Abstract
Some hemodialysis patients are not suitable for creation of an arteriovenous fistula (AVF) or arteriovenous graft (AVG). However, they can receive a tunneled cuffed central venous catheter (tcCVC), but this carries risks of infection and mortality. We aimed to evaluate the safety and effectiveness of brachial artery transposition (BAT) versus those of tcCVC. This retrospective study evaluated hemodialysis patients who underwent BAT or tcCVC placement because of severe heart failure, hand ischemia, central venous stenosis or occlusion, inadequate vessels for creating standard arteriovenous access, or limited life expectancy. The primary outcome was whole access circuit patency. Thirty-eight patients who underwent BAT and 25 who underwent tcCVC placement were included. One-year patency rates for the whole access circuit were 84.6% and 44.9% in the BAT and tcCVC groups, respectively. The BAT group was more likely to maintain patency (unadjusted hazard ratio: 0.17, 95% confidence interval: 0.05–0.60, p = 0.006). The two groups did not have significantly different overall survival (log-rank p = 0.146), although severe complications were less common in the BAT group (3% vs. 28%, p = 0.005). Relative to tcCVC placement, BAT is safe and effective with acceptable patency in hemodialysis patients not suitable for AVF or AVG creation.
Similar content being viewed by others
Introduction
The United States, Japan, and Europe have aging populations of patients with end-stage renal disease1,2,3. Their comorbidities and/or blood vessel conditions can make these patients unsuitable for creation of an arteriovenous fistula (AVF) or arteriovenous graft (AVG) for hemodialysis (HD). Furthermore, cardiovascular disease, including congestive heart failure (CHF), is highly prevalent and the primary cause of death among HD patients4,5,6,7,8. Moreover, a retrospective analysis revealed that creating an AVF or an AVG was associated with right ventricular (RV) dilation, which was independently associated with worsening of CHF and an increased risk of death among HD patients9. Vascular access (VA)-induced ischemia is also common among patients who have peripheral circulatory disorders, such as older patients, patients with diabetes, and patients who undergo frequent operations for arteriovenous (AV) access10,11.
The European Society for Vascular Surgery guidelines indicate that long-term placement of a tunneled cuffed central venous catheter (tcCVC) may be appropriate for HD patients with VA-induced ischemia, CHF, or a limited life expectancy12. The Kidney Disease Outcomes Quality Initiative guidelines also indicated that a tcCVC can be used in cases where VA is difficult because of outflow vein problems, such as central venous occlusion or stenosis13. However, some observational studies have identified a poorer survival rate among HD patients who received a tcCVC than among patients who underwent creation of an AVF or AVG14,15,16,17,18. Moreover, placement of a CVC is associated with an increased risk of infection19,20,21.
Some reports have described using an artery as an alternative VA strategy22,23, and brachial artery transposition (BAT) is a recognized alternative to tcCVC placement in patients who are not suitable for AVF or AVG creation1,24,25. The BAT strategy for VA uses the brachial artery for blood delivery and the superficial veins for blood return. Therefore, if either the superficial brachial artery or superficial vein has a problem and cannot function as VA or be dialyzed, the entire access circuit will be lost.
Moreover, cannulation of the brachial artery must be delayed for > 2 weeks until the brachial artery has completely adhered to the connective tissue. Finally, although several reports have described BAT, few studies have compared the outcomes to those from tcCVC as a more standard option. Therefore, this study evaluated the safety and effectiveness of BAT and tcCVC as tertiary access strategies in HD patients who were not suitable for AVF or AVG creation.
Results
Patient characteristics
One hundred and three patients underwent surgery for BAT or tcCVC. Thirty-three patients had an AVF attached to the BAT and were excluded because an AVF could be created in these patients and the indications were not met. In these patients, the length of the AVF was less than 10 cm, and the BAT was created to increase the area available for puncture. Patients who changed VA from BAT to tcCVC (n = 3) and tcCVC to BAT (n = 3) were excluded because of the possible influence of the reciprocal relationship. Patients with tcCVC who underwent surgery with poorly controlled infections were excluded (n = 1). This study finally included 38 patients who underwent BAT and 25 patients who underwent permanent tcCVC placement for HD during the study period (Fig. 1). The baseline characteristics were generally similar, although the BAT group had a higher proportion of patients with heart failure with reduced ejection fraction (HFrEF) (Table 1). The detailed causes of CHF and hand ischemia are shown in Supplementary Table 1.
The study flowchart. Among patients who underwent BAT (n = 74) or tcCVC placement (n = 29), we excluded patients who created both AVF and BAT, had previously undergone BAT (n = 3) or tcCVC placement (n = 3), as well as 1 patient who had a tcCVC implanted with inadequate infection control. AVF arteriovenous fistula, BAT brachial artery transposition, tcCVC tunneled cuffed central venous catheter, VA vascular access.
Dialysis progress
The dialysis progress is shown in Table 2. The mean follow-up intervals were 537 ± 435 days for the BAT group and 196 ± 291 days for the tcCVC group. In the BAT group, the brachial artery was cannulated after approximately 3 weeks if wound healing was observed, which involved the use of a 17-gauge needle on the blood delivery side and puncture of any superficial vein using a 16–17-gauge needle on the blood return side. The mean interval from the BAT operation to its first successful use for dialysis was 28.9 ± 14.5 days. The proportions of cases with adequate dialysis (Kt/V of > 1.2) were 93.3% in the BAT group and 78.9% in the tcCVC group, although the difference was not significant (p = 0.19).
Patency rates for the whole access circuit
In the BAT group, the surgical success rate was 94.7%, as 2 patients died because of VA-unrelated acute coronary syndrome before the first successful cannulation. During the observation period, the BAT group had a primary patency rate of 92.9% and a secondary patency rate of 100%. The 1-year patency rates for the whole access circuit were 84.6% in the BAT group and 44.9% in the tcCVC group (p = 0.002) (Fig. 2). Univariable Cox proportional hazard analyses revealed that the BAT group had a greater likelihood of whole access circuit patency (HR: 0.17, 95% CI 0.05–0.60, p = 0.006, Table 3). Furthermore, the BAT group had a higher patency rate in the analyses that were adjusted for age, hypertension, HFrEF, and antiplatelet drugs (all p = 0.022 to 0.002, Table 4).
In the BAT group, loss of whole access circuit patency was observed in 3 patients, which was related to loss of the return side veins in all 3 cases. There were no cases of hand ischemia requiring amputation due to occlusion or ischemia of the brachial artery. In the tcCVC group, loss of whole access circuit patency was observed in 8 patients, which was related to catheter dysfunction (2 patients) or catheter-related bloodstream infection (6 patients). Information regarding the new or additional VA strategies is provided in Supplementary Table 2.
Overall survival rates
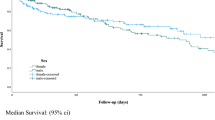
During the observation period, 19 patients died in the BAT group and 13 patients died in the tcCVC group. The causes of death are described in Supplementary Appendix 1. The 1-year overall survival rates were 70.3% in the BAT group and 43.0% in the tcCVC group. No significant differences were observed in the Kaplan–Meier analysis (p = 0.146, Fig. 3) or the unadjusted Cox regression analysis (Supplementary Table 3).
Complications
The complications are listed in Table 5. The BAT group had a significantly lower rate of severe complications (3% vs. 28%, p = 0.005). In addition, the BAT group was significantly less likely to develop VA-related infection (0% vs. 24%, p = 0.003). Only 1 patient in the BAT group experienced a severe complication, which involved a CTCAE grade 3 brachial artery aneurysm, and secondary patency of the BAT was restored after surgical resection of the brachial artery aneurysm. All other complications in the BAT group were CTCAE grade ≤ 2 and did not require surgery, with patency of the whole access circuit preserved throughout the observation period. No patients died because of BAT-related complications.
In the tcCVC group, 6 patients experienced VA-related catheter-related bloodstream infection, which were generally CTCAE grade 3, and 5 patients experienced catheter-related thrombosis. One patient developed superior vena cava syndrome and pulmonary embolism, and ultimately underwent open heart surgery because of CTCAE grade 4 thrombosis in the superior vena cava. No patients died because of tcCVC-related complications.
Discussion
Previous studies have indicated that the catheter-related bloodstream infection is several times more common than AVF and AVG, and 12-month rate of catheter-related bloodstream infection is approximately 50% among patients who receive HD26,27, and infection is the second most common cause of hospitalization and death among HD patients28,29. Furthermore, the incidence of catheter-related infection can reach 0.28–1.7 episodes per 1000 CVC days30,31,32. Thus, strategies are needed to reduce the rate of VA-induced infection. The frequency of catheter infections may be affected by its definition. The definition of CRBSI in our study was based on the KDOQI-2019 guideline. The present study revealed an incidence of 1.28 episodes per 1000 CVC days in the tcCVC group, which is comparable to the previously reported rates, although the BAT group had a lower risk of VA-induced infection. Moreover, the BAT group had a significantly lower rate of serious complications.
Venous access through superficial veins may be difficult in the early stages due to unstable puncture sites, although the puncture site is stabilized subsequently, and venous puncture becomes easier in many cases. If the puncture site is unstable, a return blood route cannot be obtained, and the whole BAT access circuit is abandoned. In the present study, the whole BAT access circuit was abandoned within 1 year and, therefore, loss of the whole BAT access circuit beyond 1 year could not be observed. Therefore, although the BAT circuit required more time to prepare (vs. tcCVC placement), it may be a useful option for patients who have a life expectancy of > 1 year, given the lower likelihood of severe complications and longer patency of the whole access circuit. Furthermore, we observed that the BAT group had relatively good primary and secondary patency rates throughout follow-up. This may be because, in cases with AVG or AVF creation, the major causes of lost patency are stenosis of the graft-vein anastomosis and thrombotic occlusion due to intimal hyperplasia plus impaired remodeling (related to reduced blood flow) at the juxta-anastomotic stenosis33,34,35,36. In contrast, the BAT may provide relatively physiological hemodynamics and a lower shear stress (vs. AVF and AVG), which may prolong the patency of the circuit. In previous reports, primary patency at 1 year of BAT has been as high as over 90%24,37. In these studies, although about 2–4% of patients developed brachial artery embolism, the majority of patients were asymptomatic, and there were no cases of serious hand ischemia such as upper limb amputation. When BAT was created, side branches were ligated generally. The formation of collateral blood vessels may have improved the tolerability of brachial artery thrombosis. Therefore, BAT is considered to be a relatively safe VA to use, even considering the risk of thromboembolism.
Among patients who undergo dialysis 3 times per week, the recommended Kt/V target is 1.4 and the lower limit is 1.238. A prospective observational study has also indicated that the rates of inadequate dialysis (Kt/V of < 1.2) were 19.2% among patients with an AVF, 9.9% among patients with an AVG, and 27.5% among patients with a CVC39. The present study revealed that only 6.7% of patients in the BAT group had inadequate dialysis, which may be related to the independent inflow and outflow routes in these patients. A nationwide Japanese survey has also indicated that the mean Kt/V values were 1.37 for artery transposition, 1.28 for tcCVC, and 0.97 for temporary CVC40. Thus, the Kt/V value in our tcCVC group was relatively good, and it is possible that further accumulation of cases might identify a significantly better Kt/V value for BAT (vs. tcCVC).
Antiplatelet drugs are used to reduce AV access thrombosis, although these drugs do not significantly reduce AV access failure41,42. The KDOQI-2019 guideline suggests that the use of systemic anticoagulants for improvement of the patency of CVCs has more disadvantages than advantages13. There is also no information regarding whether antiplatelet drugs can prolong CVC patency. The present study revealed that loss of the whole BAT access circuit was only related to loss of the superficial veins as the return side vessels. Therefore, it is unclear whether antiplatelet drugs can prolong patency in this setting.
Limitations
The present study has several limitations. First, this retrospective single-center study only evaluated patients who were considered eligible for BAT or tcCVC placement, suggesting that the results are not representative of all dialysis patients. Second, we did not consider long-term patency of the whole access circuit, and short-term loss of patency in the BAT group was only related to loss of the return side veins. Thus, we suggest that strategies may be needed to protect the return side vessels and preserve the patency of the whole BAT access circuit. Third, we observed that BAT provided sufficient blood flow (140–250 mL/min), although various required flow rates have been reported in the United States (300 mL/min), the United Kingdom (250 mL/min), Germany (200 mL/min), and Japan (160 mL/min)43. A nationwide Japanese survey has indicated that artery transposition can provide a flow rate of > 300 mL/min; however, such cases accounted for approximately 1.5% of the studied population40, which suggests that further international studies are needed. Fourth, several observational studies have suggested that the relatively poor prognosis of patients with tcCVC placement (vs. AVF) is related to their poor general condition, which may suggest that physicians prefer CVCs for patients who have a poor general condition44,45. The same thing could happen in the selection of BAT and tcCVC. Fifth, we adjusted the analyses for known confounding factors, although unidentified confounding factors (e.g., patient status) might also have contributed to our findings. Finally, it is possible that the tcCVC was preferentially selected if there was a paucity of superficial veins as a return route.
Conclusion
The present study revealed that, relative to tcCVC placement, BAT had a longer patency period for the whole access circuit and a lower risk of severe complications, especially VA-related infection. Thus, BAT may be a safe and effective tertiary access strategy in HD patients who are not suitable for AVF or AVG creation. However, further studies may be needed to confirm the long-term rates of patency, complications, and overall survival.
Materials and methods
Ethics
This study complies with the “Ethical Principles for Medical Research Involving Human Subjects” of the Ministry of Health, Labour and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology. In accordance with these guidelines, the Shizuoka General Hospital Research Ethical Committee determined that individual patient informed consent was not necessary because this study was an analytical study of existing information and patients were given the right to refuse participation through disclosure. With the approval of the committee (SGHIRB#2020089), the disclosure document was made available on the website of Shizuoka General Hospital, and the information of individuals was anonymized for analysis.
Study design, setting, and patients
We retrospectively identified HD patients who underwent BAT (BAT group) or tcCVC placement (tcCVC group) as a tertiary VA strategy at our local general hospital between January 2016 and September 2020. Patients were included if their original VA strategy involved no VA, AVF creation, or AVG creation. Patients were excluded if their original VA strategy involved BAT or tcCVC placement. In addition, 1 patient was excluded because the tcCVC was implanted with inadequate infection control.
Data collection
The patients’ medical records were reviewed to collect data regarding age, sex, body mass index, diabetes, hypertension, ischemic heart disease, peripheral artery disease, stroke, HFrEF, left ventricular ejection fraction of < 40%, heart valve disease, chronic obstructive pulmonary disease, cancer, antiplatelet drugs, warfarin, cause of end-stage renal disease, indications for tertiary VA, original type of VA, and dialysis history (in years). Patient outcomes were monitored through visits at our hospital and the last visit was defined as the last follow-up date. In cases where patients underwent dialysis at other hospitals, their physicians were contacted to confirm VA patency, complications, and survival.
Indications for tertiary vascular access
The indications for BAT or tcCVC placement were severe CHF, hand ischemia, central venous occlusion or stenosis, inadequate vessels for standard AV access, or limited life expectancy. Severe CHF was identified based on the presence of HFrEF, previous/concurrent severe ischemic heart disease, and/or previous/concurrent heart valve disease. Hand ischemia was defined as signs and symptoms of stage ≥ 2 VA-induced ischemia (loss of pain sensation, or pain during HD or exercise). Cases with central venous occlusion or stenosis were identified based on inadequate or delayed contrast enhancement or non-enhancement during digital subtraction angiography, which had been performed to investigate the cause of inadequate dialysis and/or edema caused by VA. Inadequate vessels for standard AV access were identified based on artery and vein diameters of < 2 mm at the planned anastomosis site. Limited life expectancy was defined as expected survival of < 1 year.
Procedures
All patients in the tcCVC group received a Palindrome Precision catheter (Chronic Dialysis Catheter, Minneapolis, MN, USA).
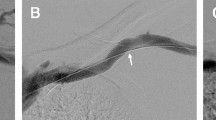
The schema for the BAT surgery is shown in Fig. 4. Local or conduction anesthesia was used for the BAT group. A continuous 15–20-cm skin incision was made over the brachial artery from the elbow to the axilla. The brachial artery was then liberated entirely from the surrounding subcutaneous tissue, and all brachial artery branches were carefully ligated using 4-0 silk sutures before they were divided. A subcutaneous fat pocket was created to hold the brachial artery, which was relocated from the ulnar side to the upper arm's radial side. The subcutaneous fat was sewn using absorbable 4-0 nylon sutures under the brachial artery to prevent prolapse from the subcutaneous pocket before skin closure.
Schema of brachial artery transposition surgical procedure. (A) Continuous skin incision was made over the brachial artery from the elbow to the axilla. (B) The brachial artery is then liberated entirely from the surrounding subcutaneous tissue. (C) A subcutaneous fat pocket was created to hold the brachial artery. The subcutaneous fat was sewn using absorbable 4-0 nylon sutures under the brachial artery. (D) After closing the wound, the transposed brachial artery is positioned slightly radially. The black arrows indicate the brachial artery. The white arrows indicate a superficial vein used as a return route. The superficial vein has not been moved.
Outcomes
The primary outcome was the patency rate of the whole access circuit. In the BAT group, primary patency was calculated from the initial operation to the first re-operation for BAT-related complications, and secondary patency was calculated from the initial operation to the abandonment of BAT (i.e., secondary patency was judged present for all re-operations that restored patency and did not lead to abandonment). The patency of the whole BAT access circuit was calculated from the initial operation to the abandonment of BAT as a functional VA (e.g., because of blood return dysfunction), and patency of the whole circuit was not considered lost until an unsuccessful reoperation. The whole access circuit also includes the transposed brachial artery and the veins for blood return. In the tcCVC group, primary patency was calculated from the initial catheter placement until any intervention that did not involve catheter removal or replacement. Patency of the whole tcCVC access circuit was defined as the interval between catheter placement and catheter exchange or removal because of any cause, which did not include adjustments to catheter positioning or anticoagulant use (e.g., heparin or urokinase) unless the catheter was removed at that time.
The secondary outcomes were the rates of complications and mortality. Complications were evaluated based on version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE), and severe complications were defined as grade ≥ 3 complications. Overall survival was calculated from the initial BAT operation or tcCVC placement to death because of any cause. In the BAT group, surgical success was defined as successful puncture for maintenance HD after the BAT procedure.
Statistical analysis
Variables were reported as mean ± standard deviation, median (range), or number (percentage), as appropriate. Categorical variables were analyzed using the χ2 test, and continuous variables were analyzed using the t-test. The Kaplan–Meier method and log-rank test were used to evaluate rates of patency and overall survival. Cox proportional hazard models were used to evaluate whether the patients’ baseline characteristics were associated with the outcomes and the results were reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Differences were considered statistically significant at p-values of < 0.05. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R software (The R Foundation for Statistical Computing, Vienna, Austria)46.
Ethical approval
The retrospective study protocol was approved by the ethics committee of Shizuoka General Hospital (SGHIRB#202089).
Data availability
Patient consent was not obtained for disclosure of the raw data, which are not freely available. However, an anonymized dataset can be requested from the Clinical Trials Management Office (Shizuoka General Hospital, 4-27-1 Kita-Ando, Aoi-ku, Shizuoka, 420-8527 Japan, E-mail: chiken-sougou@shizuoka-pho.jp. All requests must be approved by the Steering Committee and must be accompanied by data analysis plan.
References
Nitta, K. et al. Annual dialysis data report 2017, JSDT Renal Data Registry. Ren. Replace. Ther. 5, 1 (2019).
Kramer, A. et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: A summary. Clin. Kidney J. 12, 702–720 (2019).
United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. https://adr.usrds.org/2020/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities (2019).
Verbeke, F. et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: Outcome of the calcification outcome in renal disease (CORD) study. Clin. J. Am. Soc. Nephrol. 6, 153–159 (2011).
Muntner, P., He, J., Hamm, L., Loria, C. & Whelton, P. K. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J. Am. Soc. Nephrol. 13, 745–753 (2002).
Bibbins-Domingo, K. et al. Renal insufficiency as an independent predictor of mortality among women with heart failure. J. Am. Coll. Cardiol. 44, 1593–1600 (2004).
Wang, A. Y. & Sanderson, J. E. Current perspectives on diagnosis of heart failure in long-term dialysis patients. Am. J. Kidney Dis. 57, 308–319 (2011).
MacRae, J. M., Levin, A. & Belenkie, I. The cardiovascular effects of arteriovenous fistulas in chronic kidney disease: A cause for concern. Semin. Dial. 19, 349–352 (2006).
Reddy, Y. N. V. et al. Long-term cardiovascular changes following creation of arteriovenous fistula in patients with end stage renal disease. Eur. Heart J. 38, 1913–1923 (2017).
Malik, J. et al. Understanding the dialysis access steal syndrome. A review of the etiologies, diagnosis, prevention and treatment strategies. J. Vasc. Access 9, 155–166 (2008).
Schanzer, H. & Eisenberg, D. Management of steal syndrome resulting from dialysis access. Semin. Vasc. Surg. 17, 45–49 (2004).
Schmidli, J. et al. Editor’s choice-vascular access: 2018 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 55, 757–818 (2018).
Lok, C. E. et al. KDOQI clinical practice guideline for vascular access: 2019 update. Am. J. Kidney Dis. 75, S1–S164 (2020).
Allon, M. et al. Effect of change in vascular access on patient mortality in hemodialysis patients. Am. J. Kidney Dis. 47, 469–477 (2006).
Lacson, E., Wang, W., Lazarus, J. M. & Hakim, R. M. Change in vascular access and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 54, 912–921 (2009).
Polkinghorne, K. R., McDonald, S. P., Atkins, R. C. & Kerr, P. G. Vascular access and all-cause mortality: A propensity score analysis. J. Am. Soc. Nephrol. 15, 477–486 (2004).
Xue, J. L., Dahl, D., Ebben, J. P. & Collins, A. J. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am. J. Kidney Dis. 42, 1013–1019 (2003).
Malas, M. B. et al. Trends in incident hemodialysis access and mortality. JAMA Surg. 150, 441–448 (2015).
Ryan, S. V., Calligaro, K. D. & Dougherty, M. J. Management of hemodialysis access infections. Semin. Vasc. Surg. 17, 40–44 (2004).
Ravani, P. et al. Associations between hemodialysis access type and clinical outcomes: A systematic review. J. Am. Soc. Nephrol. 24, 465–473 (2013).
Arhuidese, I. J., Orandi, B. J., Nejim, B. & Malas, M. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J. Vasc. Surg. 68, 1166–1174 (2018).
Weyde, W. et al. Superficialization of the radial artery—An alternative secondary vascular access. J. Vasc. Access 13, 504–507 (2012).
Brittinger, W. D. et al. [Shuntless hemodialysis by means of puncture of the subcutaneously fixed superficial femoral artery. First dialysis experiences]. Klin. Wochenschr. 47, 824–826 (1969) (in German).
Nakamura, T., Suemitsu, K. & Nakamura, J. Superficialization of brachial artery as effective alternative vascular access. J. Vasc. Surg. 59, 1385–1392 (2014).
Nakagawa, K., Yamada, S., Matsukuma, Y., Nakano, T. & Mitsuiki, K. Survival comparison between superficialization of the brachial artery and tunneled central venous catheter placement in hemodialysis patients with heart failure: A retrospective study. Ther. Apher. Dial. 24, 408–415 (2020).
Lee, T., Barker, J. & Allon, M. Tunneled catheters in hemodialysis patients: Reasons and subsequent outcomes. Am. J. Kidney Dis. 46, 501–508 (2005).
Pietro, R. et al. Examining the association between hemodialysis access type and mortality: The role of access complications. Clin. J. Am. Soc. Nephrol. 12, 955–964 (2017).
Allon, M. et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J. Am. Soc. Nephrol. 14, 1863–1870 (2003).
Lok, C. E. & Mokrzycki, M. H. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 79, 587–598 (2011).
Brunelli, S. M. et al. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection. J. Am. Soc. Nephrol. 29, 1336–1343 (2018).
Murea, M. et al. Risk of catheter-related bloodstream infection in elderly patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 9, 764–770 (2014).
Wang, P. et al. A retrospective study of preferable alternative route to right internal jugular vein for placing tunneled dialysis catheters: Right external jugular vein versus left internal jugular vein. PLoS ONE 11, e0146411 (2016).
Tordoir, J. et al. EBPG on vascular access. Nephrol. Dial. Transplant. 22(Suppl 2), ii88-117 (2007).
Gelbfish, G. A. Clinical surveillance and monitoring of arteriovenous access for hemodialysis. Tech. Vasc. Interv. Radiol. 11, 156–166 (2008).
Roy-Chaudhury, P. et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 59, 2325–2334 (2001).
Ravani, P., Spergel, L. M., Asif, A., Roy-Chaudhury, P. & Besarab, A. Clinical epidemiology of arteriovenous fistula in 2007. J. Nephrol. 20, 141–149 (2007).
Murakami, M. et al. Multicentre study on the efficacy of brachial artery transposition among haemodialysis patients. Eur. J. Vasc. Endovasc. Surg. 61, 998–1006 (2021).
Daugirdas, J. T. et al. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am. J. Kidney Dis. 66, 884–930 (2015).
Ethier, J. et al. Vascular access use and outcomes: An international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant 23, 3219–3226 (2008).
Nakai, S. et al. Overview of regular dialysis treatment in Japan (as of 31 December 2008). Ther. Apher. Dial. 14, 505–540 (2010).
Irish, A. B. et al. Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: A randomized clinical trial. JAMA Intern. Med. 177, 184–193 (2017).
Tanner, N. C. & da Silva, A. F. Medical adjuvant treatment to improve the patency of arteriovenous fistulae and grafts: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 52, 243–252 (2016).
Rayner, H. C. et al. Creation, cannulation and survival of arteriovenous fistulae: Data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 63, 323–330 (2003).
Quinn, R. R. & Ravani, P. Fistula-first and catheter-last: Fading certainties and growing doubts. Nephrol. Dial. Transplant 29, 727–730 (2014).
Murea, M. & Satko, S. Looking beyond “Fistula First” in the elderly on hemodialysis. Semin. Dial. 29, 396–402 (2016).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Author information
Authors and Affiliations
Contributions
Y.S., M.M., S.T., K.M., and A.S. contributed to study conception and design. E.N. and Y.S. contributed to the data analysis and interpretation. Y.S., M.M., E.N., Y.S., S.T., K.M., and A.S. wrote the manuscript, critically revised it, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soma, Y., Murakami, M., Nakatani, E. et al. Brachial artery transposition versus catheters as tertiary vascular access for maintenance hemodialysis: a single-center retrospective study. Sci Rep 12, 306 (2022). https://doi.org/10.1038/s41598-021-03860-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03860-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.