Abstract
In this study, a novel magnetic mesoporous silica with yolk–shell structure (Fe3O4@Void@m.SiO2) was successfully synthesized via a polymer-template assisted method. The Fe3O4@Void@m.SiO2 was characterized by using FT-IR, EDS, SEM, TEM, VSM, PXRD and nitrogen adsorption–desorption analyses. The Fe3O4@Void@m.SiO2 nanocomposite showed high efficiency in adsorption of an organic dye and water pollutant called methylene blue (MB) with 98.2% removal capability. Furthermore, the effect of different parameters in the adsorption of MB was investigated. Different models of kinetic were examined and compared with each other. The recoverability and reusability of designed Fe3O4@Void@m.SiO2 material were also studied under applied conditions.
Similar content being viewed by others
Introduction
After the first report about interesting materials with yolk–shell (YS) structure1, many researches have developed the synthesis methods and properties of these materials with different names such as nanorattle2, movable core/shell1, core/shell with hollow interiors3 and yolk/shell4. These nanomaterials are in the center of attention due to interesting properties such as high surface area, interstitial hollow space and low density5,6. These special properties make yolk–shell nanocomposites suitable to use in the fields of biomedical7, lithium batteries8,9, sensors10,11, catalysis12,13,14,15,16 and adsorption17,18. Some of recently reported yolk–shell structured materials are Sn4P3@C19, Sn@SnO/SnO220, Al@TiO2 NPs21 and Au-CeO2@ZrO222. Among different shells, silica-based ones is more important in catalysis and adsorption processes owing to its high loading capacity compared to other shells23,24. The preparation methods effect on features of the yolk, space, thickness, porosity and shape of YS materials. The more common methods used for the preparation of YSs are hard temple-assisted, soft template-assisted and template-free25. Among these, the soft template method has attracted more attention due to easy removal of template and also economically friendly26,27.
Moreover, owing to superparamagnetic properties, easy separation and low toxicity, magnetic nanoparticles (MNPs) have been so interested in different fields such as biomedical28,29, magnetic resonance imaging30, drug delivery31 and separation32. However, the most of MNPs suffer from disadvantages of aggregation, biodegradation and low capacity. In order to increase the stability and capacity, these NPs are composited with different species. Among different species, mesoporous silica is more attracted because of its high surface area, high pore volume and high capacity33,34,35.
As regards to water purification importance and lots of different pollutants that made by pharmaceutical, paper making, textile, leather, etc., different methods such as photocatalytic degradation, adsorption and oxidation have been used to eliminate water pollutants36,37. To date many adsorbents have been applied for the elimination of organic and inorganic pollutants from water. Among these, magnetic NPs have attracted more attention due to the advantages of easy magnetically separation, economically friendly and high efficiency. Some of recently developed systems are magnetic zeolites38, magnetic carbon nanotubes composites39, Zn/ferrite/graphene oxide40 and activated carbon/NiFe2O441. Although these adsorbents gave good to high efficiency in the removal of water pollutants, however, in spite of high adsorption capacity of the yolk–shell based magnetic nanocomposites, according to our knowledge, there is no report in the study of efficiency of these type nanomaterials in the removal of water pollutants. In view of the above, herein, a novel magnetic Fe3O4@mesoporous silica nanocomposite (Fe3O4@Void@m.SiO2) with yolk–shell structure and high magnetic properties is prepared for adsorption of methylene blue (MB) from water. The kinetics, isotherm and equilibrium data for adsorption of MB have been analyzed and different models have been employed to understand the mechanism.
Experimental section
Preparation of Fe3O4@Void@m.SiO2 nanomaterial
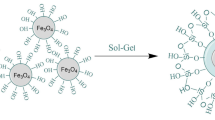
To do this, firstly magnetic Fe3O4 NPs were prepared42. In the second step, the Fe3O4 NPs were modified with resorcinol–formaldehyde polymer to form Fe3O4@RF material. In order to prepare Fe3O4@RF, the Fe3O4 NPs (100 mg) were added to a mixture of EtOH (20 mL) and H2O (10 mL) in an ultrasonic bath. Then, ammonium hydroxide (0.5 g, aqueous solution, 28 wt %), HCHO (0.1 g, 37 wt %) and resorcinol (0.1 g, 0.09 mM) were added while stirring for 2 h. After polymerization, the resulted Fe3O4@RF was collected and washed completely with H2O and EtOH. Next, the as-made Fe3O4@RF (0.12 mg) was dispersed in a mixture of CTAB (0.45 g), ammonia (2 mL, 28 wt %), H2O (100 mL) and EtOH (150 mL). The resulted combination was stirred to form a homogeneous mixture. After adding of TMOS (1.5 mL), stirring was continued for 6 h. The resulted Fe3O4@RF@m.SiO2 material was washed completely with EtOH and H2O. Finally, the RF layer and CTAB surfactant were removed after heating of the Fe3O4@RF@m.SiO2 material at 550 °C for 6 h (Fig. 1). The final magnetic nanomaterial with yolk–shell structure was denoted as Fe3O4@Void@m.SiO2.
Adsorption process
For this, 5 mg of the Fe3O4@Void@m.SiO2 was added in 15 mL of an aqueous solution of MB. This mixture was shaken continuously and the Fe3O4@Void@m.SiO2 was magnetically separated immediately. The MB concentration was measured by UV–vis at 665 nm. The adsorption efficiency for MB in water solution was calculated by using Eq. (1).
On the other hand, the amount of adsorbed MB on the adsorbent (qe) was calculated by using Eq. (2).
Herein, C0 and Ce are, respectively, the concentration of the initial dye solution and the residual dye solution that quantitatively estimated by linear regression equations resulted at different dye concentrations, V is solution volume in liters and m is adsorbent amount (mg).
Results and discussion
The magnetic mesoporous silica material (Fe3O4@Void@m.SiO2) with yolk–shell structure was prepared from an intermediate structure (Fe3O4@RF) which was made by polymerization of HCHO and resorcinol (Fig. 2) on the Fe3O4NPs surface. The mesoporous silica shell was coated on the Fe3O4@RF by using CTAB and TMOS via a sol–gel approach. Finally, RF and CTAB were eliminated to deliver Fe3O4@Void@m.SiO2 (Fig. 1).
Characterization
The SEM image of Fe3O4@Void@m.SiO2 yolk shell nanomaterial is illustrated in Fig. 3. As shown, the designed material has particles with spherical morphology and average size of 84 nm. These types of NPs are excellent candidates for adsorption processes.
The TEM image of the Fe3O4@Void@m.SiO2 nanomaterial also showed a yolk–shell structure with black cores (Fe3O4 NPs) and mesoporous silica shell (Fig. 4).
The low-angle PXRD (LA-PXRD) pattern of Fe3O4@Void@m.SiO2 showed an intense peak centered at 2 theta of 1 degree that is characteristic of nanomaterials with an ordered 2D hexagonal mesostructure (Fig. 5). These types of materials have high surface area and are very effective in the adsorption processes.
The wide-angle PXRD of Fe3O4@Void@m.SiO2 showed six reflection peaks at 2θ of 63°, 57°, 54°, 43°, 35° and 30° (Fig. 6). This pattern is in good agreement with the PXRD pattern of Fe3O4 NPs confirming high stability of the magnetite cores during material preparation. This analysis also showed a broad peak at 2 theta about 20 degree that is attributed to mesoporous silica shell42.
Figure 7 shows the vibrating sample magnetometer (VSM) analysis of the designed nanomaterial. As shown, this material has good magnetic property. As expected, due to the nonmagnetic mesoporous silica shell and the large void space, the magnetization is reduced from 60 for Fe3O4 to 15 emu/g for Fe3O4@Void@m.SiO214.
The FT-IR spectra of Fe3O4 and Fe3O4@Void@m.SiO2 before and after calcination, are shown in Fig. 8. For all materials, the absorption peaks of Fe–O bonds are observed about 580 cm−1. The bands at 710–810 cm−1 are due to C–Si. The peaks cleared at 1100 and 935 cm−1 are due to Si–O–Si bonds. The peak at 3500 cm−1 is owing to O–H bonds of material surface. Before surfactant and RF removal, the peaks at 2850 and 2920 cm−1 are corresponded to aliphatic C–H bonds of CTAB and peaks about 3020 cm-1 are related to aromatic C–Hs of RF (Fig. 8B). Interestingly, the latter peaks are disappeared in Fig. 8C confirming successful removal of CTAB and RF during calcination. It is important to note that, to prepare a material with mesoporous yolk–shell structure, both CTAB (containing aliphatic C–H) and RF (containing aromatic C–H) should be romed. After removal of CTAB a mesoporous silica shell is resulted, while after removal of RF the void space between shell and core is resulted.
The EDS was performed for elemental analysis of the material before adsorption process (Fig. 9). This analysis clearly showed the existence of oxygen, iron and silicon confirming high stability and well incorporation of expected magnetite cores and silica shells in the material network.
The nitrogen adsorption–desorption isotherm of the Fe3O4@Void@m.SiO2 nanomaterial before adsorption process shows a type IV isotherm with an H2 hysteresis loop, which is characteristic of ordered mesostructures with high regularity (Fig. 10). The sharp capillary condensation steps occurred at a relative pressure of 0.45–0.97, indicate the large void space of Fe3O4@Void@m.SiO2 and the porous silica shell43. The Brunauer–Emmett–Teller (BET) surface area and total pore volume of the material were also found to be 666.16 m2/g and 1.28 cm3/g, respectively. The Barrett–Joyner–Halenda (BJH) pore size distribution isotherm showed a bimodal size distribution at 7.1 and 12.2 nm related to the mesoporous shell and void space between yolk and shell, respectively (Fig. 11). These data are in good agreement with LA-PXRD and TEM results confirming the presence of a mesoporous shell and yolk–shell structure for the designed nanomaterial.
Adsorption Studies
The elimination of MB, as organic dye, was examined as a model to study adsorption ability of synthesized Fe3O4@Void@m.SiO2 material. The time, dye concentration, amount of adsorbent and adsorption pH were optimized as well as different models were checked in kinetic and isotherm studies.
Effect of pH
The effect of pH was investigated due to the structure and ionization degree of the MB dye can be influenced by pH value44. The pKa of MB is 3.845, therefore, at pH values above this, the preponderant MBs are cationic. The effect of pH values was studied during 5 min using 0.005 g of the adsorbent (Fig. 12). At acidic pH, the –OHs of mesoporous silica shell and magnetic core interact with MBs via H-bonding. At basic pH, Si–O− and Fe–O− are the greater groups due to the deprotonation of Si–OH and Fe–OH, respectively. These sites improve the electrostatic interaction between the cationic dye and the anionic charged surfaces (Fig. 13). Similar results have been reported for MB elimination using clay46,47 and activated coal48. Another important factor for this adsorption process is H-bonding interactions between N-sites of dye and OH sites of adsorbent. It is important to note that the aforementioned interactions can occur on both inner and outer surfaces of mesoporous silica shell and also on outer surface of magnetite cores49,50. According these findings, a suitable mechanism for this adsorption process is proposed in Fig. 13. According to this experiment, at pH 9.0 the best result was obtained. Therefore, this was used as optimum pH in the subsequent tests.
Effect of adsorbent dosage and dye concentration
In next step, the amounts of adsorbent and dye concentration were optimized (respectively, Figs. 14 and 15). It is clear that the adsorbent amount and the numbers of active positions on its surface affect the adsorption rate. By increasing the amount of adsorbent, the vacant and unoccupied positions are increased and thus the percentage of removal is increased. While, after this, there is a little change in the adsorption process. As shown in Fig. 14, the optimum amount of adsorbent is 0.005 g for 15 mL of MB aqueous solution (5 ppm) with 98.2% removal. This confirms high performance of the designed material in the MB removal. In the next, the effect of dye concentration using a constant amount (0.005 g) of adsorbent was studied. As shown in Fig. 15, by increasing the amount of MB concentration, the removal performance of a certain amount of Fe3O4@Void@m.SiO2 is reduced which it refers to disproportionate in amount of active sites and dye molecules. Accordingly, for 0.005 g of Fe3O4@Void@m.SiO2 adsorbent, the optimum dye concentration was 5 ppm.
Effect of time
Figure 16 illustrates the time effect under optimum conditions. As seen, the rate of adsorption is high at first 3 min that can refer to adsorption by the outer surface of the shell. After that, this process is slowly increased with time. The latter adsorption can be happened on internal surface of the shell and also surface of the core. As shown, after 15 min the maximum adsorption is resulted.
Kinetics evaluation
To study the kinetics evaluation, the kinetic models of pseudo-first-order, second-order and Elovich were employed (Table 1). The pseudo-first-order model is as follows:
where qt is the adsorbed dye at t time (mg/g); qe is adsorbed dye at equilibrium (mg/g) and k1 is the rate constant of adsorption (min−1). The pseudo-second-order model is also as follows:
the qe and k2 were obtained via the plot of \(\frac{t}{{q_{t} }}\) versus t (Fig. 17, Table 1). The Elovich equation is also as follows:
As shown in Table 1 and Fig. 17, the pseudo-second-order model with a linear regression correlation coefficient (R2) value of 0.997 is completely applicable for the process.
Isotherm study
To determine the maximum capacity of sorption, the Langmuir, Freundlich and Temkin isotherm models were employed. The Langmuir model is as follows:
where qe is the equilibrium concentration of dye, qm is the maximum dye uptake and KL is Langmuir constant. These are determined by linearizing of Eq. (6) as shown in Eq. (7),
The Freundlich model is also as follows:
The linear form of above equation is Eq. (9) in which Kf and n are Freundlich constants
The other isotherm model is Temkin with a linear form as follows:
As shown in Table 2 and Fig. 18, the Langmuir model with an R2 > 0.99 is the best isotherm for the process.
Thermodynamic study
Thermodynamic parameters (ΔG0, ΔH0 and ΔS0) were calculated by using following equations51:
These parameters are listed in Table 3. The plot of ln Kc versus 1/T is illustrated in Fig. 19. As shown, a satisfactory adsorption is resulted at RT and by increasing the temperature from 25 to 55 °C, a slight decrease in MB adsorption is observed indicating the process is exothermic. This was also confirmed by the negative ΔH°. The negative amount of ΔG0 confirms that the MB adsorption on Fe3O4@Void@m.SiO2 is achievable and spontaneous. The negative ΔS° also suggests a decrease in randomness at solid/solution interface52. Moreover, the amounts of ΔH0 and ΔG0 successfully indicate that MB molecules are both physically and chemically adsorbed into/onto adsorbent material.
EDS spectrum
To investigate the successful adsorption of MB molecules into/onto Fe3O4@Void@m.SiO2, the EDS analysis after adsorption process was performed (Fig. 20). As shown, the existence of new peaks of carbon, nitrogen, sulfur and chlorine in this spectrum confirms successful adsorption of MB molecules into/onto material.
Nitrogen adsorption–desorption and BJH pore size distribution isotherms
The nitrogen adsorption–desorption analysis of the Fe3O4@Void@m.SiO2 nanomaterial after adsorption of MB was performed (Fig. 21). According to this analysis, the BET surface area and total pore volume of the Fe3O4@Void@m.SiO2 nanomaterial after adsorption process were reduced to 142.32 m2/g and 0.35 cm3/g, respectively. The BJH pore size distribution isotherm after adsorption process also showed that the sizes of shell pores and void space between yolk and shell are 5.1 and 7.9, respectively (Fig. 22). Moreover, as shown in the Figs. 21 and 22, after the adsorption process the intensity of both adsorption–desorption and BJH isotherms are reduced in comparison to the fresh material. These observations confirm that both cavities and pores of the adsorbent are occupied by MB molecules.
Recoverability and reusability studies
In next step, the recoverability and reusability of the Fe3O4@Void@m.SiO2 were investigated. To do this, the adsorbed MB molecules on Fe3O4@Void@m.SiO2 were desorbed by acidic ethanol (pH 2). Then, the adsorbent was recovered and reused under the same conditions as the first run. As Fig. 23 shown, there is an insignificant decrease in adsorption ability of adsorbent after three cycles indicating the high stability and efficiency of designed Fe3O4@Void@m.SiO2 for MB removal.
Comparison study
Next, the adsorption efficiency of our designed Fe3O4@Void@m.SiO2 nanomaterial was compared with some reported adsorbents in MB adsorption (Table 4). As shown, in the most of previous studies the adsorbent is not recovered and also in some cases higher temperature than RT is needed for absorption. Moreover, the time required for absorption in most of former reports is high. These findings successfully confirm higher tendency and excellent capacity of the Fe3O4@Void@m.SiO2 for adsorption of MB molecules in comparison to previous adsorbents.
Conclusion
In summary, a magnetic yolk–shell structured nanomaterial with mesoporous shell (Fe3O4@Void@m.SiO2) was prepared, characterized and used as an effective adsorbent for the removal of MB dye from aqueous solution. The adsorption study showed that the designed material is so efficient with high removal capacity. Optimum conditions to achieve maximum removal of 98.2% for adsorption process were 0.005 g of adsorbent, pH 9 and 15 min for 15 mL of 5 mg/mL MB solution. The kinetic and isotherm studies of adsorption process were investigated and different models were evaluated for the equilibrium data. The results showed that the Langmuir isotherm model and pseudo-second-order kinetic are successfully fitted. The maximum adsorption capacity of the material was 163.93 mg/g. The comparison study illustrated that the present adsorbent is much more efficient than previously reported adsorbents in the removal of MB dye.
References
Kamata, K., Lu, Y. & Xia, Y. Synthesis and characterization of monodispersed core–shell spherical colloids with movable cores. J. Am. Chem. Soc. 125, 2384–2385 (2003).
Kim, M., Sohn, K., Na, H. B. & Hyeon, T. Synthesis of nanorattles composed of gold nanoparticles encapsulated in mesoporous carbon and polymer shells. Nano Lett. 2, 1383–1387 (2002).
Guo, C.-W., Cao, Y., Xie, S.-H., Dai, W.-L. & Fan, K.-N. Fabrication of mesoporous core-shell structured titania microspheres with hollow interiors. Chem. Commun., 700–701 (2003).
Wang, W., Dahl, M. & Yin, Y. Hollow nanocrystals through the nanoscale Kirkendall effect. Chem. Mater. 25, 1179–1189 (2013).
Fang, X. et al. Hollow mesoporous aluminosilica spheres with perpendicular pore channels as catalytic nanoreactors. ACS Nano 6, 4434–4444 (2012).
Liu, J. et al. Yolk–shell hybrid materials with a periodic mesoporous organosilica shell: Ideal nanoreactors for selective alcohol oxidation. Adv. Funct. Mater. 22, 591–599 (2012).
Cipolatti, E. P. et al. Nanomaterials for biocatalyst immobilization—State of the art and future trends. RSC Adv. 6, 104675–104692 (2016).
Ni, W., Wang, Y. & Xu, R. Formation of Sn@ C yolk–shell nanospheres and core–sheath nanowires for highly reversible lithium storage. Part. Part. Syst. Charact. 30, 873–880 (2013).
Song, H., Cui, H. & Wang, C. Abnormal cyclibility in Ni@ graphene core–shell and yolk–shell nanostructures for lithium ion battery anodes. ACS Appl. Mater. Interfaces 6, 13765–13769 (2014).
Li, X. et al. Design of Au@ ZnO yolk–shell nanospheres with enhanced gas sensing properties. ACS Appl. Mater. Interfaces 6, 18661–18667 (2014).
Rai, P. et al. Design of highly sensitive and selective Au@ NiO yolk–shell nanoreactors for gas sensor applications. Nanoscale 6, 8292–8299 (2014).
Wang, S., Zhang, M. & Zhang, W. Yolk− shell catalyst of single Au nanoparticle encapsulated within hollow mesoporous silica microspheres. ACS Catal. 1, 207–211 (2011).
Zhang, J., Li, B. & Yang, W. Synthesis and photocatalytic performance of novel hierarchical hollow silica sphere supported TiO2 nanoparticles. Mater. Lett. 117, 252–255 (2014).
Norouzi, M. & Elhamifar, D. Magnetic yolk–shell structured methylene and propylamine based mesoporous organosilica nanocomposite: A highly recoverable and durable nanocatalyst with improved efficiency. Colloids Surf. A Physicochem. Eng. Asp. 615, 126226 (2021).
Mirbagheri, R., Elhamifar, D. & Hajati, S. Ru-containing magnetic yolk–shell structured nanocomposite: A powerful, recoverable and highly durable nanocatalyst. RSC Adv. 11, 10243–10252 (2021).
Shaker, M. & Elhamifar, D. Cu-containing magnetic yolk–shell structured ionic liquid-based organosilica nanocomposite: A powerful catalyst with improved activity. Compos. Commun. 24, 100608 (2021).
Fan, H., Lei, Z., Pan, J. H. & Zhao, X. Sol–gel synthesis, microstructure and adsorption properties of hollow silica spheres. Mater. Lett. 65, 1811–1814 (2011).
Zhang, J., Li, B., Yang, W. & Liu, J. Synthesis of magnetic Fe3O4@ hierarchical hollow silica nanospheres for efficient removal of methylene blue from aqueous solutions. Ind. Eng. Chem. Res. 53, 10629–10636 (2014).
Liu, J. et al. Uniform yolk–shell Sn 4 P 3@ C nanospheres as high-capacity and cycle-stable anode materials for sodium-ion batteries. Energy Environ. Sci. 8, 3531–3538 (2015).
Cho, J. S. & Kang, Y. C. Nanofibers comprising yolk–shell Sn@ void@ SnO/SnO2 and hollow SnO/SnO2 and SnO2 nanospheres via the Kirkendall diffusion effect and their electrochemical properties. Small 11, 4673–4681 (2015).
Li, S. et al. High-rate aluminium yolk–shell nanoparticle anode for Li-ion battery with long cycle life and ultrahigh capacity. Nat. Commun. 6, 1–7 (2015).
Evangelista, V. et al. Highly active Au-CeO2@ ZrO2 yolk–shell nanoreactors for the reduction of 4-nitrophenol to 4-aminophenol. Appl. Catal. B 166, 518–528 (2015).
Du, X. & He, J. Spherical silica micro/nanomaterials with hierarchical structures: Synthesis and applications. Nanoscale 3, 3984–4002 (2011).
Li, Z. et al. Measurement of uptake and release capacities of mesoporous silica nanoparticles enabled by nanovalve gates. J. Phys. Chem. C 115, 19496–19506 (2011).
Purbia, R. & Paria, S. Yolk/shell nanoparticles: Classifications, synthesis, properties, and applications. Nanoscale 7, 19789–19873 (2015).
Wang, Q., Liu, Y. & Yan, H. Mechanism of a self-templating synthesis of monodispersed hollow silica nanospheres with tunable size and shell thickness. Chem. Commun., 2339–2341 (2007).
Zoldesi, C. I., van Walree, C. A. & Imhof, A. Deformable hollow hybrid silica/siloxane colloids by emulsion templating. Langmuir 22, 4343–4352 (2006).
Park, J.-I. & Cheon, J. Synthesis of “solid solution” and “core-shell” type cobalt–platinum magnetic nanoparticles via transmetalation reactions. J. Am. Chem. Soc. 123, 5743–5746 (2001).
Piao, Y. et al. Wrap–bake–peel process for nanostructural transformation from β-FeOOH nanorods to biocompatible iron oxide nanocapsules. Nat. Mater. 7, 242–247 (2008).
Lee, J.-H. et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 13, 95–99 (2007).
Wu, X. et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 21, 41–46 (2003).
Dubertret, B. et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298, 1759–1762 (2002).
Elhamifar, D., Mofatehnia, P. & Faal, M. Magnetic nanoparticles supported Schiff-base/copper complex: An efficient nanocatalyst for preparation of biologically active 3, 4-dihydropyrimidinones. J. Colloid Interface Sci. 504, 268–275 (2017).
Elhamifar, D., Ramazani, Z., Norouzi, M. & Mirbagheri, R. Magnetic iron oxide/phenylsulfonic acid: A novel, efficient and recoverable nanocatalyst for green synthesis of tetrahydrobenzo [b] pyrans under ultrasonic conditions. J. Colloid Interface Sci. 511, 392–401 (2018).
Mousavi, F., Elhamifar, D. & Kargar, S. Copper/IL-containing magnetic nanoporous MCM-41: A powerful and highly stable nanocatalyst. Surf. Interfaces 25, 101225 (2021).
Dutta, K., Mukhopadhyay, S., Bhattacharjee, S. & Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 84, 57–71 (2001).
Lachheb, H. et al. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 39, 75–90 (2002).
Oliveira, L. C., Petkowicz, D. I., Smaniotto, A. & Pergher, S. B. Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water. Water Res. 38, 3699–3704 (2004).
Peng, X., Luan, Z., Di, Z., Zhang, Z. & Zhu, C. Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb (II) and Cu (II) from water. Carbon 43, 880–883 (2005).
Fei, P., Zhong, M., Lei, Z. & Su, B. One-pot solvothermal synthesized enhanced magnetic zinc ferrite–reduced graphene oxide composite material as adsorbent for methylene blue removal. Mater. Lett. 108, 72–74 (2013).
Jiang, T., Liang, Y.-D., He, Y.-J. & Wang, Q. Activated carbon/NiFe2O4 magnetic composite: A magnetic adsorbent for the adsorption of methyl orange. J. Environ. Chem. Eng. 3, 1740–1751 (2015).
Mirbagheri, R., Elhamifar, D. & Norouzi, M. Propylamine-containing magnetic ethyl-based organosilica with a core–shell structure: An efficient and highly stable nanocatalyst. New J. Chem. 42, 10741–10750 (2018).
Dai, J. et al. Yolk–shell Fe3O4@ SiO2@PMO: Amphiphilic magnetic nanocomposites as an adsorbent and a catalyst with high efficiency and recyclability. Green Chem. 19, 1336–1344 (2017).
Wang, L. et al. Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: Kinetics, isotherms, pH, and ionic strength studies. Bioresour. Technol. 101, 5808–5814 (2010).
Sousa, H. R. et al. Evaluation of methylene blue removal by plasma activated palygorskites. J. Mater. Res. Technol. 8, 5432–5442 (2019).
Şahin, Ö., Kaya, M. & Saka, C. Plasma-surface modification on bentonite clay to improve the performance of adsorption of methylene blue. Appl. Clay Sci. 116, 46–53 (2015).
Adeyemo, A. A., Adeoye, I. O. & Bello, O. S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 7, 543–568 (2017).
El Qada, E. N., Allen, S. J. & Walker, G. M. Adsorption of basic dyes from aqueous solution onto activated carbons. Chem. Eng. J. 135, 174–184 (2008).
Chandrasekhar, S. & Pramada, P. Rice husk ash as an adsorbent for methylene blue—Effect of ashing temperature. Adsorption 12, 27–43 (2006).
Hamdaoui, O. Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 135, 264–273 (2006).
You, L., Wu, Z., Kim, T. & Lee, K. Kinetics and thermodynamics of bromophenol blue adsorption by a mesoporous hybrid gel derived from tetraethoxysilane and bis (trimethoxysilyl) hexane. J. Colloid Interface Sci. 300, 526–535 (2006).
Alkan, M., Demirbaş, Ö. & Doğan, M. Adsorption kinetics and thermodynamics of an anionic dye onto sepiolite. Microporous Mesoporous Mater. 101, 388–396 (2007).
Li, Y. et al. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 95, 501–507 (2013).
Dural, M. U., Cavas, L., Papageorgiou, S. K. & Katsaros, F. K. Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: Kinetics and equilibrium studies. Chem. Eng. J. 168, 77–85 (2011).
Ayad, M. M., El-Nasr, A. A. & Stejskal, J. Kinetics and isotherm studies of methylene blue adsorption onto polyaniline nanotubes base/silica composite. J. Ind. Eng. Chem. 18, 1964–1969 (2012).
Haque, E., Jun, J. W. & Jhung, S. H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal–organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 185, 507–511 (2011).
Ghorai, S. et al. Enhanced removal of methylene blue and methyl violet dyes from aqueous solution using a nanocomposite of hydrolyzed polyacrylamide grafted xanthan gum and incorporated nanosilica. ACS Appl. Mater. Interfaces 6, 4766–4777 (2014).
Wu, X.-L., Shi, Y., Zhong, S., Lin, H. & Chen, J.-R. Facile synthesis of Fe3O4-graphene@ mesoporous SiO2 nanocomposites for efficient removal of Methylene Blue. Appl. Surf. Sci. 378, 80–86 (2016).
Zheng, J. et al. Synthesis of yolk–shell magnetic magnesium silicate with tunable yolk morphology for removal of methylene blue in water. J. Alloys Compd. 596, 5–9 (2014).
Tan, X., Lu, L., Wang, L. & Zhang, J. Facile synthesis of bimodal mesoporous Fe3O4@SiO2 composite for efficient removal of methylene blue. Eur. J. Inorg. Chem. 2015, 2928–2933 (2015).
Acknowledgements
The authors thank the Yasouj University and the Iran National Science Foundation (INSF) for supporting this work.
Author information
Authors and Affiliations
Contributions
R.M.: Investigation, Writing—Original Draft, Resources, Formal analysis. D.E.: Conceptualization, Writing—Review and Editing, Supervision, Visualization. M.S.: Resources, Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mirbagheri, R., Elhamifar, D. & Shaker, M. Yolk–shell structured magnetic mesoporous silica: a novel and highly efficient adsorbent for removal of methylene blue. Sci Rep 11, 23259 (2021). https://doi.org/10.1038/s41598-021-02699-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02699-w
This article is cited by
-
Magnetic-based adsorbing platforms for water and wastewater purification
International Journal of Environmental Science and Technology (2024)
-
Fe3O4@C@MCM41-guanidine core–shell nanostructures as a powerful and recyclable nanocatalyst with high performance for synthesis of Knoevenagel reaction
Scientific Reports (2023)
-
Fe3O4@void@C-Schiff-base/Pd yolk-shell nanostructures as an effective and reusable nanocatalyst for Suzuki coupling reaction
Scientific Reports (2023)
-
Adsorptive Removal of Copper and Chromium Ion by Using Azospirillum Biofertilizer as Low-cost Biosorbent in Aqueous Medium
Water, Air, & Soil Pollution (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.