Abstract
Several seaweed extracts have been reported to have potential antimethanogenic effects in ruminants. In this study, the effect of three brown seaweed species (Undaria pinnatifida, UPIN; Sargassum fusiforme, SFUS; and Sargassum fulvellum, SFUL) on rumen fermentation characteristics, total gas, methane (CH4), carbon dioxide (CO2) production, and microbial populations were investigated using an in vitro batch culture system. Seaweed extract and its metabolites, total flavonoid and polyphenol contents were identified and compared. For the in vitro batch, 0.25 mg∙mL−1 of each seaweed extract were used in 6, 12, 24, 36 and 48 h of incubation. Seaweed extract supplementation decreased CH4 yield and its proportion to total gas production after 12, 24, and 48 h of incubation, while total gas production were not significantly different. Total volatile fatty acid and molar proportion of propionate increased with SFUS and SFUL supplementation after 24 h of incubation, whereas UPIN was not affected. Additionally, SFUS increased the absolute abundance of total bacteria, ciliate protozoa, fungi, methanogenic archaea, and Fibrobacter succinogenes. The relative proportions of Butyrivibrio fibrisolvens, Butyrivibrio proteoclasticus, and Prevotella ruminicola were lower with seaweed extract supplementation, whereas Anaerovibrio lipolytica increased. Thus, seaweed extracts can decrease CH4 production, and alter the abundance of rumen microbial populations.
Similar content being viewed by others
Introduction
Methane (CH4) is the second largest contributor to greenhouse gas (GHG) emissions after carbon dioxide (CO2) and has a global warming potential (GWP) approximately 28 times greater than that of CO21,2. Ruminants produce CH4 as a metabolic end-product of enteric fermentation in the rumen, representing between 2 and 12% of the gross energy intake3. Research on strategies to reduce CH4 production is necessary owing to the global awareness and threat.
Although numeral feed additives have been used to decrease CH4 production by manipulating the ruminal microbial fermentation, seaweed has shown to be some of the most promising worldwide4,5,6,7. Seaweed has rich and diverse bioactive compounds, particularly halogenated and polyphenolic metabolites that can suppress methanogenesis in the rumen8,9,10,11. A species of red seaweed, Asparagopsis taxiformis (A. taxiformis), including bromoform and dibromochloromethane, specifically inhibits enzymatic activities by binding to vitamin B1212; this is chemically similar to the coenzyme F430, a cofactor needed for methanogenesis13. A species of brown seaweed is the only species to accumulate a variety of polyphenol compounds (e.g., phlorotannins) as an adaptive defense strategy against stress conditions and herbivory14,15. A previous study reported that phlorotannins purified from Ascophyllum nodosum (A. nodosum), a species of brown seaweed, had an antimethanogenic effect without affecting ruminal microbial fermentation11. However, the exact mechanism has not yet been understood thus far. Notably, phlorotannins can induce an antimicrobial effect by inactivating extracellular enzymes and proteins necessary for the growth and metabolism of microorganisms16. Due to the similarity of chemical structures between phlorotannins and terrestrial tannin, the antimicrobial effect of phlorotannins against rumen methanogens may be worth further exploration11.
The current study consider three brown seaweed species (Undaria pinnatifida, UPIN; Sargassum fusiforme, SFUS; and Sargassum fulvellum, SFUL) that were selected based on cultivation potential, biochemical profile, and sustainability as animal feed in ruminant production17,18,19. In addition, our previous in vitro batch culture studies classified dried UPIN, SFUS, and SFUL as consisting of minerals, heavy metals, and metabolites, and also found that the three seaweeds enhanced rumen volatile fatty acid (VFA) concentrations when fed at 1% dry matter (DM) content17,18,19. Moreover, Li et al.14 reported that UPIN, SFUS, and SFUL, accumulate phlorotannins with health beneficial biological activities, which in some cases can demonstrate antimicrobial and antimethanogenic effects in ruminants. A reduction in CH4 production was observed at the rate of 1% DM of SFUS, but not for UPIN and SFUL. Seaweed in the form of an extract supplemented at 0.25 mg∙mL−1 with only timothy hay, exhibited a greater reduction in CH4 production after 48 h of incubation20. A recent study reported that the supplementation of the red seaweed A. taxiformis reduced the rumen methanogens abundance along with a CH4 reduction via an in vitro continuous culture system9. In contrast, brown seaweed (A. nodosum and Laminaria digitate (L. digitata)) supplementation did not affect the abundance of rumen methanogens, fungi, and total bacteria15. However, the impact of UPIN, SFUS, and SFUL on the rumen microbial abundance is still not fully understood.
Therefore, we hypothesize that the supplementation of three different seaweed extracts would reduce enteric CH4 production and enhance rumen fermentation characteristics. The main objectives of this study were to: (1) identify the metabolites and potential antioxidants (including the flavonoid and phenol content) of the three brown seaweed extracts; (2) determine if the polyphenolic compounds in the seaweed extracts reduced enteric CH4 and CO2 production; (3) examine the effect of seaweed extracts on the abundance of rumen bacterial, ciliate protozoal, fungal and methanogenic archaea using real-time polymerase chain reaction (qPCR); and (4) investigate the rumen fermentation characteristics and mode of action of seaweed extracts using in vitro batch culture.
Results
Metabolite, total flavonoid and polyphenol profiles of seaweed extracts
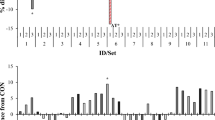
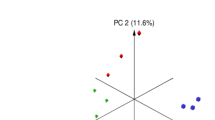
1H-NMR analysis of three seaweed extracts (UPIN, SFUS, and SFUL) identified 149 metabolites in the three seaweed extracts (Fig. 1 and Table 1). However, only 24 metabolites (including guanidoacetate, ethylene glycol, alanine, galactose, and inter alia) were classified into four chemical classes (organic acids, carbohydrates, amino acids, and lipids). The PCA plot showed separated clusters among the three seaweed extracts and revealed differences that were considerably separated in PC 1 (30.5%), and PC 2 (24.1%) in PCA (Fig. 1A). Further PLS-DA analysis of relative intensities of the metabolites revealed the significant differences of identified metabolites in seaweed extracts with 20 of them being significantly different (VIP score > 1.5) (Fig. 1B). We found gallate in the extract that obtained highest VIP score, which possesses antioxidant properties, and 14 phenolic metabolites were detected in SFUL (69.20 ± 7.86 µM) and SFUS (2.10 ± 0.06 µM) but not in UPIN. The total flavonoid and polyphenol contents are shown in Table 1. Total flavonoid content varied between species and was highest for SFUL (25.21 ± 1.72 mg CE/g) followed by UPIN (1.66 ± 0.26 mg CE/g) and lowest in SFUS (0.49 ± 0.12 mg CE/g). The total polyphenol content was highest in SFUL (5.77 ± 0.07 mg GAE/g) followed by SFUS (1.89 ± 0.05 mg GAE/g) and lowest in UPIN (1.59 ± 0.16 mg GAE/g). In the present study, SFUL had higher total flavonoid and polyphenol contents than the other seaweed extracts.
Multivariate score plots for brown seaweed extracts. (A) Principal component analysis (PCA) score plot, ellipses represent 95% confidence intervals. (B) Variable importance in projection (VIP) scores of the top 20 metabolites with VIP scores > 1.5. UPIN (red ellipse), Undaria pinnatifida; SFUS (yellow ellipse), Sargassum fusiforme; SFUL (blue ellipse), Sargassum fulvellum; 2-HIC, 2-hydroxyisocaproate; 3,5-diBrTy, 3,5-dibromotyrosine; 3-MPA, 3-methyladipate; N6-AcLys, N6-acetyllysine; N-ALT, N-acetyltyrosine; N-NDMA, N-nitrosodimethylamine; N,N-DMG, N,N-dimethylglycine; O-Acart, O-acetylcarnitine.
Effects of seaweed extracts on rumen fermentation characteristics
Rumen fermentation characteristics are shown in Table 2 and Fig. 2. Compared to the CON, UPIN, SFUS, and SFUL supplementation resulted in the pH being greater (P = 0.001) at 6 h of early incubation time. The apparent DM digestibility was estimated by a nylon bag, and it tended to be affected by supplementation, wherein SFUS was lower (P = 0.059) than CON at 12 h of incubation, and no differences were observed afterward. The ammonia nitrogen (NH3-N) concentration was considerably lower by supplementation with UPIN, SFUS, and SFUL compared with CON from 12 h up to 48 h of incubation. The most pronounced decrease in NH3-N concentration was observed (P < 0.001) at 12 h of incubation. The concentration of total VFA did not differ among treatments at 12 h of incubation. However, it was considerably higher (P < 0.05) with SFUS and SFUL than CON at 24 h of incubation. None of the seaweed extracts affected the molar percentage of acetate concentration at both 12 and 24 h of incubation. SFUS and SFUL showed a significantly higher (P < 0.001) molar percentage of propionate at 24 h of incubation. A tendency (P = 0.057) indicated that SFUS showed a lower molar percentage of butyrate at 24 h of incubation, whereas UPIN and SFUL did not affect the butyrate molar percentage in the cultures. The molar percentage of isobutyrate increased by with SFUL supplementation at both 12 (P < 0.01) and 24 h (P < 0.001) of incubation compared to the CON. In contrast, the molar percentage of isovalerate was greater in CON among treatments at both 12 (P < 0.001) and 24 h (P < 0.001) of incubation. SFUS resulted in a significantly higher (P < 0.01) molar percentage of valerate at 12 h of incubation but did not differ among the treatments at 24 h of incubation. As a result, the acetate to propionate (AP) ratio was lower (P = 0.054, P < 0.001) for SFUL at 12 and 24 h of incubation.
Effects of brown seaweed extracts on volatile fatty acid production after 12 and 24 h of in vitro incubation. CON (gray box), without seaweed extracts; UPIN (red box), Undaria pinnatifida; SFUS (yellow box), Sargassum fusiforme; SFUL (blue box), Sargassum fulvellum; Data were analyzed using seaweed extracts dose amount: 0.25 mg-mL−1. Error bars are standard error of the mean. a,bMeans (n = 5) with different superscript letters indicate a significant difference (P < 0.05).
Effects of seaweed extracts on total gas, CH4, and CO2 production
Total gas production was significantly lower (P < 0.001) in UPIN, SFUS, and SFUL supplementation compared with CON at an early incubation time (Fig. 3). The CH4 production lowered gradually up to 48 h during the incubation for all treatments. Compared to CON, the most pronounced reduction was observed in the UPIN, SFUS, and SFUL supplementation at 12 (reduction of 26.8%, 23.4%, 26.3%, P < 0.001, respectively), and 24 h (reduction of 21.3%, 24.4%, 24.6%, P < 0.05, respectively) of incubation. Compared with CON, the CH4 proportion to total gas production by all seaweed extract supplementations was significantly lower at 12 (P < 0.01), 24 (P < 0.01), and 48 h (P < 0.001) of incubation. The CO2 production tended to be significantly lower with UPIN, SFUS, and SFUL supplementation compared with CON at 12 (P < 0.001) and 48 h (P < 0.05) of incubation.
Effects of brown seaweed extracts on (A) total gas, (B) CH4, (C) CO2 production, and (D) total gas (% of CH4) in vitro. CON (gray circle), without seaweed extracts; UPIN (red circle), Undaria pinnatifida; SFUS (yellow circle), Sargassum fusiforme; SFUL (blue circle), Sargassum fulvellum; Data were analyzed using seaweed extracts dose amount: 0.25 mg-mL−1. Error bars are standard error of the mean. a,bMeans (n = 5) with different superscript letters indicate a significant difference (P < 0.05).
Effects of seaweed extracts on the abundance of microbial community
Regarding the microbial counts, there was no significant change in the absolute value of total bacteria among the treatment and CON (Table 3). Compared to CON, the populations of both ciliate protozoa (P < 0.01) and fungi (P < 0.01) were significantly higher with SFUS supplementation, while UPIN and SFUL supplementation were significantly lower. None of the seaweed extracts decreased (P < 0.05) the abundance of methanogenic archaea, which the microbial population directly responsible for rumen CH4 production, rather SFUS supplementation resulted in a significantly higher proportion than CON. In the relative populations of fiber-degrading bacterial species both Fibrobacter succinogenes and Ruminococcus flavefaciens were significantly higher in SFUS (P < 0.01) and SFUL (P < 0.05) supplementation, while Ruminococcus albus was not affected by any of the treatments. There was a tendency (P = 0.092) that Butyrivibrio fibrisolvens abundance reduced in SFUS and SFUL supplementation compared to CON, while the abundance of Butyrivibrio proteoclasticus was not affected by any of the treatments. In contrast, all the treatments had a significantly higher (P < 0.01) abundance of Anaerovibrio lipolytica, which includes lipolytic bacteria. Except for UPIN supplementation, both SFUS and SFUL had a significantly lower (P < 0.001) abundance of Prevotella ruminicola, which includes proteolytic bacteria.
Discussion
Supplementation of seaweed extracts in ruminant diets has been reported to alter digestion, fermentation characteristics, proteolysis, and microbial communities in the rumen9,18,21,22,23. Polyphenols in seaweeds (Table 1) protect proteins from degradation and improve the efficiency of nitrogen use in ruminants by increasing the amount of by-pass protein and reducing rumen fiber degradation by decreasing the attachment of microbes to feed particles24. In the present study, supplementation with seaweed extracts did not affect DM digestibility but lowered the NH3-N concentration and AP ratios (Table 2, Fig. 2). This could be attributed to the polyphenolic content in seaweed extracts, which can decelerate ruminal proteolysis, peptidolysis, and deamination7. The reduced molar proportion of isovalerate supports this premise7,10. Isovalerate is produced by microbial deamination and the decarboxylation of leucine in the rumen25; the polyphenolic content could possibly reduce this process. This result is also supported by the higher abundance of fibrolytic bacterial populations, including F. succinogenes and R. flavefaciens (Table 3) because isovalerate is a well-known requirement for their growth26. In addition, the lower abundance of proteolytic bacteria, namely B. fibrisolvens, B. proteoclasticus, and P. ruminocola might also support this result.
In the present study, seaweed extract supplementation induced an evident shift in the VFA pattern compared with the CON (Fig. 2). Notably, SFUS and SFUL supplementation caused a shift in the ruminal fermentation to a greater molar percentage of propionate without affecting the molar percentage of acetate. Wettstein et al.27 reported that decreased rumen methanogenesis sometimes shifts rumen fermentation from acetate to propionate because the propionate synthesis pathway is favored rather than acetate synthesis pathway. Generally, CH4 formation in the rumen is regarded as a syntrophy between H2-producing microbes and H2-consuming methanogens28; the manipulation of the H2 sink plays an important role in mitigating CH4 in the rumen. The major metabolic H2 sink strategy for CH4 mitigation is the enhancement of propionate production because it acts as a sink for H2 and reduces the availability of H2 for methanogens29. Becker et al.30 reported that flavan-3-ol, ( +)-catechin has the potential to be an alternative H2 sink for CH4 precursors without reducing the VFA production. However, contrary results were obtained in the present study, as SFUS and SFUL had a greater VFA production. Further analysis of catechin from seaweed extracts and an investigation of the relationship between VFA production and CH4 formation in the rumen fluid using an in vitro batch culture system is necessary to clarify the mechanism by which seaweed extracts alter rumen fermentation. The present study showed that both SFUS and SFUL supplementation had the highest molar percentage of propionate, even though it presented a low population of P. ruminicola (Table 3), a major propionate-producing bacterial species in the rumen31. A possible explanation for the greater molar percentage of propionate is the high concentration of lipid class metabolites (e.g., ethylene glycol) in seaweed extracts and the higher relative abundance of A. lipolytica, an important rumen bacterium for lipid hydrolysis32. Our data indicate that the notable scores in SFUS and SFUL were fumarate and glycerol, respectively. Fumarate can be converted into propionate, and glycerol (a major source of lipid metabolism) is rapidly fermented to propionate in the rumen33. However, a limitation in this study was the lack of members of the ruminal microbiome that contribute to the propionate synthesis pathway. Physiological and ecological studies employing metagenomics approaches on rumen bacteria are needed to determine if the seaweed extracts directly affect succinate- and propionate- producing bacteria at the genus or species level in the rumen. This may further support our speculation that seaweed extracts have the potential to increase propionate production. Nevertheless, the type of amino acids, carbohydrates, and preformed organic acids measured in seaweed extracts may affect the molar proportion of accumulated VFAs. Taken together, the decreased AP ratio suggests that metabolic H2 is, at least in part, redistributed to propionate, which may partly explain the CH4 reduction observed in this study.
There was a trend for a slight increase in total gas production with the supplementation of seaweed extracts, possibly owing to the increase in fiber-degrading bacteria in the seaweed extracts (Table 3, Fig. 3). The novel finding in our study is that supplementation with seaweed extracts caused a 5.4% to 26.8% suppression in CH4 production up to 48 h incubation, without compromising DM digestibility or total VFA production. Moreover, the proportion of CH4 to total gas production was suppressed by seaweed extract supplementation. The CH4 is produced in the rumen and hindgut of ruminants by a group of methanogenic archaea, which are estimated to account for 0.3–3.3% of the rumen microbial population34,35. Most of them use H2 and CO2 produced by some fermentative members of the rumen microbes to produce CH4 through the hydrogenotrophic pathway36. With significantly lower CH4 production, CH4-producing microbes (ciliate protozoa and methanogenic archaea) were expected to be less with seaweed extract supplementation. However, SFUS supplementation resulted in a larger abundance of ciliate protozoa, and methanogenic archaea, and the total bacteria, fungi, fibrolytic bacteria, and A. lipolytica, which may explain the high VFA production compared with CON and other seaweed extracts (Table 3, Fig. 2). The supplementation of UPIN and SFUL significantly reduced the abundance of ciliate protozoa but did not affect methanogenic archaea. However, these findings agree with the in vitro findings reported by Molina-Alcaide et al.37, who suggested that different seaweed species may have variable effects on the abundance of ciliate protozoa. Similarly, according to Henderson et al.36, ciliate protozoa and methanogenic archaea are not always correlated, even if they have a mutualistic relationship that enhances CH4 formation in the rumen. This indicates that there are also different partner specificities within archaeal and protozoal species. Similar and contradictory results were observed in the in vivo experiment performed in this study, where ciliate protozoal abundance increased via brown seaweed species A. nodosum, but total bacteria abundance and DM digestibility were reduced. The seaweed species and the experimental system (in vitro vs. in vivo) were different in these studies, thus, suggesting that an in vivo system would enhance future studies in evaluating the antimethanogenic effects of different seaweed extracts on ciliate protozoa38.
Another factor that could affect rumen microbial abundance was linked to the properties of total flavonoids, polyphenols and phenolic metabolites (Table 1) of the three seaweed species, which act as antimicrobial. For example, it has been reported that polyphenolic compounds of seaweed (e.g., phlorotannins) can reduce CH4 production in ruminants22. However, it is unclear whether this directly affects polyphenolic compounds on CH4-producing microbes, including ciliate protozoa and methanogenic archaea. A previous in vitro batch culture study11 showed that when phlorotannins (derived from A. nodosum) was added to rumen fluid, the NH3-N concentration was lower than that without the addition of phlorotannins (Table 2). This result concurs with the present study, in which seaweed extract supplementation reduced the NH3-N concentration. The abundance of other proteolytic bacteria (e.g., Prevotella bryantii) was greater when phlorotannins was added, despite a significant reduction in NH3-N concentration. In contrast, the present study found that all three seaweed extracts lowered the abundance of proteolytic bacteria (B. fibrisolvens, B. proteoclasticus, and P. ruminocola) (Table 3). Thus, our results indicate that the polyphenol content from UPIN, SFUS, and SFUL also have a function in the formation of protein-phenol complexes and discrepancies in the proteolytic bacteria abundance may be partly attributable to the different types of polyphenolic compounds produced by the interspecies difference of seaweed. Nonetheless, additional research is needed to elucidate the mechanism related to CH4 production and/or rumen microbial abundance to explain the effects of polyphenols from seaweed-species.
Another finding of this study was that the interaction between seaweed and basal substrates plays an important role in the effectiveness of rumen methanogenesis23. Indeed, in our previous research20, we found that the supplementation of timothy hay with five, brown seaweed extracts (Ecklonia stolonifera, Eisenia bicyclis, UPIN, SFUS, and SFUL) decreased CH4 production only after long-term in vitro incubation (48 h). However, in the present study we found that CH4 production was effectively reduced after short-term to long-term in vitro incubation (12, 24, and 48 h) (Fig. 3). This discrepancy might be attributable to the corn grain (concentrate) and timothy hay combination, which led to alterations in rumen fermentation (e.g., a higher molar proportion of propionate and lower methane production) as a result of microbial selection (Table 3). Therefore, to enable the wider application of seaweed extracts as a novel candidate additive in the future, the potential of seaweed extracts must be evaluated at various forage-to-concentrate ratios under in vitro and in vivo studies. Seaweed extract supplementation resulted in lower CO2 production than the CON at 12 and 48 h of incubation, which could indicate a high conversion rate of CO2 to CH4 by methanogenic archaea. Nevertheless, our results indicate that bioactive compounds in seaweed extracts are responsible for reducing methanogenic archaea to utilize free CO2 during methanogenesis, which could reduce CH4 production without decreasing methanogenic archaea (Tables 1, 3). Moreover, bioactive compounds such as phloroglucinol (monomeric unit of phlorotannins) decreased CH4 production with the reduction of methanogens39. Overall, our findings suggest that CH4 and CO2 decrease at a greater rate than the total gas production and this should direct more energy into VFA production.
In the rumen, it is known that CH4 production results from a mutualistic association between ciliate protozoa and methanogenic archaea40 (Table 3). Although there was no reduction of methanogenic archaea, a decrease in CH4 production may have been caused either by the suppressed metabolism of a CH4-producing microbe (independent of species) or the changed composition of the methanogenic community, or both41. Additionally, Zhou et al.42 reported that decreases in methanogenic archaea populations may not necessarily lead to a reduction in CH4 production, at least within short-term in vitro incubation. The discrepancy between CH4 production and the dynamics of the methanogenic archaea population might be partly attributable to the insensitivity of some ruminal methanogenic archaea to seaweed extracts.
A suitable compound for the reducing of methanogenesis in ruminants should be effective in reducing CH4 production and increasing propionate. The present study indicated that seaweed extracts can significantly decrease CH4 production, NH3-N concentrations, and shift the abundance of rumen microbial populations, but DM digestibility and total VFA production are not affected. Additionally, seaweed extracts possess the potential for CH4 reduction but do not always result in antiprotozoal activity suggesting that the unidirectional relationship between methanogenesis and protozoal numbers, as affected by seaweed extract, is not compulsory. Metagenomic and metabolomic approaches are essential for understanding how certain seaweed extracts impact the rumen microbiome and whether these effects hold promise as rumen modulators to improve rumen fermentation characteristics and productivity.
Materials and methods
Ethics statement
This study was performed in accordance with the principles of the Basel Declaration and recommendations of Laboratory Animals Guidelines of Gyeongsang National University (Jinju, Gyeongsangnam-do, Korea). All management and experimental protocols involving animals were approved by Gyeongsang National University Animal Research Ethical Committee (GNU-191011-E0050). This study followed standard procedures and ARRIVE guidelines to ensure an appropriate animal care.
Brown seaweed extract preparation
The collection of seaweed material complied with institutional, national, and international guidelines and legislation concerning Undaria pinnatifida (UPIN), Sargassum fusiforme (SFUS), and Sargassum fulvellum (SFUL) seaweed. In accordance with guidelines and regulations for biosafety in Korea, extraction the seaweed (UPIN, SFUS, and SFUL), and residues was discarded according to protocol.
Three different types of brown seaweed extracts, i.e., UPIN, SFUS, and SFUL were purchased from the Jeju Biodiversity Research Institute (Jeju-do, Korea). Voucher specimens of the seaweed and other information regarding the seaweed extracts are available at this institute. Each fresh seaweed was cut or crushed into small pieces, freeze-dried and ground into a fine powder. Subsequently, powder was extracted with 80% (v/v) ethanol solvent (Daejung Chemical and Metals CO., Ltd, Siheung, Korea), and then placed in ultrasonic cleaner (Branson Ultrasonics Corporation, Danbury, CT, USA). Afterward, dimethylsulfoxide (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was infused to dissolve the stock solution (50-mg∙mL−1) of each extract and diluted using culture media.
Chemical analysis
Chemical composition of substrates used on in vitro is shown in Table 4. Timothy hay and corn grain samples were ground through a 1 mm screen (Wiley Mill, Arthur Thomas Co., Philadelphia, PA), prior to in vitro and chemical analysis. Official methods of AOAC43 were used to analyze dry matter (DM), crude protein (CP, % of DM), ether extract (EE, % of DM) and Ash (% of DM) contents in the substrate. Neutral and acid detergent fiber (NDF and ADF, % of DM) contents were analyzed according to previously described method of Van Soest et al.44. Non-fibrous carbohydrate (NFC, % of DM) was calculated by following equation:
Total flavonoid content was determined using the method of Zhishen et al.45 and Woisky and Salatino46 with slight modifications. In short, an aliquot of 100 μL of each seaweed extract solutions or standard (( +)-catechin hydrate) were mixed with 7.5 μL of sodium nitrite (5%), 15 μL of aluminium chloride (10%), 100 μL of 1 M sodium hydroxide, and 25 μL of distilled water and allowed to react 30 min. The total flavonoid concentration of each seaweed extracts was measured by microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA) at 510 nm.
Total polyphenol content was determined using the method of Singleton et al.47 with minor modifications. Briefly, 100 μL of seaweed extracts or standard (gallic acid) were infused into an Eppendorf tube followed by 100 uL of 1 N Folin–Ciocalteu reagent solution. Afterward, 100 uL of 2% sodium carbonate solution was infused and tubes were thoroughly mixed by vortexing and allowed to stand for 30 min at room temperature. The total polyphenol concentration of each seaweed extracts was measured by microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA) at 750 nm.
Experiment procedures
Rumen fluid was collected from two non-lactating cannulated Hanwoo cows (average body weight = 440 kg) before morning feeding. The cows were fed a twice daily (0900 and 1700) for 2% DM of their body weight of timothy hay and commercial concentrate in a 6:4 ratios with free access to clean drinking water and a mineral block. Rumen fluid samples were filtered through four layers of cheesecloth, immediately transferred to the laboratory kept in a water bath at 39 °C. Afterward, filtered rumen fluid mixed with a buffer medium48 at a ratio of 1:2 (v/v) and maintained at anaerobic environment. Then, mixture (40 mL/bottle) was accurately infused into a 120 ml serum bottle, under a stream of O2-free N2, containing 500 mg of substrate which was composed of 300 mg of timothy hay and 200 mg of corn grain; substrates were placed into nylon bags, which were later sealed and poured into the serum bottles. Seaweed extract mixtures were used at 1 dose amount: (0.25 mg∙mL−1) 5% of substrate of in vitro incubation medium. A CON (without seaweed extracts) was included in parallel. The dose amount (0.25 mg∙mL−1) was determined by previous studies20,49. Bottles were capped with a butyl rubber and placed in shaking incubator (120 rpm) at 39 °C for 6, 12, 24, 36 and 48 h incubation. A total of 115 bottles were used for 4 treatment including CON with 5 replicates each time points (6, 12, 24, 36 and 48 h incubation). The following treatments were used: (1) CON, (2) supplementation 0.25 mg∙mL−1 of UPIN extract, (3) supplementation 0.25 mg∙mL−1 of SFUS extract 5%, (4) supplementation 0.25 mg∙mL−1 of SFUL extract. There were also 15 bottles that were used for without substrate as a blank with 3 replicates each time points (6, 12, 24, 36 and 48 h incubation).
Sampling and measurements
In vitro gas production during 6, 12, 24, 36 and 48 h incubation was determined by using a pressure transducer (Laurel Electronics, Inc., Costa Mesa, CA, USA) as described by Theodorou et al.50. All pressure values were converted to gas volume (mL) from the following equation defined by our laboratory conditions
where V is gas volume (mL), P is measured pressure (psi).
Headspace gas (6 mL) was collected from each bottle and moved into vacuum test tube (Vacutainer, Becton Dickinson, Franklin Laker, NJ, USA). Concentration of CH4 and CO2 in the gas samples were determined by a gas chromatography (Shimadzu, GC-2010 PLUS, Japan) equipped with HP-PLOT Q capillary column (I.D. 0.53 mm, L.30 m) and flame ionization detector (FID). The temperature of the column, injector and detector were set at 50, 150 and 200 °C, respectively. Helium and H2 gases were used as carrier and combustion gases, respectively. The total production of CH4 and CO2 was calculated according to López et al.51 as follows:
The pH value of each sample recorded using a pH meter (S220, Mettler-Toledo, Greifensee, Switzerland). A 2 mL of liquid sub-sample was collected from 6, 12, 24, 36 and 48 h incubations and then immediately centrifuged at 20,000 × g for 10 min at 4 °C for analyze NH3-N. The NH3-N was analyzed as described in Chaney and Marbach52, where the NH3-N concentration was adapted for 96 well plates with absorbance at 630 nm with a spectrometer (Model 680, Bio-Rad Laboratories, Hercules, CA, USA). A rumen fluid (2 mL) was collected from 12 and 24 h incubation and then immediately centrifuged at 20,000 × g for 10 min at 4 °C for analyze VFA. The VFA concentration was measured with a high performance liquid chromatography (L-2200, Hitachi, Tokyo, Japan) according to the method of Adesogan et al.53. Remaining rumen fluid (2 mL) of 24 h incubation was centrifuged at 20,000 × g for 15 min at 4 °C, supernatant was discarded, and the pellet was stored at − 80 °C in a freezer until use for DNA extraction and microbial community analysis. The apparent DM digestibility of the substrate was estimated after drying the residues collected in the nylon bags and the initial substrate at oven dried at 105 °C for 24 h.
Microbial DNA extraction and quantitative real-time-polymerase chain reaction
Total DNA was extracted from the pellet stored at − 80 °C using the repeated bead beating plus column method54. Genomic DNA was extracted from triplicate samples using QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) following manufacturer recommendations. The quality and quantity of extracted DNA were analyzed with a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Real-time quantitative PCR assays for enumeration of total bacteria, ciliate protozoa, fungi, methanogenic archaea, fibrolytic bacteria (Fibrobacter succinogenes, Ruminococcus albus, Ruminococcus flavefaciens), proteolytic bacteria (Butyrivibrio fibrisolvens, Butyrivibrio proteoclasticus, Prevotella ruminicola, Anaerovibrio lipolytica) were conducted as described by Denman and McSweeney55 and Khafipour56 on a CFX 96 Touch system (Bio-Rad Laboratories). Specific information on primer sequences for rumen microbes is presented in Table 5. All the reaction were carried out triplicate, total reaction volumes of 20 μL. Reaction mixture consisted of 0.5 μL of 10 mM dNTP mix (BioFACT, Daejeon, Korea), 2 μL of 10 × buffer (BioFACT, Daejeon, Korea), 1 μL of tenfold diluted genomic DNA, each 1 1 μL of 10 μM primer-set, 0.1 μL of taq polymerase (BioFACT, Daejeon, Korea), 1 μL of Evagreen (SolGent, Daejeon, Korea), and 13.4 μL of bio-grade water. For absolute quantification of each microbes, using a standard plasmid DNA to the respective target sequence. All the detail procedure of PCR condition and manufacturing each microbe plasmids were proceeded according to Kim et al.57 and Hamid et al.58.
NMR spectroscopy and metabolite identification and quantification
Five hundred microliters of methanol-d4, 400 μL of 0.2 M phosphate buffer solution (0.2 M of sodium hydrogen phosphate, 0.2 M sodium dihydrogen phosphate in D2O, pH 7.0 ± 0.1), and 100 μL of 5 mM TSP (3-trimethylsilyl propionic-2, 2, 3, 3-d4 acid sodium salt) were added into an Eppendorf tube containing 50 ± 0.5 mg of each seaweed extract. 1H-NMR experiments were carried out on Ascend 800 MHz, Avance III HD Bruker spectrometer (Bruker Biospin AG, Fällanden, Switzerland) equipped with 5 mm CPTIC 1H-13C/15 N/D Z-GRD Z119427/0011 cryogenic probe. 1H-NMR spectra were processed and analysed using Chenomx NMR Suite 8.4 (Chenomx, Edmonton, AB, Canada). All 1H-NMR spectra were calibrated, phased and baseline-corrected manually using the processor module of Chenomx NMR Suite. The concentration and profiling of metabolites were estimated using the profiler module of Chenomx NMR Suite. The detail procedures of such analyses has been reported in a previous article Choi et al.23.
Statistical analysis
The online open-source platform, MetaboAnalyst 5.0 was used for the multivariate analyses including principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) of metabolites in seaweed extracts. To refine analysis of metabolites in seaweed extracts, the variable importance in projection (VIP) score along the predictive component were acquired. The VIP score exceeding 1.5 were selected as differentially expressed metabolites. Detail procedures of such analyses has been reported in a previous article64. The data of in vitro was statistically analyzed using the PROC MIXED procedure of SAS 9.4. The statistical model used in this study as follow:
where Yijk is the experimental data; µ is the overall mean, ti is the fixed effect of dietary treatments; rj is the random effect of replication; and γk is the unexplained random error. Tukey’s multiple range test was used to identify differences between treatments. Statistical significance was declared at P ≤ 0.05, and a trend was discussed when 0.05 < P ≤ 0.10.
References
Stocker, T. F. et al. Climate change 2013. The physical science basis. Working group I contribution to the fifth assessment report of the intergovernmental panel on climate change-abstract for decision-makers; Changements climatiques 2013. Les elements scientifiques. Contribut. (2013).
Zhao, L. et al. Ozone decreased enteric methane production by 20% in an in vitro rumen fermentation system. Front. Microbiol. 11, 1–13 (2020).
Johnson, K. A. & Johnson, D. E. Methane emissions from cattle. J. Anim. Sci. 73, 2483–2492 (1995).
Odongo, N. E. et al. Long-term effects of feeding monensin on methane production in lactating dairy cows. J. Dairy Sci. 90, 1781–1788 (2007).
Immig, I., Demeyer, D., Fiedler, D., Van Nevel, C. & Mbanzamihigo, L. Attempts to induce reductive acetogenesis into a sheep rumen. Arch. Anim. Nutr. 49, 363–370 (1996).
Kim, S. H., Mamuad, L. L., Kim, D. W., Kim, S. K. & Lee, S. S. Fumarate reductase-producing enterococci reduce methane production in rumen fermentation in vitro. J. Microbiol. Biotechnol. 26, 558–566 (2016).
Patra, A. K. & Yu, Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 78, 4271–4280 (2012).
Kinley, R. D. et al. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 259, 120836 (2020).
Roque, B. M. et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 1, 1–14 (2019).
Wang, Y., Xu, Z., Bach, S. J. & McAllister, T. A. Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol. 145, 375–395 (2008).
Wang, Y., Alexander, T. W. & Mcallister, T. A. In vitro effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on rumen bacterial populations and fermentation. J. Sci. Food Agric. 89, 2252–2260 (2009).
Wood, J. M., Kennedy, F. S. & Wolfe, R. S. Reaction of multihalogenated hydrocarbons with free and bound reduced vitamin B12. Biochemistry 7, 1707–1713 (1968).
Allen, K. D., Wegener, G. & White, R. H. Discovery of multiple modified F430 coenzymes in methanogens and anaerobic methanotrophic archaea suggests possible new roles for F430 in nature. Appl. Environ. Microbiol. 80, 6403–6412 (2014).
Li, Y.-X., Wijesekara, I., Li, Y. & Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 46, 2219–2224 (2011).
Belanche, A., Jones, E., Parveen, I. & Newbold, C. J. A metagenomics approach to evaluate the impact of dietary supplementation with Ascophyllum nodosum or Laminaria digitata on rumen function in Rusitec fermenters. Front. Microbiol. 7, 1–14 (2016).
Scalbert, A. Review article number 63 antimicrobial properties of tannins. Phytochemistry 30, 3875–3883 (1991).
Choi, Y. Y. et al. In vitro and in situ evaluation of Undaria pinnatifida as a feed ingredient for ruminants. J. Appl. Phycol. 32, 729–739 (2020).
Choi, Y. Y. et al. The potential nutritive value of Sargassum fulvellum as a feed ingredient for ruminants. Algal Res. 45, 101761 (2020).
Choi, Y. Y. et al. New challenges for efficient usage of Sargassum fusiforme for ruminant production. Sci. Rep. 10, 1–13 (2020).
Choi, Y. Y. et al. In vitro five brown algae extracts for efficiency of ruminal fermentation and methane yield. J. Appl. Phycol. 33, 1253–1262 (2021).
Machado, L., Magnusson, M., Paul, N. A., De Nys, R. & Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 9, e85289 (2014).
Belanche, A., Ramos-Morales, E. & Newbold, C. J. In vitro screening of natural feed additives from crustaceans, diatoms, seaweeds and plant extracts to manipulate rumen fermentation. J. Sci. Food Agric. 96, 3069–3078 (2016).
Maia, M. R. G., Fonseca, A. J. M., Oliveira, H. M., Mendonça, C. & Cabrita, A. R. J. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci. Rep. 6, 1–10 (2016).
Makkar, H. P. S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 49, 241–256 (2003).
Stewart, C. S., Flint, H. J. & Bryant, M. P. The rumen bacteria. In The Rumen Microbial Ecosystem (eds Hobson, P. N. & Stewart, C. S.) 10–72 (Springer, Dordrecht, 1997).
Andries, J. I., Buysse, F. X., De Brabander, D. L. & Cottyn, B. G. Isoacids in ruminant nutrition: Their role in ruminal and intermediary metabolism and possible influences on performances—A review. Anim. Feed Sci. Technol. 18, 169–180 (1987).
Wettstein, H.-R., Machmüller, A. & Kreuzer, M. Effects of raw and modified canola lecithins compared to canola oil, canola seed and soy lecithin on ruminal fermentation measured with rumen simulation technique. Anim. Feed Sci. Technol. 85, 153–169 (2000).
Kobayashi, Y. Abatement of methane production from ruminants: Trends in the manipulation of rumen fermentation. Asian-Australas. J. Anim. Sci. 23, 410–416 (2010).
Henderson, C. The influence of extracellular hydrogen on the metabolism of Bacteroides ruminicola, Anaerovibrio lipolytica and Selenomonas ruminantium. J. Gen. Microbiol. 119, 485–491 (1980).
Becker, P. M. et al. Evidence for a hydrogen-sink mechanism of (+)catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics 10, 179–189 (2014).
AlZahal, O., Li, F., Guan, L. L., Walker, N. D. & McBride, B. W. Factors influencing ruminal bacterial community diversity and composition and microbial fibrolytic enzyme abundance in lactating dairy cows with a focus on the role of active dry yeast. J. Dairy Sci. 100, 4377–4393 (2017).
Bauman, D. E., Perfield, J. W., De Veth, M. J. & Lock, A. L. New perspectives on lipid digestion and metabolism in ruminants. In Proceedings of Cornell Nutrition Conference, vol. 65, 175–189 (Cornell University, 2003).
Lee, S. Y. et al. Glycerol as a feed supplement for ruminants: In vitro fermentation characteristics and methane production. Anim. Feed Sci. Technol. 166–167, 269–274 (2011).
Janssen, P. H. & Kirs, M. Structure of the archaeal community of the rumen. Appl. Environ. Microbiol. 74, 3619–3625 (2008).
O’Hara, E., Neves, A. L. A., Song, Y. & Guan, L. L. The role of the gut microbiome in cattle production and health: Driver or passenger?. Annu. Rev. Anim. Biosci. 8, 199–220 (2020).
Henderson, G. et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 5, 1–15 (2015).
Molina-Alcaide, E. et al. In vitro ruminal fermentation and methane production of different seaweed species. Anim. Feed Sci. Technol. 228, 1–12 (2017).
Zhou, M. et al. Air-dried brown seaweed, Ascophyllum nodosum, alters the rumen microbiome in a manner that changes rumen fermentation profiles and lowers the prevalence of foodborne pathogens. mSphere 3, e00017-18 (2018).
Sarwono, K. A., Kondo, M., Ban-Tokuda, T., Jayanegara, A. & Matsui, H. Effects of phloroglucinol and the forage: Concentrate ratio on methanogenesis, in vitro rumen fermentation, and microbial population density. Adv. Anim. Vet. Sci 7, 164–171 (2019).
Belanche, A., de la Fuente, G. & Newbold, C. J. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 90, 663–677 (2014).
Machmüller, A., Soliva, C. R. & Kreuzer, M. Effect of coconut oil and defaunation treatment on methanogenesis in sheep. Reprod. Nutr. Dev. 43, 41–55 (2003).
Zhou, M. et al. Relationship between rumen methanogens and methane production in dairy cows fed diets supplemented with a feed enzyme additive. J. Appl. Microbiol. 111, 1148–1158 (2011).
AOAC. Official methods of analysis. In Association of Official Analytical Chemists, 15th edn. (1990).
Van Soest, P. J., Robertson, J. B. & Lewis, B. A. Symposium: Carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. J. Dairy Sci. 74, 3583–3597 (1991).
Zhishen, J., Mengcheng, T. & Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64, 555–559 (1999).
Woisky, R. G. & Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 37, 99–105 (1998).
Singleton, V. L., Orthofer, R. & Lamuela-Raventós, R. M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299, 152–178 (1999).
McDougall, E. I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 43, 99–109 (1948).
Lee, S. J. et al. Effect of Rhodophyta extracts on in vitro ruminal fermentation characteristics, methanogenesis and microbial populations. Asian-Australasian J. Anim. Sci. 31, 54–62 (2018).
Theodorou, M. K., Williams, B. A., Dhanoa, M. S., McAllan, A. B. & France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 48, 185–197 (1994).
López, S. et al. Some methodological and analytical considerations regarding application of the gas production technique. Anim. Feed Sci. Technol. 135, 139–156 (2007).
Chaney, A. L. & Marbach, E. P. Modified reagents for determination of urea and ammonia. Clin. Chem. 8, 130–132 (1962).
Adesogan, A. T., Krueger, N., Salawu, M. B., Dean, D. B. & Staples, C. R. The influence of treatment with dual purpose bacterial inoculants or soluble carbohydrates on the fermentation and aerobic stability of bermudagrass. J. Dairy Sci. 87, 3407–3416 (2004).
Yu, Z. & Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36, 808–812 (2004).
Denman, S. E. & McSweeney, C. S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 58, 572–582 (2006).
Khafipour, E., Li, S., Plaizier, J. C. & Krause, D. O. Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl. Environ. Microbiol. 75, 7115–7124 (2009).
Kim, H., Kim, B., Cho, S., Kwon, I. & Seo, J. Dietary lysophospholipids supplementation inhibited the activity of lipolytic bacteria in forage with high oil diet: An in vitro study. Asian-Australas. J. Anim. Sci. 33, 1590–1598 (2020).
Hamid, M. M. A. et al. Rumen fermentation, methane production, and microbial composition following in vitro evaluation of red ginseng byproduct as a protein source. J. Anim. Sci. Technol. 62, 801–811 (2021).
Sylvester, J. T., Karnati, S. K. R., Yu, Z., Morrison, M. & Firkins, J. L. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 134, 3378–3384 (2004).
Wang, R. F., Cao, W. W. & Cerniglia, C. E. A universal protocol for PCR detection of 13 species of foodborne pathogens in foods. J. Appl. Microbiol. 83, 727–736 (1997).
Stevenson, D. M. & Weimer, P. J. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl. Microbiol. Biotechnol. 75, 165–174 (2007).
Paillard, D. et al. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 91, 417–422 (2007).
Tajima, K. et al. Diet-Dependent Shifts in the Bacterial Population of the Rumen Revealed with Real-Time PCR. Appl. Environ. Microbiol. 67, 2766–2774 (2001).
Eom, J. S. et al. Metabolomics comparison of rumen fluid and milk in dairy cattle using proton nuclear magnetic resonance spectroscopy. Anim. Biosci. 34, 213–222 (2021).
Acknowledgements
This project was supported by a Grant from the National Research Foundation (NRF) of Korea, funded by the Korean Government (Grant Number NRF-2015R1A6A1A03031413). The authors have not stated any conflicts of interest.
Author information
Authors and Affiliations
Contributions
Y.C and S.J.L. wrote the main manuscript text, prepared tables and figures, and performed data analysis. H.S.K. and J.S.E. analyzed and interpreted the NMR results. S.U.J. and H.K. analyzed and interpreted the rumen PCR results. L.L.G. and J.S. assisted drafting of the manuscript and results interpretation. S.S.L. contributed statistical expertise and assisted in drafting of the manuscript. S.S.L. was responsible for experimental concept and design, supervised all work, and critically revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, Y., Lee, S.J., Kim, H.S. et al. Effects of seaweed extracts on in vitro rumen fermentation characteristics, methane production, and microbial abundance. Sci Rep 11, 24092 (2021). https://doi.org/10.1038/s41598-021-03356-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03356-y
This article is cited by
-
Improving the nutritional values of yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) larvae as an animal feed ingredient: a review
Journal of Animal Science and Biotechnology (2023)
-
Anti-methanogenic potential of seaweeds and seaweed-derived compounds in ruminant feed: current perspectives, risks and future prospects
Journal of Animal Science and Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.