sensor node in the subsequent sections. Figure 1, describes the concept of the ABC setup, surface biopotential signals are acquired by a custom-designed sensor node that then transmits the signal using ABC through the subcutaneous tissues of the animal body using EQS-ABC. These signals are picked up by a receiver connected to the ground isolated conductive surface. In this setup, we also transmit the signals using Bluetooth as a method to compare the ABC transmitted signal with an established communication modality. In this pilot study, we use the concept of body communication and extend it into the animal domain enabling long term benefits in energy consumption along with size, weight, and area benefits. The low power requirement enables the use of smaller batteries or coils in the case of energy harvested nodes. We show the concept of ABC applied to animal biomedical studies, this modality can be extended into the neuroscience domain. This work explores the recent advances in Electro-Quasistatic Human Body Communication (EQS-HBC) and adapts it to facilitate animal biopotential recording. Experiments were performed with EKG signals of the rat as the chosen surface biopotential signal.
State of the art in wireless biopotential recording
The first biopotential discovery dates back to 1666 when Francesco Redi measured the EMG from a specialized muscle in the electric eel5, the field of animal biopotential recordings evolved from tethered systems to wireless systems in the year 1948 when Fuller and Gordon first used radio communication for biopotential signal transmission6. Presently, multi-channel recording devices with wireless power transfer is being implemented, this coupled with smart devices and experimental arenas permits in-sensor analytics. The evolution and detailed comparison of the state of the art in biopotential recording has been described in a later section.
Biopotential signals, both non-invasive (skin surface) and invasive, have been studied as a means of building bio-electronic medical devices. The central nervous system controls the body and this control can be observed by studying the changes in the peripheral physiological factors such as changes in the heart rate, muscle activity, and breathing. To study these changes, long term monitoring of these physiological signals is necessary7. EKG is one of the most widespread diagnostic tools in medicine and the similarity between human and rat EKG8 has permitted the study of various physiological conditions and cardiac diseases9,10. Along with EKG signals, other surface biopotentials such as sEMG and EEG are studied in rats, analysis of these signals is used in sleep studies, epilepsy, locomotive analysis, and effect of spinal cord injuries11,12.
The study of the brain along with the body is essential in understanding the control mechanisms of the brain on physiology. Sican Liu described a novel neural interface system for simultaneous stimulation and recording of EEG/EMG and ENG (Electroneurogram) signals13. Along with surface biopotential signals, invasive recording allows for localized, high fidelity signal analysis. Neural biopotential signal analysis is a topic of extensive research in experimental neuroscience, with the aim of improving the quality of life of people with severe sensory and motor disabilities. Wireless neural recording systems have been described in insects, rodents and non-human primates. In rodents particularly, various neural interface systems which include bidirectional communication has been explored14,15. Application-specific integrated circuit (ASICs) for neuro-sensing applications has been described for implantable neurosensors4,16,17,18.
Chronic multi-channel neural recording is a powerful tool in studying dynamic brain function. Multi-electrode arrays permit recording of more than one channel simultaneously enabling neuroscientists to explore different regions of the brain in response to a particular stimulus. Bandwidth constraints limit the number of channels that can be recorded simultaneously resulting in a trade-off between the number of channels that can be simultaneously recorded, power requirements, and the form factor of the device. For example, Borton et al. designed an implantable hermetically sealed device that was capable of sending neural signal information via a wireless data link to a receiver placed 1 meter away. This system permitted 7-h of continuous operation17. Chae et al. describes a 128-channel 6 mW wireless neural recording IC with on the fly spike detection for one selected channel. A sequential turn-on method is used to minimize the power requirement19. Similarly, Miranda et al. developed a 32-channel system that can be used for 33 h continuously but requires two 1200 mAh batteries20. To achieve a meaningful experimental duration, the power consumption is often \(>10\) mW, generally dominated by the communication (radio) power. Thus, it is evident that wireless neural interfaces are power-hungry and there is a need for constant replacement of the batteries or selective channel selection in a chronic setting. To overcome these constraints wirelessly powered neural interfaces were developed, which eliminates the need for constant replacement of the batteries. Implantable devices, in particular, need wirelessly powered devices to reduce the need for a battery at the implant site. Enriched experimental arenas allow for the constant transmission of power facilitating chronic recordings. Yeager et al. developed a wireless neural interface, NeuralWISP capable of sending neural information over a 1-m range21. Lee et. al describes an EnerCage-HC2 to inductively transfer power to a 32-channel implantable neural interface4. Wireless power transfer though ensures longer experimental duration, one has to take into account the exposure to high electromagnetic fields along with concerns regarding excessive heat dissipation. Thus, it is evident that neural recordings are limited by size constraints and overall power consumption. This leads to the next advancement in wireless biopotential recording with electro-quasistatic animal body communication, which aims to use the animal body as the transmitting medium similar to the concept of human body communication. In the following section we describe the concept of body communication and also describe how the ABC differs from HBC while still having similar advantages.
Body Communication Basics
Body communication-based wearable technology has gained prominence over recent times as a communication modality for sending real-time information.
Animal Body Communication model: (a) Represents the field lines corresponding to the rat body and the transmitter ground plane. (b) The rat body couples to the conductive plane and the associated capacitances are depicted. The conductive plane is ground isolated and forms the capacitance C\(_{CSG}\). The node consists of the ground plane which couples with the earth’s ground to form the capacitive return path. (c) The circuit model associated with the experimental setup is shown in the figure. The simplified model of the animal body communication circuit shows that the output voltage is proportional to the conductive plane to ground capacitance C\(_{CSG}\), return path capacitance C\(_{G\_TX}\), and load capacitance C\(_{L}\). (d) Simplified Human Body Communication circuit model and transfer function as depicted by Maity et al.22. The rat models in (a) and (b) were created using the software Paint 3D by Microsoft Corporation (Version 6.2009.30067.0).
Recent advances in using the human body as a channel for bio-physical communication has resulted in an energy-efficient secure information exchange modality23. HBC was first proposed as a method to connect devices on a Personal Area Network (PAN) by Zimmerman24, using a capacitively coupled HBC model where the return path is formed by the electrode to ground capacitance. The transmitter capacitively couples the signal into the human body which is then picked up at the receiver end. Galvanic coupling-based HBC introduced by Wegmueller et al.25, the signal is applied and received differentially by two electrodes respectively. HBC utilizes the conductivity of the human body for a low transmission loss, high-efficiency transmission modality making it ideal for energy-constrained devices. Traditional wireless body area networks (WBAN) use EM signals that radiates outside the body all around us, resulting in only a fraction of the energy being received. This radiative nature and high frequencies in WBANs are typically high energy and of the order of 10 nJ/bit26. Recent advances have shown impulse-radio ultra wideband (IR-UWB) to be more energy efficient than traditional WBANs, with a energy of 1 nJ/bit27. Now, if the body’s conductivity is used, it provides a low loss broadband channel that is private (the full bandwidth is available for communication). This low loss and wide bandwidth availability along with the low-frequency operation results in ultra-low power body communication at 415 nW28 as well as very low energy communication at 6.3 pJ/bit29. Low-frequency HBC was not widely adopted due to the high loss at these frequencies because of resistive (50 \(\Omega\)) termination30. Recently we demonstrated, by using capacitive termination, the loss in the EQS region is reduced by a factor of \(>100\), making it usable29,31. The first bio-physical model for EQS-HBC was developed by Maity et al.22 and a detailed understanding of the forward path32 and return path33 was described. Datta et al.34 describes an advanced biophysical model to capture channel variability. EQS-HBC is presently the most promising low-power, low-frequency communication alternative for WBAN. It has also been shown that the EQS-HBC adheres to the set safety standards35.
The state of the art in body communication has been restricted to human body communication. In this work we propose to utilize the recent developments in the concept of body communication and apply it to the animal body for biopotential and neural recordings, reducing the size, weight, area, and power of the device. We propose a capacitive termination EQS communication from a sensing node on the rat’s body and also device an experimental arena to pick up these EQS signals most efficiently. This form of communication utilizes electro-quasistatic transmission through the conductive layers of the rat below the skin surface. The skin is a high impedance surface while the inner tissue layers are conductive. The transmission of the electro-quasistatic signals through the body with a capacitive return path at frequencies below 1 MHz ensures that the signal is contained within the body.
Animal Body Communication—Biophysical Theoretical Model
As already established, Human Body Communication has been explored as a viable communication model, extending this to an animal body allows for a low loss, efficient channel model, compared to the traditional wireless modalities currently used. Figure 2a,b depicts the concept of Animal Body Communication, the rat body capacitively couples with the signal plane. The transmitter placed on the body of the rat modulates this electric field to transmit OOK (On–Off Keying) sequences corresponding to the sensed biopotential signal. The experimental arena is designed such that the animal moves around on a conductive surface, which is isolated from the earth’s ground. This surface picks up the EQS signals coupled onto the animal’s body and is received through ground-referenced receiver. Hence, the received voltage is inversely proportional to the capacitance of the signal plane to ground (the less the capacitance the easier it is for the wearable device on the animal to modulate the potential of the animal body and the surface).
The circuit model for Animal Body Communication is described in Fig. 2c. At lower frequencies the skin impedance and the series body and foot impedance is negligible compared to the capacitance between the signal plane and ground. Given the operation of ABC in the electro-quasistatic regime, these impedances can be neglected in the computation of the channel loss. From the simplified circuit model, the output voltage V\(_{o}\) and the input voltage V\(_{In}\) are related as follows:
In Fig. 2c,d we compare the animal body circuit model along with the established human body circuit model. The output voltage is a function of C\(_{CSG}\) as shown in Eq. (1) for ABC, while it a function of C\(_{Body}\) in case of HBC. The approximate value of C\(_{CSG}\) was found to be 50pF. This value was computed for an output voltage, V\(_{o}\) of 45mV, which was experimentally determined and an input voltage, V\(_{In}\) of 3.3 V. The value of the return path capacitance was computed using the equation \(C_{G\_TX} = 8\varepsilon _0a\), where a is the radius of the ground plane33. This value was calculated to be 0.9pF for a radius of 0.0127 m. The receiver load capacitance from the datasheet was found to be 14pF. Body communication-based systems heavily depend on the body surface and ground sizes. Maity et al. describes the Bio-Physical Model for HBC22, Fig. 3 compares the ABC model with the HBC model. Traditional HBC systems have the human body connected to a transmitter and a receiver placed on a different part of the body. The human body has a much larger surface area when compared to an animal. In this ABC setup, the sensor node is placed on the body of the rat, while the receiver is a large conductive plane. This large conductive plane ensures that the movement of the rat is not restricted, and data can be continuously recorded. In contrast, in human body communication, the body is on the earth’s ground and there exists a trunk path to ground. Due to this, the output voltage is affected by the body capacitance, unlike in the animal body setup. Figure 3 illustrates the key components of HBC and ABC. The capacitance of the body varies from ABC and HBC due to the fact that the ABC channel model consists of the additional conductive surface on which the rat is free to move. Another important component is the rat foot impedance, in ABC the rat’s feet rest on the conductive surface. C\(_{Foot}\) and R\(_{Foot}\) change depending on the position of the rat’s foot on the conductive plane.
In the human model, the received signal is collected from the body surface itself, thus the output voltage depends on the capacitive return path of both the transmitter and the receiver. In the ABC model, the conductive surface is ground isolated and connected to an oscilloscope which acts as the receiving unit. The transmitter couples to the floating body and the return path capacitance C\(_{G\_TX}\) from the earth’s ground plane to the transmitter ground plane completes the loop, allowing for signal transmission. The receiver in ABC is the oscilloscope signal probe, which can be modeled as the load capacitance C\(_{L}\) in parallel with the load resistance R\(_{L}\). This oscilloscope is earth ground referenced and hence eliminates the capacitive return path of HBC. The low-loss in ABC coupled with low-carrier frequency communication (as a wire) enables ABC power consumption to be much lower when compared to wireless communication modalities such as Bluetooth. This reduced power enables longer duration experiments with small form factor devices.
Results
Animal Body Communication was explored as a new modality for the transmission of biopotential signals. The sensing and transmitting devices are built using off the shelf components and consist of a communication module, a processing module, a power source, and an interface to connect it to the rat body. Surface electrodes are placed on the skin surface of the rat, after employing appropriate skin preparation techniques and then connecting the electrodes to the front end of the device.
Experimental Test Setup: The sensor node is placed on the rat skin surface and connected to the surface electrodes (Right Arm (RA), Left Arm (LA), and Right Leg (RL)) for EKG sensing. The rat’s feet are taped on a conductive copper plate, the plate is then connected to a receiver for ABC transmission. The Bluetooth receiver and the ABC receiver are connected to the computer for signal acquisition and processing. The setup also consists of the anesthetizing unit which delivers the anesthetizing drug and oxygen to rat. The rat model was created using the software Paint 3D by Microsoft Corporation (Version 6.2009.30067.0).
Biopotential information is sensed and modulated for transmission, simultaneously transmitting the signal over Bluetooth and through the body of the rat as Animal Body Communication. Bluetooth has long been used as a wireless communication modality and widely cited in literature as a means to transmit biopotential information. In this work, we use this gold standard of communication to compare the biopotential information received from the ABC transmitter and Bluetooth module. In an ideal situation, a tethered system acts as the gold standard, however, body communication cannot be achieved when the system is ground connected (as in the case of a tethered system), with a ground connected system, the results would be optimistic and incorrect22. A correlation analysis is performed to compare both signals. Experiments were performed on a rat to prove the feasibility of Animal Body Communication.
Animal Body Communication Experimental Setup
The Animal Body Communication setup is tested on Sprague Dawley rats, experiments were performed on anesthetized rats. In this study, capacitive coupling is used as a means to achieve Animal Body Communication. The details of the sensor node are described in the methods section.
Anesthetized rats are placed on a non-conductive surface, the sensor node, in a casing, is placed on the rat skin surface and patch connectors are used to connect to the surface electrodes. The feet of the rat are placed on a conductive copper plate, signals are acquired using the sensing unit, then transmitted via Bluetooth to a receiver connected to a computer as shown in Fig. 4. The device is capable of transmitting both over Bluetooth and through ABC simultaneously. Only the feet are connected to the conductive plane while leaving the body on a non-conductive surface. This depicts a case when the rat moves in a cage with only the feet on the bottom plane. ABC happens through the transmission of OOK sequences from the node through the body, to the conductive copper plate. These signals are picked up using an oscilloscope connected to the conductive plane. The oscilloscope signal probe is connected to the conductive plane while the ground probe is left floating. EKG signals are acquired using a three-electrode setup with the electrodes placed on the Right Arm (RA), Left Arm (LA), and Right Leg (RL). The RL serves as the right leg drive, common to EKG recording systems. Additional monitoring systems such as the anesthetizing setup and body vital measurement systems are present in this experimental setup not part of the communication setup. This setup aims to mimic the setup as described in Fig. 1. The copper plates act as the conductive surface which in an awake recording setup will form the base on which the rat is free to move.
Time division multiplexing
Biopotential signal measurements require the body to be grounded to improve the CMR of the entire system. Grounding the body eliminated the floating nature which is essential for body communication. Thus, to sense and transmit biopotential signals, time-division multiplexing is used. Such multiplexing between each sensing cycle and transmission cycle ensures that surface biopotentials can be sensed accurately and also transmitted via body communication.
Time multiplexed sensing and transmission cycles: (a) Sensing cycles consists of acquiring the single-lead EKG signal using three electrodes, Right Arm, Left Arm and Right Leg. ABC Transmission starts at the end of the sensing cycle and has the same duration as the sensing cycle. Bluetooth transmission duration is almost twice the ABC transmission duration. Upon the completion of both transmissions, the sensing cycle restarts, and this cycle repeats. (b) Post-processing steps on ABC transmitted sequence and Bluetooth transmitted sequence transmission.
In the event of simultaneous sensing and transmission, given that the transmitter is placed on the surface of the body, the sensing electrodes pick up the OOK sequences used in the transmission, resulting in a corrupted sensed signal. To avoid this, sensing and transmission are time multiplexed. This technique is critical for body communication with surface biopotentials. Simultaneous surface biopotential sensing and EQS body communication should be possible and is part of the future work. Figure 5a describes the time multiplexing cycles, data is sensed for a period of 5s followed by the transmission for 10s. The transmission of ABC and Bluetooth occurs simultaneously, however Bluetooth sequences take longer to transmit due to packet constraints resulting in a longer transmission time as compared to the sensing time. Following the transmission cycle, the sensing cycle repeats. ABC data is sent as OOK sequences which are then demodulated and decoded to retrieve the EKG sample as shown in Fig. 5b. Bluetooth samples are transmitted as characters corresponding to the ADC codes, which are then converted to corresponding samples to compare with the transmitted ABC signal.
Time Domain Correlational Analysis on Acquired EKG signal
EKG signals are chosen for testing the animal body communication setup. The experiment was conducted on a total of 8 Rats over 2 months. This current set-up ensures continuous synchronized transmission of the biopotential signal from both the Bluetooth module and the ABC transmitter.
Rat Electrocardiogram (EKG) Analysis: (a) Bluetooth and ABC transmitted signal. (b) Overlaid Bluetooth and ABC transmitted signal data, depicting overlap of the EKG peaks. (c) Complete 5s Bluetooth and ABC transmitted EKG Signal. (d) Time multiplexed ABC transmission cycles, 5s transmission time followed by a 10s wait time to allow for Bluetooth transmission and next cycle sensing. (e) Overlaid plots of EKG Signals from eight rats with correlational analysis between Bluetooth and ABC transmitted signals. (f) Time varying correlational analysis of one 5s sensing cycle of rat EKG signal.
As mentioned before, the signals are time-multiplexed allowing Animal Body Communication. The EKG signal is sensed for a period of 5s followed by simultaneous transmission of ABC and Bluetooth. Figure 6 shows the EKG sample comparison, Fig. 6a shows the Bluetooth and the ABC EKG data for a period of 0.6s, these two signals are overlaid in Fig. 6b, the PQRST peaks of the characteristic EKG signal align, similarly, the data is compared for all 8 rats and correlation coefficients across each trial is depicted in Fig. 6e. The correlation coefficient for all the rats was seen to be \(> 99\%\). In Fig. 6c, we can see the complete overlaid 5s sample. Time multiplexing results in an ABC transmission period followed by a wait time for the completion of the Bluetooth transmission and sensing. Figure 6d depicts this time-multiplexed ABC data, with this cycle being continuous. Figure 6f depicts the variation of correlation across the entire 5s window, the correlation between Bluetooth and ABC is approximately 1 throughout the 5s window depicting a reliable transmission system.
Since we are using commercial off the shelf (COTS) components not designed for body communication, the sensitivity is significantly worse compared to what can be achieved with custom-designed transceivers. Due to this, the BER (bit error rate) of the system is higher. We are able to show that even with a high BER, good correlations between the Bluetooth transmitted data and ABC transmitted data. We have further elaborated on this in the Discussion section.
Effect of Distance of Foot from Conductive Surface to Received ABC Signal
A key component of animal body communication is the dependence of the received signal on body resistance and capacitance. Variation of the distance of the foot from the conductive surface changes the magnitude of the received signal which then tests the robustness of the system. Experimental analysis with only one foot on a conductive surface with varying distances shows that even with the foot raised, OOK sequences can be picked up from the conductive strip. The distance from the conductive surface was varied from 3 cm (Foot Raised) to a negligible distance when the rat foot is taped to the conductive surface (Foot Completely on Surface). It is evident that as the distance from the conductive surface reduces, the amplitude of the coupled signal increases. However, even at large distances, though the signal amplitude is lower, the received Bluetooth and ABC signal can be decoded and display \(>97\%\) correlation.
Effect of distance on received signal strength: (a) Experimental setup to measure the effect of distance variation on received OOK signal; In position 6, the rat foot is closest to the conductive surface and in position 1 the foot is furthest from the conductive surface. (b) Received OOK signals of different distance variations and the decoded signals for the different distances (c) Correlation between Bluetooth and ABC received signals as a function of distance, each depicting correlation \(>97\%\). (d) Variation in the amplitude of the received signal as a function of distance, for a transmitter amplitude of 3.3 V.
This case evaluated only with one foot coupling to the conductive surface. In reality, the entire rat body would couple to the conductive surface increasing the received signal. When the rat foot is raised above the conductive surface, the foot resistance R\(_{Foot}\) becomes infinite, however, even in that case C\(_{Foot}\) and C\(_{B\_CS}\) exists as shown in Fig. 2 and the body as a whole will couple to the signal plane. Here C\(_{Foot}\) and C\(_{B\_CS}\) are the capacitances of the foot to the conductive surface and the body to the conductive surface respectively. This ensures the necessary path for transmission of the signal. Since body communication works on capacitive coupling, even without complete contact with the conductive surface, the OOK sequences couple to the conductive surface. It is highly unlikely that the rat would have all feet raised above the conductive plane, for a long time. In the event of improper contact with the conductive surface or when the rat jumps, it is shown that the signals can still be received on the conductive plane and can be successfully decoded. In the event that the rat has all of its feet and body away from the conductive surface, which is not a common occurrence, C\(_{Foot}\) and C\(_{B\_CS}\) would reduce and the signal may be lost. For such cases, bi-modular redundancy can be introduced in the system in which case, the lost data could be retrieved by transmitting it at a later instance. This form of error correction used for short burst errors, can ensure robust transmission. In Fig. 7 the variation of the distance of the rat foot from the conductive surface is shown, position 1 is furthest away from the conductive strip, while in position 6, the rat foot is completed taped on the conductive surface. The amplitude of the received signal increases with the reduction in distance for a set transmitter voltage of 3.3V. It can be seen that in all cases the sequences can be decoded, and all show high correlations with Bluetooth.
Discussions
Capacitive coupling from the transmitter ground plane to the earth’s ground ensures the return path necessary for animal body communication.
Effect of an External Ground Plane: (a) Experimental setup depicting the electric field lines from the transmitter ground plane to the external ground plane; Capacitive return path from the transmitter ground to the external ground plane. (b) In vivo test setup with a hand-held external ground plane. The external ground plane is placed above the rat body similar to the experimental setup, mirroring the top of a rat cage. The rat models in (a) were created using the software Paint 3D by Microsoft Corporation (Version 6.2009.30067.0).
The presence of a large conductive signal plate prevents the existence of such a capacitive path to ground. The addition of a conductive plane connected to the receiver ground placed above the rat body provides the necessary return path. The transmitter ground plane along with this floating ground plane forms the capacitance C\(_{G\_TX}\). In the setup with a rat cage, as shown in Fig. 8b the top and bottom surfaces of the rat cage are made conductive, with the top plate connected to the receiver ground, while the bottom plate, which acts as the signal plane is connected to the signal probe of the receiver. During in vivo tests, the ground plane consisted of a hand-held conductive plane above the anesthetized rat body. Only the feet of the rat are connected to the signal plane, with a slot in the conductive plane to allow for the placement of the rat. Figure 8a describes the need for the addition of the conductive ground plane in a model rat cage. Similar to Fig. 2b, the capacitive coupling from the device ground plane to the external ground plane provides the necessary return path. The sensor node placed on the body of the rat has the transmitter ground plane on the top surface and the signal electrode touches the body of the rat. The addition of this floating ground plane allows for the use of a large signal plane, providing a larger experimental arena for the rat to move on without being limited by the loss of the signal return path.
Limitations and scope for future work
Some neuroscientific behavioral experiments involve swimming or require the animal to walk on treadmills and mazes. Due to the nature of the signal path, a modified setup would be needed to accommodate a conductive plane. In some cases, such as swimming, this may not be possible. However, there is a possibility to extend this setup into cases involving mazes with a special setup where the maze bottom surface is made conductive to receive the ABC transmitted signals. Similarly, in the case of treadmills, a copper strip can be stuck on the belt which can then be connected to the receiver. Conductive textile could be used as the plane through which ABC signals are received. Also, given that the signal electrode needs to be interfaced with the body of the animal, this could act as a limitation in certain applications. For this setup, we consider Bluetooth as the gold standard and compare the ABC signal with the Bluetooth signal. For the receiver, we use an oscilloscope-based system to recover the data. The sensitivity of this system is low, similar to traditional oscilloscopes which results in low SNR and higher BER. The \(\hbox {SNR}_{{dB}}\) of the received signal was computed to be in the range of 7-8dB. Based on this SNR, the BER of the system for OOK modulation is of the range of 10\(^{-3}\) to 10\(^{-2}\) as stated by Salehi and Proakis36. Our system has a similar BER of 10\(^{-2}\). Even with a high BER, the system achieves good correlations between the two communicated signals. With a custom-designed receiver with higher sensitivity, it is possible to achieve a much lower BER of the order of 10\(^{-4}\) for a 500 kHz carrier28
In this system, we use time-division multiplexing to achieve ABC communication. There is a need for simultaneous sensing and monitoring in many neuroscientific studies. The basic physics of body communication does not change, and we have evaluated that body communication does not affect the actual electrophysiological signal. Given this, there is a path to simultaneous sensing and transmission which will involve a change in the engineering design of the current system. It is also possible to extend this system to support data recordings from multiple animals. For animals that are not singly housed, Frequency Division Multiple Access (FDMA) can be used, which allows us to use different carrier frequencies that can be separated on the receiver end allowing continuous transmission from multiple animals on a common conductive plane. For Electro-Quasistatic body communication, the carrier frequency is below 20 MHz. The IEEE 802.15.6 standard for Wireless Body Area Networks (WBAN) specifies short-range, wireless communication in the vicinity of, or inside a human. The standard specifies the center frequency at 20 MHz with a bandwidth of 5 MHz double-sided supporting 2.5 Mbps with OOK. For a 16 QAM (Quadrature amplitude modulation) system, the data rate can be as high as 2.5 \(\times\) 10\(^{6}\,\times\) 4. Thus, this standard specifies communication at extremely low power and data rate up to 10 Mbps. In this work, we have focused on single-channel recording up to 50 Kbps. In the future, increasing the carrier frequency would allow us to increase the data rate up to 10 Mbps.
Conclusion
To conclude, in this work we demonstrate a novel communication modality in the animal studies domain and demonstrate how the advances in Electro-Quasistatic Human Body Communication (EQS - HBC) can be adapted to animal biopotential recording.
Biopotential signals were acquired from the rat and transmitted using Animal Body Communication. The theory and channel model for animal body communication was developed and a custom-designed sensor node was built and tested in vivo. The correlation between standard wireless transmission systems and ABC was found to be \(> 99\%\) in these tests. The power consumption for Bluetooth transmission was observed to be 29.5 mW, while the power consumption for ABC transmission was found to be 0.5 mW. This depicts a \(> 50\times\) reduction in power. If a custom-designed IC is built with only ABC transmission, the device size and power can be further significantly reduced, along with the possibility to make these high bandwidth systems. The effect of variation of distance of the foot of the rat from the receiver signal plane was observed and it is clear that reliable signals can be received even with improper contact or raised feet, adding to the reliability of this communication channel. A modified test setup was explored as an additional technique to ensure robust communication. While in this study, EKG was the chosen biopotential, it can be extended to neural signal acquisition and transmission, where low power communication modalities are essential. In Fig. 9 the evolution of animal biopotential recording was studied, the key differences between tethered, wireless and EQS-ABC was compared and it was found that EQS-ABC can prove to be the next advancement in this domain, allowing for an ultra-low power, efficient channel model.
Methods
System Architecture
Size, weight, area, and power consumption of wireless recording devices have the potential to significantly affect animal behavior and compromise the quality and length of recordings, thereby hindering scientific studies. Overcoming these obstacles formed the core design objectives for the custom node for the acquisition of biopotential signals and wireless transmission of data and resulted in the following initial specifications. Physical dimensions were constrained to one cubic inch, which is sufficiently small to be placed on a rodent and large enough to house the various components. The net weight and power consumption were capped at 50g, and 50 mW respectively. This posed a significant challenge since the analog front end for sensing, micro-controller for computing, wireless communication for comparison purposes, power management, and animal body communication had to be miniaturized and integrated into the device while meeting the power budget.
The system architecture as shown in Fig. 10a can be broadly divided into three blocks, the custom-wireless signal acquisition node, the Bluetooth receiver connected to the data logging system (computer), and the animal body communication receiver. The custom node consisted of two vertically stacked custom-designed printed circuit boards (PCB) which were populated with commercially available integrated circuits and discrete components. The top board in the stack contained the micro-controller and Bluetooth System on Chip (SoC), along with the antenna and matching network on the top layer. The bottom layer consisted of the power management system and charging connector. The analog front end was housed on the top layer of the bottom stack, with the bottom layer serving as the electrode for animal body communication. The detailed view and the assembled view of the sensor node is shown in as shown in Fig. 10b,c respectively.
A System on Chip (NRF52840, Nordic Semiconductors) which integrates an ARM Cortex-M4F micro-controller and a Bluetooth 5.0 transceiver was selected to form the core of the node since it would minimize the device footprint and power consumption. The SoC utilizes Bluetooth 5.0 - Bluetooth Low Energy (BLE), which is the latest version of the Bluetooth wireless communication. The on board 1MB flash memory and 256 KB RAM was sufficiently large to store the sampled signals and implement in-sensor analytics in the future. Power efficiency was further improved by utilizing the on-chip DC-DC converters. A 3.7 V 150 mAh Lithium Polymer rechargeable battery is directly soldered onto the board, along with the battery management circuitry. In this custom node, we use a battery management integrated circuit, MCP73831 by Microchip Technologies. This linear charge management controller was selected for its small physical size and the need for a low number of external components.
The custom node collected the EKG signals from a zero-insertion force connector placed on the PCB. Signal conditioning and sampling of the EKG signal was performed by another SoC (ADS1298, Texas Instruments). This analog front-end chip incorporates a programmable gain differential amplifier and right-leg drive generation for conditioning EKG signals, which were subsequently sampled at 500 Hz by a 24-bit analog to digital converter. The SoC was programmed to optimize signal acquisition quality and power consumption. The sampled signals were sent to the micro-controller through an on-chip Serial Peripheral Interface.
The sampled data was stored in a buffer in the micro-controller until the transmission window started. The samples were then converted to characters and transmitted as a string over Bluetooth after adding delimiters to differentiate between subsequent samples. For Animal Body Communication, the sample was transmitted in its original 24-bit binary integer form after creating packets by adding two bits (binary 1) at the start and end of the sample. Each bit in ABC was represented by on-off keying, wherein a 500 kHz, 50% duty cycle square wave was turned on (binary 1) or off (binary 0). The amplitude of each bit is 3.3 V, which is the output of the micro-controller. ABC data was transmitted at 25 Kbps, which was significantly lower than the minimum required Bluetooth bandwidth of 45 Kbps, which excludes the overhead added by the Bluetooth stack.
The custom-designed node was packaged in a 3D-printed housing of dimensions 25 mm \(\times\) 25 mm \(\times\) 10 mm, which is equivalent to 0.39 cubic inches. It had a net weight of 20g and average power consumption of 29.5 mW (with Bluetooth transmission for data comparison purposes) which resulted in approximately 20 h of battery life. This is 19 times smaller and has more than twice the battery life when compared to a commercial wireless unit (Bio-Radio). We expect a much longer lifetime when the Bluetooth transmission is turned off and only ABC transmission is turned on. The power required for sensing is typically orders of magnitude lower than the power required for communication, thus the system power is dominated by this communication power. The ABC transmission power is 50\(\times\) lower when compared to the Bluetooth transmission power and this translates into an order of magnitude improvement in the device lifetime and reduction in the battery size.
The Bluetooth receiver was essentially another NRF52840 SoC connected via USB to the data logging system, which in this case was a computer. This setup was used instead of the inbuilt Bluetooth device of the computer since it would be easier to collate data from multiple transmitters.
The conductive signal plane is connected to the high impedance receiver probe. A computer-based oscilloscope, by Pico Technologies, was used as the ABC receiver. The OOK sequences are sampled at 3.9 MSamples/s and collected for post-processing.
Signal Processing
OOK sequences collected from the ABC receiver are sent to a computer for processing. Signals are first band-passed between 400 to 600 kHz with 80 dB attenuation software filters. Filtered sequences are demodulated using envelop detection and thresholding. Sequences are then decoded using the start and stop bit followed by software error correction. Bluetooth sequences in the form of ADC codes are converted to corresponding voltage values and compared to the received ABC signals.
Communication Protocols
-
Time Multiplexed Data
As discussed earlier, a requisite for animal body communication especially while recording surface biopotential signals is the need to time multiplex the sensing and transmission periods.
-
Error-Correcting Algorithms
There is a possibility to bring in redundancy into the communication channel to ensure the robustness of this communication modality. We have shown that if the rat foot is lifted from the conductive surface, the received signal can still be picked up by the receiver. The goal of this paper is to ensure that long term recordings of freely moving animals can be obtained. To ensure that there is a successful transmission of data, error-correcting algorithms become a necessity.
Bi-modular Redundancy can be introduced by repeating packets over time. In the event of a jump or signal drop, repeated packets ensure that the signal information is faithfully transmitted. This technique reduces the data rate due to the added redundancy. Block Codes a common error-correcting technique of encoding the data in blocks, such that the code is a linear combination of the message and parity bits in a linear block code.
Surgery
All surgical procedures were performed under aseptic conditions at Purdue Animal Facility. 5% Isoflurane gas and oxygen were used to anesthetize the rat in an induction chamber, followed by a continuous flow of 2.5% Isoflurane gas with oxygen delivered through a nose cone. The dosage of Isoflurane and the flow of oxygen is continuously monitored to ensure that the rat does not respond to the toe pinch while still maintaining a steady breathing rhythm and observable pink extremities. A heating pad is placed below the rat to maintain the body temperature and lubricating drops are added to the eyes of the rat to prevent drying. The skin surface is shaved and cleaned for the placement of the surface electrodes. The device is placed on a shaved surface on the belly of the rat with the signal plane touching the skin surface. The surface electrodes are connected to the device using patch connectors. The experiment was performed on 8 Sprague Dawley rats which is sufficient to show the science and working of ABC.
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and all experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals. The experiments were closely monitored and reviewed by Purdue Animal Care and Use Committee (PACUC).
References
Harrison, R. et al. A low-power integrated circuit for a wireless 100-electrode neural recording system. In 2006 IEEE International Solid State Circuits Conference-Digest of Technical Papers, 2258–2267, https://doi.org/10.1109/JSSC.2006.886567 (IEEE, 2006).
Liu, X. et al. A fully integrated wireless compressed sensing neural signal acquisition system for chronic recording and brain machine interface. IEEE Trans. Biomed. Circ. Syst. 10, 874–883. https://doi.org/10.1109/TBCAS.2016.2574362 (2016).
Szuts, T. A. et al. A wireless multi-channel neural amplifier for freely moving animals. Nat. Neurosci. 14, 263. https://doi.org/10.1038/nn.2730 (2011).
Lee, B. et al. An implantable peripheral nerve recording and stimulation system for experiments on freely moving animal subjects. Sci. Rep. 8, 6115. https://doi.org/10.1038/s41598-018-24465-1 (2018).
Reaz, M. B. I., Hussain, M. S. & Mohd-Yasin, F. Techniques of EMG signal analysis: detection, processing, classification and applications. Biol. Proc. Online 8, 11–35. https://doi.org/10.1251/bpo115 (2006).
Fuller, J. & Gordon, T. The radio inductograph—a device for recording physiological activity in unrestrained animals. Science 108, 287–288. https://doi.org/10.1126/science.108.2802.287 (1948).
Shikano, Y., Sasaki, T. & Ikegaya, Y. Simultaneous recordings of cortical local field potentials, electrocardiogram, electromyogram, and breathing rhythm from a freely moving rat. J. Vis. Exp. JoVEhttps://doi.org/10.3791/56980 (2018).
Konopelski, P. & Ufnal, M. Electrocardiography in rats: a comparison to human. Physiol. Res.https://doi.org/10.33549/physiolres.933270 (2016).
Pereira-Junior, P. P., Marocolo, M., Rodrigues, F. P., Medei, E. & Nascimento, J. H. Noninvasive method for electrocardiogram recording in conscious rats: feasibility for heart rate variability analysis. Anais da Academia Brasileira de Ciencias 82, 431–437. https://doi.org/10.1590/S0001-37652010000200019 (2010).
Zigel, Y. et al. A surface ecg-based algorithm to determine the atrial refractoriness of rodents during electrophysiological study. Cardiovasc. Eng. Technol. 2, 388–398. https://doi.org/10.1007/s13239-011-0055-5 (2011).
Keller, A. V. et al. Electromyographic patterns of the rat hindlimb in response to muscle stretch after spinal cord injury. Spinal Cord 56, 560–568. https://doi.org/10.1038/s41393-018-0069 (2018).
Sitnikova, E., Hramov, A. E., Grubov, V. & Koronovsky, A. A. Rhythmic activity in eeg and sleep in rats with absence epilepsy. Brain Res. Bull. 120, 106–116. https://doi.org/10.1016/j.brainresbull.2015.11.012 (2016).
Liu, S. et al. A novel neural interfacing electrode array for electrical stimulation and simultaneous recording of eeg/emg/eng. In 2019 International Conference on Intelligent Informatics and Biomedical Sciences (ICIIBMS), 1–5, https://doi.org/10.1109/ICIIBMS46890.2019.8991516 (IEEE, 2019).
Angotzi, G. N., Boi, F., Zordan, S., Bonfanti, A. & Vato, A. A programmable closed-loop recording and stimulating wireless system for behaving small laboratory animals. Sci. Rep. 4, 5963. https://doi.org/10.1038/srep05963 (2014).
Hampson, R. E., Collins, V. & Deadwyler, S. A. A wireless recording system that utilizes bluetooth technology to transmit neural activity in freely moving animals. J. Neurosci. Methods 182, 195–204. https://doi.org/10.1016/j.jneumeth.2009.06.007 (2009).
Yin, M., Borton, D. A., Aceros, J., Patterson, W. R. & Nurmikko, A. V. A 100-channel hermetically sealed implantable device for chronic wireless neurosensing applications. IEEE Trans. Biomed. Circ. Syst. 7, 115–128. https://doi.org/10.1109/TBCAS.2013.2255874 (2013).
Borton, D. A., Yin, M., Aceros, J. & Nurmikko, A. An implantable wireless neural interface for recording cortical circuit dynamics in moving primates. J. Neural Eng. 10, 026010. https://doi.org/10.3791/569800 (2013).
Biederman, W. et al. A fully-integrated, miniaturized (0.125 mm2) 10.5 μw wireless neural sensor. IEEE J. Solid-State Circ. 48, 960–970. https://doi.org/10.3791/569801 (2013).
Chae, M. S., Yang, Z., Yuce, M. R., Hoang, L. & Liu, W. A 128-channel 6 mw wireless neural recording IC with spike feature extraction and uwb transmitter. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 312–321. https://doi.org/10.3791/569802 (2009).
Miranda, H., Gilja, V., Chestek, C. A., Shenoy, K. V. & Meng, T. H. Hermesd: a high-rate long-range wireless transmission system for simultaneous multichannel neural recording applications. IEEE Trans. Biomed. Circ. Syst. 4, 181–191. https://doi.org/10.3791/569803 (2010).
Yeager, D. J., Holleman, J., Prasad, R., Smith, J. R. & Otis, B. P. Neuralwisp: a wirelessly powered neural interface with 1-m range. IEEE Trans. Biomed. Circ. Syst. 3, 379–387. https://doi.org/10.1109/TBCAS.2009.2031628 (2009).
Maity, S. et al. Bio-physical modeling, characterization, and optimization of electro-quasistatic human body communication. IEEE Trans. Biomed. Eng. 66, 1791–1802. https://doi.org/10.3791/569805 (2018).
Sen, S., Maity, S. & Das, D. The body is the network: to safeguard sensitive data, turn flesh and tissue into a secure wireless channel. IEEE Spectr. 57, 44–49. https://doi.org/10.1109/MSPEC.2020.9271808 (2020).
Zimmerman, T. G. Personal area networks: near-field intrabody communication. IBM Syst. J. 35, 609–617. https://doi.org/10.1147/sj.353.0609 (1996).
Wegmueller, M. S., Oberle, M., Felber, N., Kuster, N. & Fichtner, W. Signal transmission by galvanic coupling through the human body. IEEE Trans. Instrum. Meas. 59, 963–969. https://doi.org/10.3791/569807 (2009).
Sen, S. Context-aware energy-efficient communication for iot sensor nodes. In 2016 53nd ACM/EDAC/IEEE Design Automation Conference (DAC), 1–6, https://doi.org/10.1145/2897937.2905005 (IEEE, 2016).
Spark microsystems - products, https://doi.org/10.3791/569808 (accessed: 11.11.2020).
Maity, S. et al. A 415 nw physically and mathematically secure electro-quasistatic hbc node in 65nm cmos for authentication and medical applications. In 2020 IEEE Custom Integrated Circuits Conference (CICC), 1–4, https://doi.org/10.1109/CICC48029.2020.9075930 (IEEE, 2020).
Maity, S., Chatterjee, B., Chang, G. & Sen, S. Bodywire: a 6.3-pj/b 30-mb/s- 30-db sir-tolerant broadband interference-robust human body communication transceiver using time domain interference rejection. IEEE J. Solid-State Circ. 54, 2892–2906. https://doi.org/10.1109/JSSC.2019.2932852 (2019).
Bae, J., Cho, H., Song, K., Lee, H. & Yoo, H.-J. The signal transmission mechanism on the surface of human body for body channel communication. IEEE Trans. Microw. Theory Tech. 60, 582–593. https://doi.org/10.33549/physiolres.9332700 (2012).
Das, D., Maity, S., Chatterjee, B. & Sen, S. Enabling covert body area network using electro-quasistatic human body communication. Sci. Rep. 9, 1–14. https://doi.org/10.1038/s41598-018-38303-x (2019).
Maity, S., Mojabe, K. & Sen, S. Characterization of human body forward path loss and variability effects in voltage-mode hbc. IEEE Microw. Wirel. Compon. Lett. 28, 266–268. https://doi.org/10.1109/LMWC.2018.2800529 (2018).
Nath, M., Maity, S. & Sen, S. Towards understanding the return path capacitance in capacitive human body communication. IEEE Trans. Circ. Syst. II Express Briefshttps://doi.org/10.1109/TCSII.2019.2953682 (2019).
Datta, A., Nath, M., Yang, D. & Sen, S. Advanced biophysical model to capture channel variability for eqs capacitive hbc. arXiv preprint arXiv:2010.15339 (2020).
Maity, S. et al. On the safety of human body communication. IEEE Trans. Biomed. Eng.https://doi.org/10.1109/TBME.2020.2986464 (2020).
Salehi, M. & Proakis, J. Digital communications. McGraw-Hill Educ. 31, 32 (2007).
Piccolino, M. Luigi galvani and animal electricity: two centuries after the foundation of electrophysiology. Trends Neurosci. 20, 443–448. https://doi.org/10.33549/physiolres.9332704 (1997).
AlGhatrif, M. & Lindsay, J. A brief review: history to understand fundamentals of electrocardiography. J. Commun. Hosp. Internal Med. Perspect. 2, 14383. https://doi.org/10.3402/jchimp.v2i1.14383 (2012).
Caton, R. The electric currents of the brain. Br. Med. J. 2, 278. https://doi.org/10.33549/physiolres.9332706 (1875).
Shadid, R. & Noghanian, S. A literature survey on wireless power transfer for biomedical devices. Int. J. Antennas Propag.https://doi.org/10.1155/2018/4382841 (2018).
Sodagar, A. M., Wise, K. D. & Najafi, K. A wireless implantable microsystem for multichannel neural recording. IEEE Trans. Microw. Theory Tech. 57, 2565–2573. https://doi.org/10.33549/physiolres.9332707 (2009).
Najafi, K. & Wise, K. D. An implantable multielectrode array with on-chip signal processing. IEEE J. Solid-State Circ. 21, 1035–1044. https://doi.org/10.1109/JSSC.1986.1052646 (1986).
Acknowledgements
This work was supported by National Science Foundation CAREER Award (ECCS 1944602), Air Force Office of Scientific Research YIP Award under Grant FA9550-17-1-0450 and by the NIH Stimulating Peripheral Activity to Relieve Conditions (SPARC) program (OT2OD023847 and OT2OD028183). (The contents of this article do not necessarily represent the official views of the NIH). The authors would like to thank Dr. Shovan Maity, graduated PhD student, Shramana Chakraborty, M S Student, Jongcheon Lim, Umm E Hani Abdullah, David Yang, Arunashish Datta, Debayan Das, Mayukh Nath, PhD Students as well as Visiting Scholar Gargi Bhattacharya at Purdue University for their immense co-operation and support during the experiments.
Author information
Authors and Affiliations
Contributions
S.Sriram, S.A., M.P.W., S.S., conceived the idea, S.A. and S.Sriram designed the ABC sensor node. S. Sriram conducted the theoretical analysis and performed the experiments under the guidance of M.P.W. and S.Sen. All authors contributed to the drafting of this manuscript and have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sriram, S., Avlani, S., Ward, M.P. et al. Electro-Quasistatic Animal Body Communication for Untethered Rodent Biopotential Recording. Sci Rep 11, 3307 (2021). https://doi.org/10.1038/s41598-021-81108-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81108-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.