Abstract
Insects are now well recognized as biologically relevant alternative hosts for dozens of mammalian pathogens and they are routinely used in microbial pathogenesis studies. Unfortunately, these models have yet to be incorporated into the drug development pipeline. The purpose of this work was to begin to evaluate the utility of orange spotted (Blaptica dubia) cockroaches in early antibiotic characterization. To determine whether these model hosts could exhibit mortality when infected with bacteria that are pathogenic to humans, we subjected B. dubia roaches to a range of infectious doses of Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, and Acinetobacter baumannii to identify the medial lethal dose. These results showed that lethal disease did not develop following infection of high doses of S. aureus, and A. baumannii. However, cockroaches infected with E. coli and K. pneumoniae succumbed to infection (LD50s of 5.82 × 106 and 2.58 × 106 respectively) suggesting that this model may have limitations based on pathogen specificity. However, because these cockroaches were susceptible to infection from E. coli and K. pneumoniae, we used these bacterial strains for subsequent antibiotic characterization studies. These studies suggested that β-lactam antibiotic persistence and dose was associated with reduction of hemolymph bacterial burden. Moreover, our data indicated that the reduction of bacterial CFU was directly due to the drug activity. Altogether, this work suggests that the orange-spotted cockroach infection model provides an alternative in vivo setting from which antibiotic efficacy can be evaluated.
Similar content being viewed by others
Introduction
The utilization of animal infection models is absolutely critical for the antimicrobial drug development1. Prior to testing in vivo, typically, compounds that either inhibit microbial growth or exhibit microbicidal activity are discovered and tested in vitro2. Promising antimicrobial compounds are further tested in vivo using a mammalian infection model1,2. Because in vitro systems cannot completely simulate host factors associated with drug stability, metabolism and excretion, bioavailability, or drug inactivation, in vivo animal infection studies are crucial during preclinical drug assessment3,4.
A common in vivo model used in preclinical antibiotic characterization is the neutropenic murine thigh model5,6. In this model, 6-week old specific pathogen-free female ICR/Swiss mice are rendered neutropenic through the use of two injections of cyclophosphamide5. Following the administration of anesthesia, suspensions of bacteria are injected into the mouse thigh and the mice are treated with an antibiotic. After a period of time, mice are euthanized, and mouse thighs are removed aseptically, homogenized, and this homogenate is serially diluted and plated for CFU. The pharmacokinetic and pharmacodynamic (PK/PD) data generated allow investigators to predict the potential clinical efficacy and the most effective dosing regimens of antibiotics. For instance, the amount of time the antibiotic is present in the infected animal above minimum inhibitory concentration (T > MIC) is one particular PK/PD parameter that correlates with the efficacy of beta-lactam antibiotics7. Analyzing PK/PD data generated by these in vivo studies allows drug developers to predict the potential efficacy in humans and eliminates the risk of conducting clinical trials using drug candidates with insufficient in vivo activity.
Insects are now well recognized as biologically relevant alternative hosts for dozens of mammalian pathogens (both bacteria and fungi) and they are routinely used in molecular pathogenesis studies8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. Unfortunately, incorporation of these models into the drug development pipeline has not yet occurred in a meaningful way. We predict that in vivo PK/PD correlates for antibiotic efficacy in insect models of infection may extend to mammalian infection studies. If this were true, then utilization of insect infections early in antibiotic development may reduce the number of mammals used for experimental purposes. Therefore, the purpose of this study is to evaluate the utility of orange spotted (OS) cockroaches (Blaptica dubia) in early antibiotic characterization.
Results
We have previously established that Francisella tularensis, Burkholderia pseudomallei, and Burkholderia mallei are lethal to tropical cockroaches at very low doses and that the tissue tropism and genetic requirements for virulence in this model are similar to that seen in mammalian models8,15. Based on these findings and the fact that other major multi-drug resistant (MDR) bacteria, mycobacteria, and fungi are pathogenic toward other insect species, we hypothesize that a broad range of clinically relevant human pathogens will also establish productive infections in OS cockroaches. Therefore, we sought to determine whether four model opportunistic human pathogens were capable of causing disease in OS cockroaches. To do so, we infected OS cockroaches with a range of infectious doses of Escherichia coli, Staphylococcus aureus (MRSA), Klebsiella pneumoniae, and Acinetobacter baumannii and observed whether these bacteria were able to induce insect mortality. This showed that both E. coli and K. pneumoniae were capable of establishing lethal infections in the OS cockroaches (LD50s of 5.82 × 106 and 2.58 × 106 respectively; Table 1 and Table S1 in the Supplemental Material). However, both A. baumannii and S. aureus bacteria were unable to produce 50% mortality in the OS cockroaches, even at infectious doses greater than 2 × 107 CFU (Table 1 and Table S1 in the Supplemental Material). To ensure that these strains were at all capable of inducing lethal infection, we similarly infected Galleria mellonella [greater wax moth larvae, an established insect infection model9,10,11,12,13,14,16,17,18,19,20,21,22]. In the G. mellonella model, all bacteria were capable of mediating disease that resulted in insect mortality (LD50s of 2.42 × 108, A. baumannii; 2.42 × 104 for E. coli, 3.41 × 105 for K. pneumoniae, and 9.07 × 105 for S. aureus; Table 1 and Table S1 in the Supplemental Material). This suggests that OS cockroaches may be intrinsically resistant to particular bacterial infections. However, these cockroaches succumbed to infection from E. coli and K. pneumoniae [among others8] and may be useful for modeling certain mammalian infections and for preliminary antibiotic characterization studies.
We next sought to determine if pharmacodynamic (PD) model independence extends to the OS cockroach model. Several groups have firmly established that the PD parameters that govern efficacy are largely model-independent. Differences in the physiology of bacteria grown in vitro and in vivo can lead to changes in sensitivity profiles, but there is usually very little difference in PD correlates of protection from one in vivo model to the next. This observation is fundamental to the popular neutropenic mouse thigh model wherein the host is rendered immune-incompetent so that the PD interaction between the bacterium and the drug can be analyzed in a generic in vivo environment. Within mammals, it appears that as long as the necessary PD requirements are met at the site of infection, then the treatment will be effective.
One such PD metric is the drug duration (a pharmacokinetic variable). We therefore characterized the duration of three ß-lactam antibiotics in the OS cockroaches. Four different doses of cefotaxime, carbenicillin, or ampicillin were injected into OS cockroaches. At time points indicated (Fig. 1) hemolymph was extracted and spotted onto Whatman filter disks that were placed on TSA agar that had been lawn-streaked with E. coli. To determine the level of the ß-lactam remaining in the hemolymph, zones of inhibition were measured and compared to a standard curve. For all three antibiotics, the highest dose administered (5 mg) was still detected 24 h post-injection. For both cefotaxime and carbenicillin, the 0.5 mg dose remained above the detectable levels up to 8 h post administration. Notably, administration of any of the antibiotics used here did not affect cockroach viability, regardless of the dose (Table S2 in the supplemental material). For each of the three ß-lactam antibiotics tested, the half-life was longer in OS cockroaches than in humans or mice (Table 2). However, this difference in drug duration may not preclude the use of the OS cockroach as a model as long as this host reproduces PK/PD relationships observed in mammalian models.
Beta lactam recovery from B. dubia roaches over time. Cefotaxime (A), carbenicillin (B), or ampicillin (C) was injected into B. dubia roaches at the dose indicated. At the designated time points, hemolymph was extracted and was mixed with an anticoagulant. This material was dispensed onto a disk of sterile filter paper which was placed onto TSA containing E. coli that had been spread-plated. Following incubation, zones of inhibition were measured; to estimate the amount of antibiotic recovered, the values for the zones of inhibition generated from the recovered hemolymph were compared to a standard curve.
To determine the MIC of ampicillin, carbenicillin, and cefotaxime, the broth microdilution method was used23. MIC values are reported in Table 3. Notably, the MIC of cefotaxime was much lower than that of ampicillin and carbenicillin for both E. coli and K. pneumoniae BLS (Table 3).
Because of the low MIC for cefotaxime (Table 3) and since this antibiotic exhibited the longest duration in the OS cockroach hemolymph (Fig. 1A), cefotaxime was selected for use in the subsequent in vivo studies. We next sought to determine whether the duration of cefotaxime above the MIC correlated with a reduction in CFU in OS cockroaches that had been infected with E. coli or K. pneumoniae BLS. Here, we also infected OS cockroaches with a lethal dose of K. pneumoniae BAA 2146 (beta-lactam resistant strain) as a control as this bacterium is completely resistant to cefotaxime, and therefore should not exhibit a reduction in CFU regardless of the treatment dose. Three hours later, these roaches were injected with cefotaxime at the indicated dose (Fig. 2A–C) and were incubated at 37 °C. Twenty-four hours later, viable OS cockroaches were euthanized, and hemolymph was isolated. This material was serially diluted and plated onto solid bacterial growth medium to enumerate CFU. These data suggest that fewer E. coli and K. pneumoniae BLS bacteria were recovered from OS cockroaches from which a higher dose of cefotaxime was administered (Fig. 2A,B). However, regardless of antibiotic dose, equivalent levels of bacteria were isolated from OS cockroaches that were infected with the beta-lactam resistant K. pneumoniae BAA 2146 (Fig. 2C). This result indicated that the reduction in E. coli and K. pneumoniae BLS CFU was specific to the activity of cefotaxime. Moreover, for the OS cockroaches infected with either E. coli or K. pneumoniae BLS, significant insect mortality was observed in the groups that were mock-treated or treated with the lowest dose of antibiotic (Fig. 2A,B).
Treatment with cefotaxime reduces bacterial burden in a dose dependent manner that is specific to the activity of this drug. B. dubia cockroaches were infected with a lethal dose of E. coli (A), K. pneumoniae (BLS, Beta-lactam sensitive strain) (B), or K. pneumoniae (BAA-2146, Beta-lactam resistant) (C). Alternatively, B. dubia cockroaches were infected with a sublethal dose of E. coli (D) or K. pneumoniae BLS (E). At 24 h post infection, surviving cockroaches were euthanized and their hemolymph was isolated and mixed with an anticoagulant. This material was serially diluted and plated to enumerate CFU. CFU recovered from hemolymph was compared to input. These data were analyzed by a one way ANOVA and Tukey’s post hoc (A, P < 0.0001; P < 0.0001for 5 mg, 0.5 mg, 0.05 mg; P = 0.0784 for 0.005 mg cefotaxime. B, P < 0.0001; P < 0.0001for 5 mg, 0.5 mg, 0.05 mg; P < 0.001 for 0.005 mg cefotaxime. C, P = 0.1676. D, P < 0.0001; P = 0.0797 for 5 mg; P = 0.0007 for 0.5 mg; P = 0.3399 for 0.05 mg; P = 0.8688 for 0.005 mg cefotaxime. E, P < 0.0001; P < 0.0001 for 5 mg, 0.5 mg; P = 0.0001 for 0.05 mg; P < 0.005 mg cefotaxime).
In a similar fashion, OS cockroaches were infected with a sublethal dose of E. coli or K. pneumoniae BLS and were treated with cefotaxime three hours later (Fig. 2D,E). Twenty-four hours later, these OS cockroaches were euthanized, and their hemolymph was extracted and serially diluted and plated on solid bacterial growth medium to enumerate CFU. These data also demonstrated a dose-dependent response of the amount of cefotaxime administered relative to the reduction of bacterial burden (Fig. 2D,E).
T > MIC should exhibit a linear relationship with the log-reduction in CFU in vivo. To generate this plot for the OS cockroach model, we extrapolated the T > MIC data from the data presented in Fig. 1 and the reduction in bacterial burden from the data presented in Fig. 2. The lowest concentration of cefotaxime that we could detect by disk diffusion assay was 0.7 mg/mL, a value that was much higher than the MIC (Table 3). Because of our limitations in detecting cefotaxime from OS hemolymph, for this analysis, we defined T > MIC as the length of time we were able to recover detectable levels of antibiotic from the insects (the MIC for cefotaxime in the broth microdilution assays was lower than our limit of detection). The maximum value assigned for T > MIC was 24 h as this was the longest time-point from which we assayed for the presence of antibiotics from the OS cockroach hemolymph. This crude analysis suggests that the T > MIC of cefotaxime vs. log reduction in CFU for E. coli as well as K. pneumoniae BLS in the OS cockroach model both exhibit a linear relationship (Fig. 3A,B). Altogether, these data suggest that the OS cockroach infection model provides an in vivo setting of which β-lactam antibiotic efficacy can be evaluated on certain opportunistic pathogens.
Log-reduction in CFU vs. Time > MIC exhibits a linear relationship for E. coli and K. pneumoniae BLS in the OS cockroach model. Data presented in Figs. 1 and 2 were used to extrapolate CFU reduction and Time > MIC for E. coli (A) or K. pneumoniae BLS (B). T > MIC was defined as the length of time detectible levels of antibiotic were recovered from the insects (the MIC for cefotaxime was lower than our limit of detection). The maximum value assigned for T > MIC was 24 h as this was the longest time-point from which we assayed for the presence of antibiotics from the OS cockroach hemolymph. GraphPad Prism was used to interpolate the standard curves from the mean Log-reduction in CFU values versus T > MIC for both E. coli (A) and K. pneumoniae BLS (B). The dashed lines represent the 95% confidence intervals. Adjusted R2 = 0.9839 (A) and 0.9856 (B).
Discussion
Many previous studies have investigated the use of insects to model mammalian infection and to evaluate antibiotic efficacy8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,24,25,26,27,28,29,30. Probably the most extensively utilized insect model for studies involving microbial pathogenesis and antibiotic efficacy is the waxworm, G. mellonella14. The waxworm model has been used to investigate traditional antibiotics, novel anti-biofilm compounds, and even bacteriophage therapy24,25,31,32. Although not nearly as developed as the G. mellonella model, the B. dubia OS cockroach model has potential for use as a model for pathogenesis8. Moreover, this current study provides further evidence that the B. dubia OS cockroach model has real practical potential for evaluating antibiotic efficacy in vivo. Here, we further characterized the B. dubia infection model by challenging this insect with various bacteria that are opportunistic human pathogens. These studies indicated that while the OS cockroaches were susceptible to infections by E. coli and K. pneumoniae bacteria, these insects exhibited minimal observable disease when infected with S. aureus and A. baumannii. These results underscore the potential limitations of this model – being unable to uniformly model infection for medically relevant human pathogens. Nevertheless, the current work showed that E. coli and K. pneumoniae were capable of causing disease in OS cockroaches, mortality was associated with a higher bacterial dose, and that bacterial burden decreased with antibiotic dose. Therefore, although limited, the OS cockroach may not only be useful for modeling a variety of infections, but this model host has potential for evaluating antibiotic efficacy in a preclinical setting prior to, or as an alternative to, mammalian models.
One of the advantages to the OS cockroach infection model is the lower price associated with these host animals. Therefore, research expenditures allocated toward infection models will provide for the utilization of a much higher sample size when using the OS cockroach host which will increase statistical power and confidence level. Facility costs are also much lower when using OS cockroaches, as these can be housed in standard laboratory settings in plastic or glass terraria. Moreover, as these animals are invertebrates, institutional oversight through an animal care and use committee is unnecessary. Finally, any ethical concerns associated with using mammals in research are eliminated by using the OS cockroaches.
Data presented here suggests that there is a linear relationship between the T > MIC of the β-lactam antibiotic, cefotaxime, vs. log-reduction in bacterial CFU of infected OS cockroaches. This is especially important since this this same metric is commonly used to predict β-lactam efficacy in vivo via the neutropenic mouse thigh model5,6. Therefore, adopting the OS cockroach model to screen β-lactam efficacy prior to murine studies could potentially reduce the number of mammals used for research purposes. One limitation to the current study was the lack of sensitivity of the bioassay used to estimate antibiotic duration in vivo. Utilization of HPLC–MS may have revealed lower antibiotic concentrations in the insect hemolymph far lower than our limit of detection with the bioassay. Nevertheless, the expected relationship between log-reduction in CFU and drug availability (T > MIC) was still observed here regardless of the utilization of a less sensitive approach.
Materials and methods
Bacterial strains and growth conditions
Klebsiella pneumoniae BAA-2146 (multi-drug resistant, New Delhi metallo-beta-lactamase [NDM-1] positive) Acinetobacter baumannii (ATCC 19606), and Staphylococcus aureus BAA-1556 (MRSA) were obtained from the American Type Culture Collection. The Escherichia coli strain used in this study was isolated from a feline urinary tract infection. Klebsiella pneumoniae BLS (beta lactam-sensitive) is a general laboratory strain maintained in the West Liberty University Microbiology Culture Collection33. Single colonies obtained from tryptic soy agar (TSA) plates were used to inoculate tryptic soy broth (TSB). These broth cultures were incubated at 37 °C until the bacteria reached stationary phase.
Bacterial enumeration
Prior to preparing the inocula for infection studies, viable CFU/mL were determined for stationary phase cultures (data not shown). Here, broth cultures were normalized to OD600 = 0.3. This diluted culture was serially diluted in phosphate buffered saline (PBS) and these dilutions were plated onto TSA. After an overnight incubation at 37 °C, bacterial colonies were enumerated and CFU/mL was determined.
Determination of MIC by broth microdilution
Minimum inhibitory concentrations were determined a modified broth microdilution34,35 similar to the EUCAST method (www.eucast.org). Bacteria cultured to stationary phase were used to inoculated LB broth at concentration of ~ 2 × 105 CFU per well of a 96-well microtiter plate (200 μl/well). Antibiotics were serially diluted and the plates were incubated at 37 °C for ~ 16 h. A Synergy H1 microplate reader (Bio-Tek) was used to measure OD600. The MIC was determined to be the lowest concentration of antibiotic that inhibited bacterial growth by a difference of 0.3 OD600 units or more.
Cockroach housing
Blaptica dubia roaches were ordered from Backwater Reptiles (www.backwaterreptiles.com) and were housed in smooth plastic terraria. Each terrarium contained a petri dish with food and hydration crystals (supplied by Backwater Reptiles) as well as several pressed paper egg cartons. Roaches were housed at 37 °C until needed for each experiment. Cockroach terraria were cleaned weekly, removing dead cockroaches and replacing food and hydration crystals. For each experiment, cockroaches were grouped (between 10 and 20) into large 150 × 25 mm petri dishes and housed at 37 °C.
Cockroach infections
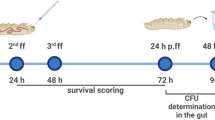
Cockroaches were infected similarly to methods previously described8. Bacteria were cultivated in TSB to stationary phase and were diluted in PBS to the concentration indicated. Cockroaches were injected with 10 μL of the desired bacterial suspension to the left of the midline at the base of the third tergum8. Cockroaches were monitored for mortality every 24 h post-infection. Mortality was determined by lack of movement following stimulation. The LD50 was determined by the probit analysis36,37,38.
To evaluate the effect of antibiotic concentration on the cockroach CFU burden in vivo, suspensions of bacteria were adjusted to ~ 100 × LD50 in PBS and the insects were subsequently infected. Three hours later, cockroaches were then injected with the antibiotic dose indicated (20 μL of each cefotaxime suspension to the right of the midline at the base of the third tergum). As a control, a group of cockroaches was infected but not treated. Cockroaches that survived at 24 h post-treatment were euthanized by decapitation, and the hemolymph was isolated8. To do so, the head and attached gastrointestinal tract were removed, and the hemolymph was dispensed into a microcentrifuge tube containing 20 μL 0.05% N-Phenylthiourea (anticoagulant). This material was serially diluted in PBS and then each dilution was plated to enumerate CFU8.
Galleria mellonella infections
The G. mellonella infection model was conducted as was previously published21,26. G. mellonella larvae were purchased from Best Bet Waxworms Inc. Healthy larvae with no visible melanization were selected. Stationary phase broth cultures were diluted in PBS to the concentrations indicated. Using a 30-gauge needle, each larva was infected with 10 µL of the bacterial suspension. Infected and control larvae were incubated at 37 °C and were monitored for viability daily. The LD50 values were calculated in a similar fashion as was described in the B. dubia cockroach infection studies.
Antibiotic rescue
In vivo antibiotic concentrations were determined using a bioassay of insect hemolymph similar to methods previously published for mammalian body fluids39,40,41,42. Cockroaches were injected with 20 μL of 250 mg/mL, 25 mg/mL, 2.5 mg/mL or 0.25 mg/mL Ampicillin, Carbenicillin, or Cefotaxime respectively. These concentrations were generated by serially diluting a stock antibiotic (250 mg/mL) in PBS. Intrahemoceol injections were administered to the right of the midline at the base of the third tergum8. Each group of cockroaches was housed in a large 150 × 25 mm petri dish at 37 °C. At the time points indicated, at least three cockroaches from each group were euthanized and hemolymph was isolated and mixed with 20 μL 0.05% N-Phenylthiourea (anticoagulant). Ten microliters of the hemolymph was dispensed onto a circular disk of sterile Whatman filter paper which was placed onto TSA containing E. coli (100 μL OD600 = 0.3 of a stationary phase culture) that had been spread-plated. TSA plates were incubated at 37 °C for 24 h. Zones of inhibition were measured in millimeters using a metric ruler. Horizontal and vertical measurements for each disk were averaged. To estimate the amount of antibiotic recovered, the values for the zones of inhibition generated from the recovered hemolymph were compared to a standard curve that was generated by dispensing known quantities of antibiotic onto the sterile Whatman circles, and carrying out the disk diffusion assays similarly as was just previously detailed here.
Statistical analysis
GraphPad Prism was used for statistical calculations. The particular analyses used are indicated in the figure legends.
References
Byrne, J. M. et al. FDA public workshop summary: Advancing animal models for antibacterial drug development. Antimicrob. Agents Chemother. 65, e01983-e2020 (2020).
da Cunha, B., Fonseca, L. P. & Calado, C. R. Antibiotic discovery: Where have we come from, where do we go?. Antibiotics 8, 45 (2019).
Lee, S.-H. et al. In-vitro and in-vivo antibacterial activity evaluation of a polyurethane matrix. J. Pharm. Pharmacol. 55, 559–566 (2003).
Nightingale, J. Clinical limitations of in vitro testing of microorganism susceptibility. Am. J. Hosp. Pharm. 44, 131–137 (1987).
Craig, W. A., Redington, J. & Ebert, S. C. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27, 29–40 (1991).
Louie, A. et al. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.45.3.845-851.2001 (2001).
Turnidge, J. D. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27, 10–22 (1998).
Eklund, B. E. et al. The orange spotted cockroach (Blaptica dubia, Serville 1839) is a permissive experimental host for Francisella tularensis. Proc. West Virginia Acad. Sci. 89, 34–47 (2017).
Brennan, M., Thomas, D. Y., Whiteway, M. & Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. https://doi.org/10.1016/S0928-8244(02)00374-7 (2002).
Loh, J. M. S., Adenwalla, N., Wiles, S. & Proft, T. Galleria mellonella larvae as an infection model for group A streptococcus. Virulence https://doi.org/10.4161/viru.24930 (2013).
Miyata, S., Casey, M., Frank, D. W., Ausubel, F. M. & Drenkard, E. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. https://doi.org/10.1128/IAI.71.5.2404-2413.2003 (2003).
Peleg, A. Y. et al. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.01533-08 (2009).
Champion, O. L. et al. Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis. Microbiology https://doi.org/10.1099/mic.0.026823-0 (2009).
Tsai, C. J. Y., Loh, J. M. S. & Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence https://doi.org/10.1080/21505594.2015.1135289 (2016).
Fisher, N. A., Ribot, W. J., Applefeld, W. & DeShazer, D. The Madagascar hissing cockroach as a novel surrogate host for Burkholderia pseudomallei, B. mallei and B. thailandensis. BMC Microbiol. https://doi.org/10.1186/1471-2180-12-117 (2012).
Seed, K. D. & Dennis, J. J. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect. Immun. https://doi.org/10.1128/IAI.01249-07 (2008).
Mylonakis, E. et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. https://doi.org/10.1128/IAI.73.7.3842-3850.2005 (2005).
Senior, N. J. et al. Galleria mellonella as an infection model for campylobacter jejuni virulence. J. Med. Microbiol. https://doi.org/10.1099/jmm.0.026658-0 (2011).
Insua, J. L. et al. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect. Immun. https://doi.org/10.1128/iai.00391-13 (2013).
Ramarao, N., Nielsen-Leroux, C. & Lereclus, D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J. Vis. Exp. https://doi.org/10.3791/4392 (2012).
Harding, C. R. et al. Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect. Immun. https://doi.org/10.1128/iai.00510-12 (2012).
Mukherjee, K. et al. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.01301-09 (2010).
Jorgensen, J. H. & Ferraro, M. J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 49, 1749–1755 (2009).
Yang, H.-F. et al. Galleria mellonella as an in vivo model for assessing the efficacy of antimicrobial agents against Enterobacter cloacae infection. J. Microbiol. Immunol. Infect. 50, 55–61 (2017).
Hoyle, A. et al. Optimising efficacy of antibiotics against systemic infection by varying dosage quantities and times. PLOS Comput. Biol. 16, 1–20 (2020).
Harding, C. R., Schroeder, G. N., Collins, J. W. & Frankel, G. Use of Galleria mellonella as a model organism to study Legionella pneumophila infection. JoVE https://doi.org/10.3791/50964 (2013).
Benthall, G. et al. Evaluation of antibiotic efficacy against infections caused by planktonic or biofilm cultures of Pseudomonas aeruginosa and Klebsiella pneumoniae in Galleria mellonella. Int. J. Antimicrob. Agents 46, 538–545 (2015).
Cutuli, M. A. et al. Galleria mellonella as a consolidated in vivo model hosts: New developments in antibacterial strategies and novel drug testing. Virulence 10, 527–541 (2019).
Milutinović, B., Stolpe, C., Peuß, R., Armitage, S. A. O. & Kurtz, J. The red flour beetle as a model for bacterial oral infections. PLoS ONE https://doi.org/10.1371/journal.pone.0064638 (2013).
Consentino, L., Rejasse, A., Crapart, N., Bevilacqua, C. & Nielsen-LeRoux, C. Laser capture microdissection to study Bacillus cereus iron homeostasis gene expression during Galleria mellonella in vivo gut colonization. Virulence 12, 2104–2121 (2021).
Wang, L., Tkhilaishvili, T., Bernal Andres, B., Trampuz, A. & Gonzalez Moreno, M. Bacteriophage-antibiotic combinations against ciprofloxacin/ceftriaxone-resistant Escherichia coli in vitro and in an experimental Galleria mellonella model. Int. J. Antimicrob. Agents 56, 106200 (2020).
Brackman, G., Cos, P., Maes, L., Nelis, H. J. & Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 55, 2655–2661 (2011).
Schmitt, D. M. et al. The use of resazurin as a novel antimicrobial agent against Francisella tularensis. Front. Cell. Infect. Microbiol. 3, 93 (2013).
Wiegand, I., Hilpert, K. & Hancock, R. E. W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008).
Schmitt, D. M., Connolly, K. L., Jerse, A. E., Detrick, M. S. & Horzempa, J. Antibacterial activity of resazurin-based compounds against Neisseria gonorrhoeae in vitro and in vivo. Int. J. Antimicrob. Agents 48, 367–372 (2016).
Akçay, A. The calculation of LD50 using probit analysis. FASEB J. https://doi.org/10.1096/fasebj.27.1_supplement.1217.28 (2013).
Wiegand, H. Finney, D. J.: Probit analysis. 3. Aufl. Cambridge University Press, Cambridge 1971. XV, 333 S., 41 Rechenbeispiele, 20 Diagr., 8 Tab., 231 Lit., L 5.80. Biom. Z. https://doi.org/10.1002/bimj.19720140111 (2007).
Finney, D. J. Probit Analysis: A Statistical Treatment of the Sigmoid. 1971 (1947).
Ito, K., Hayasaki, M. & Tamaya, T. Pharmacokinetics of cephem antibiotics in exudate of pelvic retroperitoneal space after radical hysterectomy and pelvic lymphadenectomy. Antimicrob. Agents Chemother. 34, 1160–1164 (1990).
Igawa, H. H., Sugihara, T., Yoshida, T., Kawashima, K. & Ohura, T. Penetration of flomoxef into human maxillary and mandibular bones. Scand. J. Plast. Reconstr. Surg. Hand Surg. 29, 259–262 (1995).
Hori, T., Nakano, M., Kimura, Y. & Murakami, K. Pharmacokinetics and tissue penetration of a new carbapenem, doripenem, intravenously administered to laboratory animals. In Vivo 20, 91–96 (2006).
Maehana, J. et al. Microbiological assay method for T-3761 concentration in body fluids. Jpn. J. Antibiot. 48, 610–620 (1995).
Patel, K. B., Nicolau, D. P., Nightingale, C. H. & Quintiliani, R. Pharmacokinetics of cefotaxime in healthy volunteers and patients. Diagn. Microbiol. Infect. Dis. 22, 49–55 (1995).
Pancoast, S. J. & Neu, H. C. Kinetics of mezlocillin and carbenicillin. Clin. Pharmacol. Ther. 24, 108–116 (1978).
Foulds, G. Pharmacokinetics of sulbactam/ampicillin in humans: A review. Rev. Infect. Dis. 8(Suppl 5), S503–S511 (1986).
Schrinner, E., Limbert, M., Penasse, L. & Lutz, A. Antibacterial activity of cefotaxime and other newer cephalosporins (in vitro and in vivo). J. Antimicrob. Chemother. 6, 25–30 (1980).
Aoki, H., Sakai, H., Kohsaka, M., Konomi, T. & Hosoda, J. Nocardicin A, a new monocyclic beta-lactam antibiotic. I. Discovery, isolation and characterization. J. Antibiot. (Tokyo) 29, 492–500 (1976).
Liu, C. X., Wang, J. R. & Lu, Y. L. Pharmacokinetics of sulbactam and ampicillin in mice and in dogs. Yao Xue Xue Bao 25, 406–411 (1990).
Acknowledgements
This work was supported, in part, by funding from the NIH-NIGMS (P20GM103434), NIH-NHLBI (1R15HL147135), and the WV-NASA Space Grant Consortium (NNX15AI01H). The authors thank Katharine Jennings for carefully reviewing this manuscript.
Author information
Authors and Affiliations
Contributions
J.H. and N.F. wrote the manuscript. E.C., C.M., T.B., N.F., and J.H. edited the manuscript. E.C., C.M., T.B., K.P., and S.C. conducted the experiments. E.C., C.M., T.B., K.P., S.C., N.F., and J.H. analyzed the data. N.F. and J.H. envisioned and designed the study. J.H. oversaw the work. All authors read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collins, E., Martin, C., Blomquist, T. et al. The utilization of Blaptica dubia cockroaches as an in vivo model to test antibiotic efficacy. Sci Rep 11, 24004 (2021). https://doi.org/10.1038/s41598-021-03486-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03486-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.