Abstract
In a cross-sectional study, with the use of molecular methods, we aimed to gain insight into oropharyngeal pneumococcal colonization over time in 1212 Greek children recruited in general pediatric settings throughout the country; they were fully vaccinated with PCV13 (3 + 1 schedule). A single sample was obtained from each child at a time interval of 26 days to 70 months after administration of the 4th (booster) PCV13 dose; sampling time was divided into six time intervals. Carriage of Streptococcus pneumoniae was detected by real-time PCR targeting the lytA gene and isolates were serotyped by singleplex real-time PCR assays. Multiple control procedures to avoid false-positive results were applied. We showed an overall S. pneumoniae carriage rate of 48.6%. Serotyping identified typeable isolates in 82% of the total lytA-positive samples. Non-PCV13 serotypes represented 83.8% of total isolates when excluding serogroups with mixed PCV13 and non-PCV13 serotypes. In multivariate analysis daycare/school attendance emerged as the main contributing factor. Notably, serotypes 19A and 3 were the only two PCV13 serotypes the colonization rate of which increased over time (χ2 for trend P < 0.001 and P = 0.012, respectively). The application of the SP2020 gene on lytA-positive serotyped samples showed pneumococcal colonization in 97% of cases, and the overall colonization profile over time closely resembled that of the lytA gene. With the provisions of the methodological approach and age group of our study, the use of the oropharynx emerges as a reliable alternative to the nasopharynx in estimating pneumococcal carriage in epidemiological studies.
Similar content being viewed by others
Introduction
Streptococcus pneumoniae is a diverse pathogen which causes infections that range in severity from acute otitis media and sinusitis to pneumonia, septicemia, and meningitis1,2,3,4. The upper respiratory tract is the principal reservoir of S. pneumoniae in children and, nasopharyngeal sampling has been historically considered as the gold standard for the detection of S. pneumoniae carriage4. However, the organism also resides in the oropharynx5,6,7, and colonization of any part of the upper airway may lead to pneumococcal disease and transmission4. Therefore, it is not surprising that sampling of saliva in older children5 and the posterior oropharyngeal wall in adults6,7,8 has also been used as a site for pneumococcal detection.
Pneumococcal pharyngeal colonization and serotype characterization is routinely investigated by culture of the dominant isolate, followed by serotyping with specific antisera9. However, the use of traditional culture-based methods may miss serotypes present at a lower density6,10. This problem has been addressed by the recent development of molecular diagnostic methods, which offer high-sensitivity detection of multiple serotypes from a single naso-/oropharyngeal sample and are able to unveil co-colonization of S. pneumoniae, especially in cultures of polymicrobial samples6,10.
Direct molecular methods are particularly relevant in case of antibiotic use for respiratory tract infections in the community11.
Among culture-independent assays, many researchers opt to use real-time PCR targeting the lytA (lytA-CDC)12, piaB8,13 and, recently, the SP202014 genes. The latter is a putative transcriptional regulator of the GntR-family, which belongs to the core genome of pneumococcus, and has emerged as an additional candidate gene for accurate pneumococcal identification14.
The 7-valent pneumococcal conjugate vaccine (PCV7), which included capsular antigens of S. pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F and 23F was incorporated into the national immunization program (NIP) of Greece in January 2006. Later, the higher-valent PCVs were introduced: the 10-valent (PCV10, with the additional serotypes 1, 5, 7F to PCV7) became available in May 2009 and the 13-valent (PCV13, with further addition of serotypes 3, 6A and 19A to PCV10) in June 2010, and were both incorporated directly into the NIP. Since July 2010, the PCV13 has become the most used PCV15, soon increasing to over 97% coverage of PCV vaccinated children. From January 2006 through June 2019, PCVs have been included in the NIP in a 3 + 1 schedule; specifically, immunization at 2, 4, and 6 months of life and a 4th (booster) dose − initially recommended to be administered at 12–18 months, later revised to 12–15 months as of December 2011- have been recommended. Despite the lack of an official vaccination registry in Greece, it is estimated that complete PCV-series coverage is approximately 80% among children < 5 years16, our group has previously conducted surveys of pneumococcal carriage among young children in various areas of Greece since 199515,17,18,19.
The aim of the present cross-sectional study among Greek children was to investigate potential changes of pneumococcal oropharyngeal carriage in the years that follow the 4th dose of PCV13. The choice to sample the oropharynx was made because of the limited molecular data currently available in children on S. pneumoniae carriage at this site and the convenience of the approach. We applied molecular methods of carriage detection to assess the patterns of pneumococcal carriage among children of increasing age, including their sixth year of life, who were fully immunized with PCV13 in a 3 + 1 schedule.
Methods
Study population
From January 18 to August 29, 2017, children who had completed the 4-dose course of PCV13 immunization, as per the Greek NIP recommendations, were prospectively enrolled by 45 general pediatric practice facilities in a consecutive fashion over 30 municipalities across Greece (Supplementary Fig. S1). A single sample from the posterior pharyngeal wall (oropharynx) was obtained from each subject in a standardized manner (questionnaire, procedure). Children were eligible for inclusion in the study if they: (a) were younger than seven years (≤ 83 months) of age; (b) were not diagnosed with severe chronic lung disease; (c) had received all four doses of the 3 + 1 PCV13 schedule; (d) had received the 4th dose of PCV13 at least 21 days prior to enrollment; and (e) had not received antibiotic treatment within seven days prior to enrollment. Based on the time elapsed between the 4th PCV13 dose and the sampling procedure (time interval post booster of PCV13) children were grouped for analysis into six groups: 26 days (d) to 11 months (m); 12–23 m; 24–35 m; 36–47 m; 48–59 m; and 60–70 m. Presence of respiratory tract infection (RTI) at the time of sampling was also considered. Carriage of S. pneumoniae and serotyping were evaluated by real-time PCR.
Questionnaire
The aim and procedures of the study were meticulously explained to the parents/guardians and written informed consent was obtained from at least one guardian of each participating child, attesting (a) acceptance to respond to the administered questionnaire and (b) allowance to obtain an oropharyngeal sample from their child. All methods were performed in accordance with relevant legislative guidelines and regulations. The research protocol was approved by the Ethics Committee of the General University Hospital of Larissa, Greece.
Upon recruitment, the parent(s) responded to an interviewer-administered questionnaire. Information regarding demographic characteristics and immunization with PCV13, including the exact dates of administration, was derived from each child’s health booklet and the interviewing pediatrician’s immunization data base. During the interview, an effort was made to retrieve additional data from the child’s medical files and the responses were recorded on structured report forms.
Pharyngeal specimen collection
The oropharyngeal sample was obtained from each child at the time of enrollment. A sterile cotton swab was inserted through the mouth and the posterior wall of the oropharynx was sampled. Samples were obtained using an ESwab kit containing a polypropylene screw-capped tube filled with 1 mL of liquid Amies medium (Brescia, Copan, Italy). The sampling was carried out by pressing the tongue downward to the floor of the mouth with a tongue depressor and swabbing the posterior pharyngeal wall without touching the sides of the mouth, the uvula or the tongue. Sample swabs were secured in tubes, stored at 4 °C, and transferred on ice via the Laboratory of the Division of Pediatric Infectious Disease of the University of Thessaly to the Laboratory of the University of Florence and Anna Meyer Children’s Hospital, Florence, Italy on a weekly basis.
Real-time PCR for the lytA gene
Total nucleic acid was automatically extracted from oropharyngeal swab samples, using the MagCore Genomic DNA Tissue Kit with automated Nucleic Acid Extractor HF16 (RBCBioscience, Taiwan) according to manufacturer’s instructions. Extracted DNA from the swab samples were stored at − 20 °C. All DNA samples were tested with real-time PCR for the lytA (lytA-CDC) gene as previously described12,20.
Singleplex real-time PCR assays for serotypes/serogroups
All lytA-positive samples were further molecularly serotyped using singleplex real-time PCR assays for 33 serotypes/groups (1, 2, 3, 4, 5, 6A/B/C/D, 7A/F, 7B/C, 8, 9A/L/N/V, 10A/B, 11A/D/E, 12A/B/F/44/46, 13, 14, 15A/B/C/F, 16F, 17F, 18B/C/F, 19A, 19F, 20, 21, 22A/F, 23A, 23B, 23F, 29, 31, 33F, 35B/D, 35F/37 and 38/25A/F) as previously described20,21, also taking into account the following modifications: (a) the primer and probe sets for the serotype/serogroup 7B/C, 31 and 35F/37 have been designed for this study protocol, and (b) the primer and probe sets for the serotype/serogroup 2, 5, 17F, 18B/C/F, 19A, 19F, 22A/F, 35B/D, and 38/25A/F have been redesigned to avoid false-positive signal10. All probes are 5’ FAM and 3’ TAMRA labelled (Supplementary Table S1).
Briefly the real-time amplification was performed in 20 µL reaction volumes containing 2× TaqMan Universal Master Mix (Applied Biosystem, Foster City, CA, USA); five µL of DNA extract was used for each reaction. DNA was amplified in an ABI 7500 FAST sequence detection system (Applied Biosystem, Foster City, CA, USA) using, for all the primer pairs, the same cycling parameters as follows: 95 °C for 20 s followed by 45 cycles of a two-stage temperature profile of 95 °C for three s and 60 °C for 30 s. Samples were considered positive for presence of the targeted sequence when the serotype/serogroup specific signal was ≤ 35 Cycle Threshold (CT). If no increase in fluorescent signal was observed after 35 cycles for any of the primer/probe set, the sample was assumed to be negative for the serotype/serogroup specific primers and was reported as not able to be assigned to a specific serotype.
Due to the high genotypic similarities among the capsule loci of certain serotypes, the PCR method was unable to discriminate among certain serotypes within a particular serogroup.
Procedures applied to reduce risk of false positivity
Two different procedures have been simultaneously applied to reduce the risk of false positivity. First, all samples in which serotype/serogroup CT was lower by > 2 CT than lytA CT were eliminated from the analysis of that specific serotype/serogroup as previously discribed5. Second, to estimate the background of false positivity, for each serotype/serogroup we tested 100 DNA lytA-negative samples using singleplex real-time PCR assays as described above. The rate of false positivity for each serotype/serogroup was then subtracted from positivity rate of each serotype/serogroup (Table 1). Moreover, all serotypes/serogroups showing a false positivity background over 3% were eliminated from analysis, i.e., serotypes/serogroups 4, 5, and 18B/C/F.
Real-time PCR for SP2020 gene
lytA-positive samples were tested with real-time PCR for SP2020 as previously described14. After initial handling and testing, 46 samples did not contain the necessary quantity of material for further analysis. SP2020 CT, similar to lytA, was set at ≤ 35.
Statistical analysis
Pneumococcal isolates were classified as PCV13 serotypes, non-PCV13 serotypes, those belonging to serogroups with both PCV13 and non-PCV13 serotypes and isolates that could not be assigned to a specific serotype/serogroup. To assess the six groups of children who had received the 4th PCV13 dose at different time intervals before the sampling, categorical parameters were compared using 2-sided chi-square test for trend. Results were expressed as median and interquartile range (IQR) or as mean value with 95% confidence intervals (95%CI) as deemed appropriate. For the assessment of two groups, categorical parameters were compared using 2-sided Fisher exact test. In addition to the exploratory analysis, multivariate logistic regression was used to test for the effect of the explanatory independent variables combined. Concordance for CTs between lytA and SP2020 genes was assessed by the Intraclass Correlation Coefficient (ICC). All analyses were performed with the IBM SPSS software version 26.0 (IBM Corp., Armonk, NY). Two-sided P-values < 0.05 were considered statistically significant.
Results
Study demographics
A total of 1256 children/samples were investigated. Forty-four children were excluded as 27 had not received all four doses of PCV13, 15 were ≥ 7 years old and two sample vials were empty. Eligible for further analysis were 1212 samples/children aged 14–83 m. The characteristics of the enrolled attendees appear in the first two columns of Table 2; 34.1% of evaluated children were aged ≥ 60 months. Among children aged 14–23 m 19.9% attended daycare and 46.9% had no siblings, whereas among children aged 24–35 m 61.1% attended daycare and 35.5% had no siblings.
Oropharyngeal carriage of S. pneumoniae
Of the 1212 children studied, 589 (48.6%) were identified as carriers of S. pneumoniae having a lytA-positive sample. Serotyping revealed that among carriers 483 (82%) were colonized with typeable isolates. Three hundred and sixty three of 483 (75.2%) children were carriers of a single serotype, while 120 of more than one serotypes (24.8%) (Supplementary Fig. S2). The material of 106 of the total samples (18%) could not be assigned to a specific serotype.
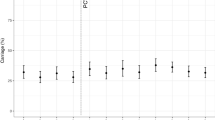
The last four columns of Table 2 depict the oropharyngeal colonization of all lytA-positive samples and typeable S. pneumoniae according to the characteristics of our study population. Figure 1 and Supplementary Fig. S3 present the typeable isolates and total lytA-positive samples, respectively, according to the post booster time interval; healthy children and those with RTI are also presented. There is a statistically significant increasing trend of S. pneumoniae colonization with increasing time interval in both typeable S. pneumoniae and total lytA-positive samples among healthy children (χ2 for trend P = 0.002 and P < 0.001, respectively). It is also evident from Fig. 1 and Supplementary Fig. S3 that this trend is due to the initial three time interval groups (i.e., 26 d–11 m, 12–23 m and 24–35 m) of healthy children. The same trend is also reflected in the total samples of both typeable and total lytA-positive groups (χ2 for trend P < 0.001; the frequencies appear to plateau in the last four post booster time interval groups of healthy and total sample children. In multivariate analysis of the risk factors presented in Table 2, daycare/school attendance emerges as the main contributing factor of pharyngeal pneumococcal colonization.
When considering the total typeable (n = 483) and total lytA-positive samples (n = 589), daycare/school attendees were more frequently colonized with S. pneumoniae vs. non-attendees (P < 0.001) and this was also the case for non-attendees who had one or more siblings at home (P = 0.006 and P < 0.001, respectively) as well as children with RTI vs. healthy children (P = 0.03 and P = 0.02, respectively) (Supplementary Fig. S4 and S5).
Serotypes of interest
Due to co-colonization, a total of 622 S. pneumoniae isolates (belonging to 25 serotypes/serogroups) were identified. Of these, 78 (12.5%) belonged to a PCV13 serotype, 23 (3.7%) to serogroups encompassing PCV13 and non-PCV13 serotypes and 521 (83.8%) to a non-PCV13 serotype/serogroup (Fig. 2).
Figure 3 shows the increase of PCV13 and non-PCV13 serotypes with increasing post booster time interval; there was no such increase in serogroups 6A/B/C/D and 9A/L/N/V which include both PCV13 and non-PCV13 serotypes.
Pneumococci belonging to serotype 19A were recovered from 26 carriers (living in 12 municipalities), serotype 3 from 25 carriers (15 municipalities) and serotype 19F from 18 carriers (11 municipalities). Serotypes 3 and 19A were the only PCV13 serotypes in which the percentage of colonization increased significantly as post booster time interval increased (Fig. 4). There was no increase in 19F carriage (Fig. 4), and there was no child carrying serotypes 23F, 14 and 7F.
SP2020 gene analysis
Of the 483 typeable samples, 437 were available for testing for the SP2020 gene. Of these, 424 (97.0%) were positive for both lytA and SP2020 gene; the 13 typeable lytA-positive, but SP2020-negative isolates belonged to serotypes/serogroups 2 (n = 1), 3 (n = 1), 11A/D/E (n = 1), 12A/B/F/44/46 (n = 1), 15A/B/C/F (n = 2), 16F (n = 1), 19F (n = 1), 20 (n = 1), 23B (n = 2), 31 (n = 1) and 35B/D (n = 1). Of the 106 lytA-positive samples that could not be assigned to a specific serotype, 84 (79.2%) were positive for the SP2020 gene. The SP2020 positivity difference between typeable and lytA-positive isolates that could not be assigned to a specific serotype is significant (P < 0.001). The CTs of the respective real-time PCR are presented in Fig. 5.
Samples tested positive for both genes: typeable (n = 424) (a) and samples not assigned to a specific serotype (n = 84) (b). Each blue circle indicates the CT values of samples tested positive for both lytA and SP2020 genes. The size of the blue circles (‘bubbles’) is proportionate to the number of samples which presented with the specific CT combination.
The concordance between CTs of the two genes was: mean 0.86; 95%CI 0.76 to 0.91 (P < 0.001) for typeable isolates (Fig. 5a), and 0.83; 95%CI 0.73 to 0.88 (P < 0.001) for isolates which could not be assigned to a specific serotype/group (Fig. 5b). When the total number which included both lytA-positive groups was analyzed the ICC rendered: mean 0.87; 95%CI 0.80 to 0.81.
The sample analysis as per both lytA and SP2020 genes according to post booster time interval is presented in Fig. 6. There is a statistically significant increasing trend of S. pneumoniae colonization with increasing post booster time interval in both typeable S. pneumoniae and total lytA- and SP2020-positive samples (χ2 for trend P = 0.001 and P < 0.001, respectively); no such trend was observed among isolates which could not be assigned to a specific serotype/serogroup (P = 0.146).
The CTs of lytA and SP2020 real-time PCRs of the two most common PCV13 serotypes, i.e., 19A and 3, and the two most common non-PCV13 serogroups, i.e., 15A/B/C/F and 11A/D/E are presented in Supplementary Fig. S6.
Co-colonization with two or more serotypes of lytA-positive samples over increasing post booster PCV13 time intervals showed an increasing trend (χ2 for trend P < 0.001); this was also the case among lytA- and SP2020-positve samples (χ2 for trend P = 0.002) (Supplementary Table S2).
Discussion
In the present cross-sectional study, with the use of molecular methods, we aimed to gain insight into S. pneumoniae colonization in a large sample of Greek children, who were fully immunized with PCV13 in a 3 + 1 immunization schedule over an age range of 14 through 83 months. The sample collection spanned over three seasons: half of winter, spring, and summer of 2017. An experienced group of investigators, who have previously conducted similar surveillance studies, was able to apply real-time PCR in order to compare S. pneumoniae colonization in various age-groups, at increasing time intervals after administration of the 4th (booster) PCV13 dose22,23. We found an overall lytA-positive carriage rate of 48.6% in our total oropharyngeal sample in a childhood population of small sized families, with relatively late entrance to daycare/school, primarily followed in pediatric private practice. Eighty-two percent were typeable isolates, of which 83.8% consisted of exclusively non-PCV13 serotypes. Co-colonization was observed in 24.8% of children. In univariate analysis there was an increasing time trend in the frequency of colonization and co-colonization for increasing time interval from completion of PVC13 immunization (four doses) among healthy children but not in those with RTI. Children attending daycare/school, those not attending but with one or more siblings at home, and those with RTI at the time of sampling were at increased risk of S. pneumoniae colonization. Importantly, an increasing trend of PCV13 serotypes 19A and 3 with increasing time interval from completion of full vaccination groups emerged.
If lytA-positive samples that could not be assigned to a specific serotype/serogroup were not included in the analysis, only slight differences in the study results were observed. These excluded lytA-positive samples most likely consisted of a mixed group of true non-typeable (non-encapsulated) pneumococcal isolates, serotypes not identified by our molecular assays, and non-pneumococcal streptococci24. The exact contribution of each of these three groups remains unknown.
It is widely appreciated that the use of conventional culture-based techniques and serotyping of pneumococci by antisera lack sensitivity in detecting S. pneumoniae isolates and render lower bacterial density in the upper airway6,10. We applied real-time PCR using the lytA-CDC gene which is the recommended method for detecting S. pneumoniae12. To obtain true positive results, the cut-off for real-time PCR when targeting the lytA gene was set at ≤ 35 CTs. Two further procedures were applied simultaneously to reduce the risk of false positivity. First, all samples in which serotype/serogroup CT was lower by > 2 CT than lytA CT were eliminated from the analysis of that specific serotype/serogroup as previously discribed5. Second, to estimate the background of false-positivity, for each serotype/serogroup we tested 100 DNA lytA-negative samples using singleplex real-time PCR assays as described above. The rate of false positivity for each serotype/serogroup was then subtracted from the positivity rate of each serotype/serogroup (Table 1). Moreover, all serotypes/serogroups showing a false positivity background over 3% were eliminated from analysis. We trust that the combination of these methodological precautions offer reasonable assurance of the true presence of S. pneumoniae in our samples and the subsequent analysis of our results. Furthermore, based on DNA sequence differences between pneumococcal lytA and its homologues, real-time PCR assays for specific identification of pneumococcus have been developed12.
Tavares et al. have proposed that, in adults, combined use of both lytA-CDC and the SP2020 gene is a powerful strategy for the identification of pneumococcus, in both pure cultures and polymicrobial samples14. In addition to lytA we applied the SP2020 gene on the available lytA-positive samples (92.2%). Ninety-seven percent of our typeable samples were positive for both genes, whereas this percentage dropped to 79.2% when the SP2020 gene was applied to those that could not be assigned to a specific serotype/serogroup.
Current real-time PCR assays are not optimized to detect all possible pneumococcal serotypes. Therefore, a proportion of samples that cannot be assigned to a specific serotype/serogroup are not detected, leading to underestimation of the frequency of serotype colonization. This is also the case with our study. In addition, our analysis was hampered by the inability to assign specific serotypes within serogroup 6, which includes serotypes 6A, 6B, 6C and 6D, and serogroup 9, which includes 9A, 9L, 9N and 9V. Both PCV13 and non-PCV13 serotypes are included in these two groups (3.7%), which are depicted as a separate category in Fig. 2. It has been shown that in case of serogroup 6, serotypes 6A and 6B which are included in PCV13, are expected to generate protective antibodies towards 6C and 6D25,26,27; however, this does not appear to be the case with serotype 9V (also included in PCV13), which does not offer cross-protection towards 9L and 9N28,29,30,31.
Although cross-sectional studies cannot resolve the direction of causality between related events, the choice of our population sample, in conjunction with the use of a molecular approach to investigate pneumococcal carriage, has rendered epidemiologically and clinically useful information. Our study population consisted of a large number (n = 1212) of fully vaccinated children spanning an age range of approximately six years. Potential risk factors such as occurrence of RTI during sampling, history of daycare/school attendance and sibship status at home were considered.
A single oropharyngeal sample was obtained from each child. Both nasopharynx and oropharynx constitute polymicrobial sites, although the oropharynx is characterized by greater bacterial diversity32. Currently, sampling of the upper respiratory tract ideally should include both the nasopharynx and the oropharynx; when only one sample is obtained, nasopharyngeal sampling has been suggested as the preferred choice9.
In our population, the overall pneumococcal carriage rate (48.6%) was comparable to that reported by other studies evaluating nasopharyngeal samples from vaccinated children of similar age33,34,35.
We observed an increasing trend for both PCV13 and non-PVC13 serotype carriage with increasing time-elapsed post-immunization groups. Non-PCV13 serotypes predominated in all groups and non-PCV13 serotype colonization reached 83.8% in the total isolates. This is consistent with the findings of other studies in similar settings33,34,35. The overall serotype distribution among carriers in our study was similar regardless of the time elapsed since the last PCV13 dose. These findings most likely reflect the full benefit of both vaccine protection and herd immunity, and offer epidemiologically relevant information regarding pneumococcal carriage among children up to 70 months post completion of PCV13.
It should be noted that the overall distribution profile when applying the SP2020 gene on lytA-positive typeable samples closely resembles that of the lytA-positive alone time trend (Supplementary Fig. S7). The similarity of findings by the two genes reinforces the validity of our results.
The 10 most prevalent non-PCV13 serotypes/serogroups in this study, in order of decreasing frequency, were 15A/B/C/F, 11A/D/E, 23B, 35F/37, 23A, 21, 35B/D, 38/25/A/F, 10A/B and 31. Several of these serotypes/serogroups were also among the most common non-PCV13 ones observed in recent studies evaluating nasopharyngeal carriage of S. pneumoniae: nine in the Kandasamy et al. (aged 13–48 months 48.7% carriage rate35), eight in the Løvlie et al. (aged 8 to 80 months 48.1% carriage rate34), and seven in the Southern et al. (< 5 years 51.9% carriage rate33) study, differing only in the ranking of frequency. Notably, the majority of the 10 most prevalent non-PCV13 serotypes/serogroups of this study were also among the 10 most common non-PCV13 serotypes in young carriers in Greece in the late PCV7 and the early PCV13 usage period15.
Interestingly, there appears to be a decrease in non-PCV13 carriage when children reach the age of six years and begin attending primary school. Since we did not obtain data beyond this age, this ‘decrease’ could be assigned to a spurious fluctuation of pneumococcal carriage over time-elapsed post-immunization curves; however, it may also, reflect the turning point of a commencing decrease in pneumococcal carriage at school age. Wyllie et al. have shown that use of two-gene quantitative PCR on enriched samples of saliva -also a highly polymicrobial upper respiratory tract source- rendered a 25% decrease of S. pneumoniae carriage rate among schoolchildren of an older (8–10 years) vs. younger (5–8 years) age5. Monitoring of carriage by consistent molecular methods over a longer period, extending into school age and adolescence, will be required to definitively answer this question.
We paid particular attention to serotypes 19A and 3; these were the only two PCV13 serotypes the colonization rate of which increased as time-elapsed post immunization progressed, and both serotypes were identified in several municipalities throughout the country; attendance of daycare/school is probably the most likely explanation. In a recent study from the UK serotypes 19A and 3 continued to circulate among PCV13 immunized children aged 24 to 48 months35. In our study, a substantial reduction of carriage rate of other PCV13 serotypes was noted and serotypes 7F, 14 and 23F were absent.
Apart from its cross-sectional design, our study has other limitations. First, to assure recruitment rates and homogeneity of the sampling procedure we opted to sample the oropharynx which is a much easier procedure than nasopharyngeal sampling. Although the oropharyngeal samples are regarded as more polymicrobial as compared to the nasopharyngeal ones32, the consistency of our results when applying a second target gene is encouraging in postulating that the oropharynx offers a reliable alternative to estimate pneumococcal carriage in epidemiological studies. Second, the use of the SP2020 target solely on lytA-positive samples precludes any comparison of sensitivity and specificity of the two genes; such comparison was not an aim of our study. Third, cost and laboratory time constraints did not allow for the application of Llull’s et al. method of separating pneumococci from mitis group streptococci36 and the Multilocus Sequence Typing (MLST) which characterizes S. pneumoniae isolates by their unique allelic profiles in our lytA- and/or SP2020-positive samples which could not be assigned to a specific serotype; thus, the distribution of 106 unassigned samples into true or false positive S. pneumoniae cannot be answered. Fourth, the description of seasonality was not a target of this study and no pharyngeal sampling was performed in autumn as well as in approximately half of the winter seasons; therefore, the sampling season was not accounted for in the multivariate analysis of our results.
In conclusion, we conducted a large cross-sectional study of S. pneumoniae oropharyngeal carriage, over 30 municipalities scattered throughout Greece, in which sampling was performed during a time interval of 26 days to 70 months after administration of the 4th (booster) PCV13 dose. By employing real-time PCR targeting the lytA gene and using carefully selected control procedures to avoid false positive results, we showed that an overall carriage rate of 48.6%; 83.8% of isolates consisted exclusively of non-PCV13 serotypes and 3.7% of serogroups 6 and 9, which are a mix of PCV13 and non-PCV13 serotypes. There was an increasing trend for carriage with increasing time interval since completion of the vaccination schedule. Notably, serotypes 19A and 3 were the only two PCV13 serotypes the colonization rate of which increased over time. The application of SP2020 as a second target gene on our lytA-positive samples rendered an overall colonization profile over time which closely resembled that of the lytA gene when used alone. With the provision of the methodological approach and age group of our study and in conjunction with standardized PCR analysis, it offers further support to the value of oropharyngeal sampling in the epidemiological assessment of pneumococcal colonization of the upper airway.
Data availability
Basic data analyzed during this study are included in this published article (and its Supplementary Information files) while part of datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gray, B. M., Converse, G. M. III. & Dillon, H. C. Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142, 923–933 (1980).
Klein, J. O. The epidemiology of pneumococcal disease in infants and children. Rev. Infect. Dis. 3, 246–253 (1981).
Faden, H. et al. Relationship between nasopharyngeal colonization and the development of otitis media in children. J. Infect. Dis. 175, 1440–1445 (1997).
Bogaert, D., De Groot, R. & Hermans, P. W. M. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154 (2004).
Wyllie, A. L. et al. Streptococcus pneumoniae in saliva of Dutch primary school children. PLoS One 9, e102045 (2014).
Almeida, S. T., Pedro, T., Paulo, A. C., de Lencastre, H. & Sá-Leão, R. Re-evaluation of Streptococcus pneumoniae carriage in Portuguese elderly by qPCR increases carriage estimates and unveils an expanded pool of serotypes. Sci. Rep. 10, 8373 (2020).
Watt, J. P. et al. Nasopharyngeal versus oropharyngeal sampling for detection of pneumococcal carriage in adults. J. Clin. Microbiol. 42, 4974–4976 (2004).
Trzciński, T. et al. Superiority of trans-oral over trans-nasal sampling in detecting Streptococcus pneumoniae colonization in adults. PLos One 8, e60520 (2013).
Satzke, C. et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 32, 165–179 (2013).
Wyllie, A. L. et al. Molecular surveillance of nasopharyngeal carriage of Streptococcus pneumoniae in children vaccinated with conjugated polysaccharide pneumococcal vaccines. Sci. Rep. 6, 23809 (2016).
Satzke, C. et al. The PneuCarriage project: a multi-centre comparative study to identify the best serotyping methods for examining pneumococcal carriage in vaccine evaluation studies. PLoS Med. 12, e1001903 (2015).
Carvalho, M. D. G. et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 45, 2460–2466 (2007).
Brown, J. S., Gilliland, S. M. & Holden, D. W. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40, 572–585 (2001).
Tavares, D. A. et al. Identification of Streptococcus pneumoniae by a real-time PCR assay targeting SP2020. Sci. Rep. 9, 3285 (2019).
Grivea, I. N. et al. Dynamics of pneumococcal carriage among day-care center attendees during the transition from the 7-valent to the higher-valent pneumococcal conjugate vaccines in Greece. Vaccine 32, 6513–6520 (2014).
Syrogiannopoulos, G. A. et al. Pneumonia with empyema among children in the first five years of high coverage with 13-valent pneumococcal conjugate vaccine. Infect. Dis. (Lond) 48, 749–753 (2016).
Syrogiannopoulos, G. A. et al. Resistance patterns of Streptococcus pneumoniae from carriers attending day-care centers in Southwestern Greece. Clin. Infect. Dis. 25, 188–194 (1997).
Syrogiannopoulos, G. A. et al. Antimicrobial use and colonization with erythromycin-resistant Streptococcus pneumoniae in Greece during the first 2 years of life. Clin. Infect. Dis. 31, 887–893 (2000).
Syrogiannopoulos, G. A., Katopodis, G. D., Grivea, I. N. & Beratis, N. G. Antimicrobial use and serotype distribution of nasopharyngeal Streptococcus pneumoniae isolates recovered from Greek children younger than 2 years old. Clin. Infect. Dis. 35, 1174–1182 (2002).
Azzari, C. et al. Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS One 5, e9282 (2010).
Azzari, C. et al. Italian group for the study of Invasive Pneumococcal Disease. Potential serotype coverage of three pneumococcal conjugate vaccines against invasive pneumococcal infection in Italian children. Vaccine 30, 2701–2705 (2012).
Grivea, I. N., Panagiotou, M., Tsantouli, A. G. & Syrogiannopoulos, G. A. Impact of heptavalent pneumococcal conjugate vaccine on nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among day-care center attendees in Central Greece. Pediatr. Infect. Dis. J. 27, 519–525 (2008).
Grivea, I. N., Tsantouli, A. G., Michoula, A. N. & Syrogiannopoulos, G. A. Dynamics of Streptococcus pneumoniae nasopharyngeal carriage with high heptavalent pneumococcal conjugate vaccine coverage in Central Greece. Vaccine 29, 8882–8887 (2011).
Simões, A. S. et al. lytA-based identification methods can misidentify Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 85, 141–148 (2016).
Grant, L. R. et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One 8, e74906 (2013).
van der Linden, M. et al. Epidemiology of Streptococcus pneumoniae serogroup 6 isolates from IPD in children and adults in Germany. PLoS One 8, e60848 (2013).
Porat, N., Benisty, R., Givon-Lavi, N., Trefler, R. & Dagan, R. The impact of pneumococcal conjugate vaccines on carriage of and disease caused by Streptococcus pneumoniae serotypes 6C and 6D in southern Israel. Vaccine 34, 2806–2812 (2016).
Bentley, S. D. et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31 (2006).
Schaballie, H. et al. Effect of previous vaccination with pneumococcal conjugate vaccine on pneumococcal polysaccharide vaccine antibody responses. Clin. Exp. Immunol. 185, 180–189 (2016).
Gaviria-Agudelo, C. L., Jordan-Villegas, A., Garcia, C. & McCracken, G. H. Jr. The effect of 13-valent pneumococcal conjugate vaccine on the serotype distribution and antibiotic resistance profiles in children with invasive pneumococcal disease. J. Pediatric. Infect. Dis. Soc. 6, 253–259 (2017).
Løchen, A., Croucher, N. J. & Anderson, R. M. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci. Rep. 10, 18977 (2020).
Stearns, J. C. et al. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 9, 1246–1259 (2015).
Southern, J. et al. Pneumococcal carriage in children and their household contacts six years after introduction of the 13-valent pneumococcal conjugate vaccine in England. PloS One 13, e0195799 (2018).
Løvlie, A., Vestrheim, D. F., Aaberge, I. S. & Steens, A. Changes in pneumococcal carriage prevalence and factors associated with carriage in Norwegian children, four years after introduction of PCV13. BMC Infect. Dis. 20, 29 (2020).
Kandasamy, R. et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J. Infect. Dis. 221, 1361–1370 (2020).
Llull, D., López, R. & García, E. Characteristic signatures of the lytA gene provide a basis for rapid and reliable diagnosis of Streptococcus pneumoniae infections. J. Clin. Microbiol. 44, 1250–1256 (2006).
Acknowledgements
Presented in part: IDWeek, Washington, DC, USA, October 2019 (Abstract no 2703). The study was supported by the 3089 grant from the Research Committee of the University of Thessaly. We thank Dr. Vasiliki Kossyvaki, pulmunologist, for her advice and statistician George Kouvatseas for his contribution to the statistical analysis. We also thank the pediatricians who enrolled children for the study: Argyzoudi Paraskevi, Almyros; Babilis Georgios, Athens; Bagourakis George, Rethymno; Bakolas Evaggelos, Preveza; Chioti Garyfalia, Kalamata; Chrysovelidi Ekaterini, Volos; Diamantopoulos Stavros, Athens; Gidaris Demitrios, Thessaloniki; Grigoriou Konstantina, Larissa; Grivea Ioanna, Larissa; Erotrokritou Savas, Trikala; Fotiadis Nikolaos, Katerini; Kantzidis Georgios, Giannitsa; Kazakou Georgia, Chania; Kolyperas Demitrios, Livadeia; Koskinas Konstantinos, Katerini; Kotsioni Kyriaki, Amaliada; Lianos Vasilios, Igoumenitsa; Liarou Maria, Thassos; Linardaki Despoina, Amaliada; Lolos Panagiotis, Thebes; Mamalis Elias, Agrinio; Mantziou Theodora, Ioannina; Mattela Lamprini, Thermi; Meristoudi Sidero, Larissa; Mitselos Christos, Pyrgos; Mixanikos Antonios, Alexandroupolis; Mpamas Michael, Rhodes; Mpouzmpoukis Ioannis, Larissa; Oikonomou Natassa, Volos; Outras Konstantinos, Trikala; Panagopoulos Alexandros, Patras; Panagopoulos Constantinos, Patras; Papageorgiou Konstantinos, Kastoria; Pateraki Irini, Rethymno; Rammos Theodoros, Athens; Siampou Ekaterini, Tripoli; Sionta Dimitria, Ptolemaida; Soultana Alexandra, Larissa; Syrogiannopoulos George, Larissa; Tasiopoulos Nikolaos, Argos; Tentolouri Dora, Trikala; Trompoukis Christos, Korinthos; Veliotis Xaralampos, Serres; Xasiotou – Touriki Eleni, Zakynthos.
Author information
Authors and Affiliations
Contributions
The study was conceived by G.A.S., I.N.G. and C.A. Data accuracy was ensured by G.A.S., I.N.G. and C.A. Formal analysis was done by G.A.S., I.N.G., M.M., F.N., A.N.M., M.R.C. and C.A. The contributors to the writing of the original draft were G.A.S. and M.A. Reviewing and editing of the whole study were done by G.A.S., I.N.G., M.M., F.N., M.R.C., M.A. and C.A. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr G.A.S. has received consulting and speaking fees from GSK, MSD and Pfizer, and received research funding (for other projects distinct from this study) from Pfizer and MSD through the Investigator Initiated Research Program, paid directly to his institution. He is an unpaid member of the Hellenic National Immunization Committee. Dr C.A. has received consulting and speaking fees from Pfizer, and received funds for unrestricted research grants from Pfizer, paid directly to her institution. Drs. I.N.G., M.M., F.N., A.N.M., M.R.C., M.A.: declare no potential competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Syrogiannopoulos, G.A., Grivea, I.N., Moriondo, M. et al. Molecular surveillance of pneumococcal carriage following completion of immunization with the 13-valent pneumococcal conjugate vaccine administered in a 3 + 1 schedule. Sci Rep 11, 24534 (2021). https://doi.org/10.1038/s41598-021-03720-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03720-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.