Abstract
Emerging studies are reporting associations between skeletal muscle abnormalities and survival in cancer patients. Cancer prognosis is associated with depletion of essential fatty acids in erythrocytes and plasma in humans. However the relationship between skeletal muscle membrane fatty acid composition and survival is unknown. This study investigates the relationship between fatty acid content of phospholipids in skeletal muscle and survival in cancer patients. Rectus abdominis biopsies were collected during cancer surgery from 35 patients diagnosed with cancer. Thin-layer and gas chromatography were used for quantification of phospholipid fatty acids. Cutpoints for survival were defined using optimal stratification. Median survival was between 450 and 500 days when patients had arachidonic acid (AA) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in muscle phospholipid below the cut-point compared to 720–800 days for patients above. Cox regression analysis revealed that low amounts of AA, EPA and DHA are risk factors for death. The risk of death remained significant for AA [HR 3.5 (1.11–10.87), p = 0.03], EPA [HR 3.92 (1.1–14.0), p = 0.04] and DHA [HR 4.08 (1.1–14.6), p = 0.03] when adjusted for sex. Lower amounts of essential fatty acids in skeletal muscle membrane is a predictor of survival in cancer patients. These results warrant investigation to restore bioactive fatty acids in people with cancer.
Similar content being viewed by others
Introduction
Essential fatty acids incorporated into membranes influence cancer progression by altering production of lipid mediators, influencing gene expression as well as activating signal transduction molecules influencing carcinogenesis1,2. Experimental studies of cancer show that arachidonic acid (AA, 20:4n-6), eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3) regulate proliferation, apoptosis, cytotoxicity, metastasis and immune cell functions in cancer cell lines3. EPA and DHA improve efficacy of anticancer treatments, chemotherapy tolerability, and inflammatory profile of the host while attenuating skeletal muscle mass depletion4,5, although the quality of studies is limited6. Collectively, the metabolic properties of essential fatty acids acting on tumor and host may influence survival of cancer patients.
Alterations in phospholipid fatty acid composition of adipose tissue, erythrocytes, neutrophils and blood plasma occur in patients with lung, colorectal and pancreatic cancers7,8,9,10,11,12. Specifically, a decrease in AA, EPA and DHA has been reported in the blood phospholipids of cancer patients compared to healthy individuals, independent of changes in caloric and fat intake7,13,14,15. Higher content of palmitoleic (16:1) and oleic acid (18:1) concurrent with a lower content of AA and linoleic acid (18:2n-6) has been observed in erythrocytes of patients with lung adenocarcinoma and small cell lung cancer compared to healthy controls7. Shorter survival of lung, pancreatic and colorectal cancer patients exhibiting lower levels of essential fatty acids in blood plasma has been observed9,16. Murphy et al. reported 59% lower AA, 26% lower EPA, and 40% lower DHA in plasma phospholipids of cancer patients who survived fewer than the median survival of 238 days compared to patients who survived > 238 days9. Having a greater proportion of EPA and DHA in plasma phospholipids associates with better prognosis (survival more than 100 days) in pancreatic cancer patients compared to patients with lower proportions16. A recent study reported lower AA in serum phospholipids of colorectal patients who exhibited disease progression after one year follow up compared to individuals without tumor progression13. Overall, measures in plasma phospholipids suggest that depletion of essential fatty acids is associated with shorter survival in cancer patients.
EPA and DHA supplementation attenuate depletion of skeletal muscle mass in individuals with cancer, a well-established prognostic factor17,18,19,20. EPA and DHA are lower in plasma phospholipids of patients with sarcopenia compared to patients with normal muscle mass9. The highest concentrations of EPA and DHA in plasma phospholipids were observed in lung cancer patients who maintained or gained skeletal muscle during cancer treatment9. Several studies have shown an association between attenuation of muscle loss and n-3 fatty acid supplementation, however, these studies assessed fatty acid composition of plasma only and not of the muscle membrane17,19.
No investigation into the alteration in fatty acid composition of phospholipids of skeletal muscle in cancer patients has been conducted. Based on observations in blood phospholipids, reduced levels of AA, EPA and DHA in skeletal muscle phospholipids are likely to be associated with shorter survival of cancer patients. The objective of the study was to investigate the relationship between fatty acid content of phospholipids in skeletal muscle and survival in patients with gastrointestinal cancers who provided biopsy during surgery. It was hypothesized that patients with higher levels of essential fatty acids in muscle phospholipids would have a better prognosis.
Materials and methods
Ethics statement
The study was approved by the Health Research Ethics Board of Alberta-Cancer and abides by the Declaration of Helsinki principles. Patients undergoing elective abdominal surgery were consecutively approached between Jun 2015 and Sept 2017 to participate in tumor and tissue banking at a hepatopancreatobiliary surgical service in Alberta, Canada. Ninety three percent of patients approached agreed to participate. Patients provided written informed consent for muscle biopsy and tissue banking and patient information (demographic, clinical, and surgical data) from medical records. The release of 35 samples from the tissue bank for analysis, as well as patient information (demographic, clinical and operative data) from medical records, was performed under the auspices of Protocol ETH-21709: The Molecular Profile of Cancer Cachexia.
Subjects and muscle biopsies
Rectus abdominis biopsies of 35 patients undergoing surgery for colorectal, pancreatic and other gastrointestinal tumors were studied. The study cohort and conditions for acquisition of muscle samples have been described previously21. Patient characteristics are shown in Table 1.
CT image analysis
CT scans completed with a spiral CT scanner for initial cancer staging and routine diagnostic purposes were used to quantify muscle area, cross section area, adipose tissue area and mean skeletal muscle radiodensity to confirm that the population sampled is representative of mean values for cancer population22. CT scans completed before surgery were analysed using SliceOmatic V4.2 software with CT image parameters that include: contrast, 5 mm slice thickness, 120 kVP, and 290 mA. Total skeletal muscle area (cm2) was evaluated on a single image at the third lumbar vertebrae (L3) using Hounsfield unit (HU) thresholds of − 29 to 150 for skeletal muscle. Total skeletal muscle area was normalized for stature (m2) and reported as skeletal muscle index (SMI) (cm2 m2). Mean muscle radiodensity (HU) is reported for the entire muscle area (i.e., quadratus lumborum, psoas, erector spinae, external obliques, transverse abdominis, internal obliques, and rectus abdominis).
Analysis of phospholipids by gas chromatography
The biopsy [≈ 50 mg] was ground using a frozen pestle and mortar without letting the muscle tissue thaw. Ground tissue was homogenized in calcium chloride [CaCl2; 0.025%] solution. A modified Folch method was used to extract lipids from muscle9,12. Lipids were extracted using chloroform/methanol (2:1, vol/vol). Thin layer chromatography chromatography (TLC) plates (G plated, Silica Gel, 20 × 20 cm, 250 microns, Analtech Inc., Newark, DE) was used to isolate phospholipids (PL). Solvent (80:20:1 petroleum ether/diethyl/ethyl ether/acetic acid [glacial; HAC]) was added to the developmental chamber. Chloroform/methanol (2:1) was added to each dried tube, vortexed and samples were spotted on plates in duplicate. Spotted plates were run in a solvent system until the solvent mixture reached ~ 1.5 cm from the top of the plate. Dried plates were sprayed with 0.1% 8-anilino-1-naphthalenesulfonic acid (ANSA) to visualize the PL bands under ultraviolet light. Bands were scraped andan internal standard, C17:0, was added for PL fatty acid quantification followed by methylation. PL fatty acid composition was analysed by gas chromatography-flame ionisation detector in a Varian 3900 gas chromatography [Varian Instruments, Georgetown, ON, Canada]. The quantity of fatty acids within the PL fraction was calculated by comparison with the known concentration of the internal standard and the sum of all fatty acids was reported as total PL. The peak area of each fatty acid was normalized against the sum of peak areas of all fatty acids to determine the relative proportions of fatty acids comprising the PL fraction.
Statistical analysis
The primary outcome was overall survival, defined as the number of days surviving after the initial visit by each patient. Patients were observed until their deaths or until November 30, 2018, at which time they were censored at the last date they were documented to have been alive. For survival analysis, univariate Cox proportional hazard model was used to determine if amounts of fatty acids (continuous variable) were associated with the number of days of survival. Given the small sample size, fatty acids that were associated with the number of days of survival with p-value less than 0.1 were selected for optimal stratification and Cox regression analysis (categorical variable). Optimal stratification, a statistical method similar to receiver operator curve analysis, was used to solve specific threshold values within continuous variables. Optimal stratification is based on log-rank statistics that best separate patients with respect to time to an event outcome (death)23. This method identifies cut-points, to establish a level of fatty acids that best separate patients at risk with respect to survival. It is appropriate to determine survival-related threshold values empirically using statistical methods such as optimal stratification, where the relationship with survival for that covariate is not known (i.e. fatty acids in skeletal muscle phospholipids). In our study, we determine the cohort specific threshold amounts of phospholipid fatty acids; thresholds for these fatty acids were examined by BMI, age, tumor site and metastasis and sex. Number of days of survival were defined as days to death from the date of surgery or as number of days between date of surgery and date of data collections (for patients with censored survival time). The Kaplan–Meier method24 was used to establish the effect of the association between the quantity of phospholipid fatty acids on the number of days of survival. Log-rank tests were used to compare the survival curves for each variable (p < 0.05). Variables were entered into a univariate Cox proportional hazards model with 95% confidence intervals. Models were adjusted for BMI, age, tumor site and metastasis and sex. Statistical significance was reported when p-value < 0.05. Data are reported as mean ± SD. Levels of significance are p values < 0.05. All statistical analyses were performed using SPSS 20.0 (Chicago, IL, USA) for Windows.
Results
The association of fatty acids with survival was first determined (Table 2). Univariate Cox regression analysis revealed association between overall survival and palmitoleic acid (16:1), AA, EPA, DPA (docosapentaenoic acid) and DHA with p-value < 0.1 (Table 2). Cut-points were then derived using optimal stratification for those fatty acids that were trending significant (p < 0.10). Using the cut-point value, the number of deaths and length of survival were determined for each of these fatty acids (Table 3). In the univariate analysis, median survival was in range of 450–500 days for patients having AA, EPA and DHA in muscle phospholipid below the established critical threshold compared 720–800 days for patients above (Table 3). Sixty-three percent of patients with AA, EPA and DHA depletion (below the cut-point) died within 2 years (median overall survival of 16 months). In contrast, 69% of patients with palmitoleic acid above 20.5 ng/mg in muscle phospholipids died (median survival of 16 months). Univariate Cox regression analysis for categorical variables revealed that having low levels of AA, EPA and DHA poses survival risk (HR 3.45–4.30; p ≤ 0.04) whereas palmitoleic acid below the cut-point is protective for survival (HR = 0.34; p < 0.05). BMI, age, tumor site and metastasis did not influence survival but being female was significant predictor of shorter survival (HR 2.7 (1.1–7.2), p = 0.04). The risk of death remained significant for AA [HR 3.5 (1.11–10.87), p = 0.03], EPA [HR 3.92 (1.1–14.0), p = 0.04] and DHA [HR 4.08 (1.1–14.6), p = 0.03] when adjusted for sex in the multivariate model.
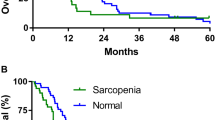
The survival distributions for patients below and above the cut-points for the essential fatty acids and adjusted for sex were statistically significantly different (p < 0.05; Fig. 1). The patients who had depletion of the essential fatty acids, AA, EPA and DHA simultaneously (all fatty acids below the cut-point), were compared to those with fatty acids above the cut-points to reveal that only one patient with these fatty acids above the cut-point had died at time of censorship, whereas 11 out of 17 (64%) patients with depleted levels of these fatty acids in muscle phospholipids died, ten of which died within a year of surgery.
Kaplein–Meier survival curve for patients with low versus high fatty acid content in skeletal muscle phospholipids. (A–C) Represent survival distribution of surgical patients with gastrointestinal cancer based on fatty acid cut-points associated with increased mortality risk obtained by optimum stratification and adjusted for sex. (D) Represents survival distribution of patients with ARA, EPA and DHA above and below the cut-points. Log-rank tests were used to compare the survival curves of each variable (p < 0.05).
When fatty acid data was expressed as a relative proportion, similar relationships were revealed. Having palmitoleic above, and EPA and DHA below, the respective cutpoints was significantly associated with shorter survival (Supplemental material Table 1).
Discussion
The main goal was to determine associations between fatty acid content and composition in skeletal muscle phospholipids and survival. The most significant finding is that having a lower mean absolute amount of the long chain essential fatty acids, AA, EPA and DHA in skeletal muscle phospholipids, is associated with poor prognosis in cancer patients, with a shortened life expectancy by 200 days (5 months).
In a cohort of patients with depletion of AA, EPA and DHA below a critical level as determined by statistical cut-points, approximately 40% more patients died compared to those with fatty acids above 136.9 ng/mg, 7.2 ng/mg and 25.2 ng/mg, respectively in muscle phospholipids. Notably, only one patient died in the group of patients with AA, EPA and DHA above this critical level but 600 days post-surgery. In contrast, over half of patients died within one year of surgery in the group of patients with AA, EPA and DHA below the critical level. Two studies have previously reported an association between levels of AA, EPA and DHA of plasma phospholipid and survival. Lower content of AA, EPA and DHA were reported in plasma of cancer patients who lived < 238 days as compared to > 238 days, determined to be the median days of survival in that study population9. Also, longer survival of pancreatic cancer patients has been associated with higher content of EPA and DHA, but not AA, in plasma16. Lower proportions of AA, EPA and DHA in plasma and erythrocyte phospholipids have been reported in bladder, lung, colorectal and pancreatic cancer as compared to healthy or non-malignant populations8,9,13,14,16. Proportions of AA, EPA and DHA in skeletal muscle in our cohort of cancer patients were lower compared to reference range of healthy person25,26. Here, we demonstrate a reduction in EPA, DHA and AA in muscle and further associate this depletion with truncated survival.
Muscle loss27,28 and plasma phospholipid fatty acid depletion9 have each been separately reported to be predictors of survival in cancer. The positive association between skeletal muscle mass and concentration of EPA and DHA in plasma phospholipids has been previously reported9. Based on this, it was hypothesised that there would be a positive association between essential fatty acids and skeletal muscle index, however, this was not observed.
A strong association is observed between essential fatty acids in phospholipid of muscle. Membrane phospholipid fatty acids regulate the biophysical properties of proteins, provide substrates for second messengers and intracellular signals to alter gene expression. Evidence suggests that EPA and DHA in membrane phospholipids can have a protective effect against cancer through several actions including protein kinase activation, enhancing cell apoptosis and modulating inflammation29,30,31. The association between dietary/plasma AA and the risk of cancer is highly controversial32. AA and its metabolites play role in muscle growth33. AA is converted into two series eicosanoids known as prostaglandins F2alpha (PGF2α) and prostaglandin E2 (PGE2) which activate the major anabolic pathway in muscle, phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signalling pathway and induce myotube hypertrophy34,35. Suppression of PGF2α in the muscle of experimental models has been shown to inhibit muscle recovery after disuse atrophy36. EPA and DHA in skeletal muscle membranes have been suggested to influence membrane properties that may influence anabolic signalling, particularly through key regulators of muscle protein synthesis37, and given the association with survival, these mechanisms are worthy of exploration.
Several limitations of the current study warrant mention. First, dietary records were not available for the patients, therefore, the effect of diet on skeletal muscle composition was not determined. The western diet38,39 and food choices cancer patients make during their cancer trajectory are foods that are good sources of AA, and its precursor linoleic acid40,41, therefore, depletion of AA observed is not likely to be related to intake, but rather a change in metabolism of essential fatty acids. Moreover, studies reported alterations in plasma and erythrocyte fatty acid composition in cancer patients compared to healthy controls independent of total calorie and fat intake12,42. Second, reliability of optimal stratification in small sample size is low23,43. The thresholds determined in this study should be considered approximate until confirmed in additional studies with larger sample size. While the first and only study to evaluate the PL composition in relation to survival, the sample size is too small to adjust for several well-known prognostic factors, which should be accounted for in future studies. There are several covariates that can be considered in the oncology setting, including tumor stage which is known to impact survival44. We did not see significant association between tumor site/metastasis and survival in our cohort. This can be due to small sample size and there is a chance of type II error. Ours is the preliminary study that provides new information that can be used to adequately power a future study to adjust for all covariates.
In conclusion, patients with AA, EPA and DHA amounts in muscle phospholipids that were below a critical level had shorter survival. The present study is a first step in establishing alterations in skeletal muscle fatty acid composition and its association with survival in cancer. This work provides rationale for conducting further studies to examine repletion of bioactive fatty acids in cancer. Further work is needed to investigate mechanisms that can explain the strong associations between essential fatty acids and survival in cancer patients observed in this study.
Data availability
Authors can confirm that all relevant data are included in the article.
Abbreviations
- AA:
-
Arachidonic acid
- CT:
-
Computed tomography
- DPA:
-
Docosapentaenoic acid
- HR:
-
Hazard ratio
- PL:
-
Phospholipid
References
Calder, P. C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 91, 791–795 (2009).
Calder, P. C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 39, 18S-32S (2015).
Catala, J. S. E.-A. Fatty Acids and Their Analogues as Anticancer Agents. in Ch. 5 (IntechOpen, 2017). https://doi.org/10.5772/intechopen.68171
Miccadei, S., Masella, R., Mileo, A. M. & Gessani, S. ω3 polyunsaturated fatty acids as immunomodulators in colorectal cancer: New potential role in adjuvant therapies. Front. Immunol. 7, 486 (2016).
Morland, S. L., Martins, K. J. B. & Mazurak, V. C. n-3 polyunsaturated fatty acid supplementation during cancer chemotherapy. J. Nutr. Intermed. Metab. 5, 107–116 (2016).
Klassen, P., Cervantes, M. & Mazurak, V. C. N-3 fatty acids during chemotherapy: Toward a higher level of evidence for clinical application. Curr. Opin. Clin. Nutr. Metab. Care 23, 82–88 (2020).
de Castro, J. et al. Erythrocyte fatty acids as potential biomarkers in the diagnosis of advanced lung adenocarcinoma, lung squamous cell carcinoma, and small cell lung cancer. Am. J. Clin. Pathol. 142, 111–120 (2014).
Murphy, R. A. et al. Aberrations in plasma phospholipid fatty acids in lung cancer patients. Lipids 47, 363–369 (2012).
Murphy, R. A., Mourtzakis, M., Chu, Q. S., Reiman, T. & Mazurak, V. C. Skeletal muscle depletion is associated with reduced plasma (n-3) fatty acids in non-small cell lung cancer patients. J. Nutr. 140, 1602–1606 (2010).
Murphy, R. A. et al. Loss of adipose tissue and plasma phospholipids: Relationship to survival in advanced cancer patients. Clin. Nutr. 29, 482–487 (2010).
Okuno, M. et al. Abnormalities in fatty acids in plasma, erythrocytes and adipose tissue in Japanese patients with colorectal cancer. In Vivo (Brooklyn). 27, 203–210 (2013).
Pratt, V. C. et al. Plasma and neutrophil fatty acid composition in advanced cancer patients and response to fish oil supplementation. Br. J. Cancer 87, 1370–1378 (2002).
Jolanta, B., Joanna, B., Diana, H. Z. & Krystyna, S. Composition and concentration of serum fatty acids of phospholipids depend on tumour location and disease progression in colorectal patients. J. Med. Biochem. 37, 39–45 (2018).
McClinton, S., Moffat, L. E. F., Horrobin, D. F. & Manku, M. S. Abnormalities of essential fatty acid distribution in the plasma phospholipids of patients with bladder cancer. Br. J. Cancer 63, 314–316 (1991).
Mikirova, N. et al. Erythrocyte membrane fatty acid composition in cancer patients. P. R. Health Sci. J. 23, 107–113 (2004).
Macášek, J. et al. Plasma fatty acid composition in patients with pancreatic cancer: Correlations to clinical parameters. Nutr. Cancer 64, 946–955 (2012).
Murphy, R. A. et al. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 117, 1775–1782 (2011).
Ryan, A. M. et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: Results of a double-blinded randomized controlled trial. Ann. Surg. 249, 355–363 (2009).
van der Meij, B. S. et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J. Nutr. 140, 1774–1780 (2010).
Weed, H. G. et al. Lean body mass gain in patients with head and neck squamous cell cancer treated perioperatively with a protein- and energy-dense nutritional supplement containing eicosapentaenoic acid. Head Neck 33, 1027–1033 (2011).
Anoveros-Barrera, A. et al. Clinical and biological characterization of skeletal muscle tissue biopsies of surgical cancer patients. J. Cachexia. Sarcopenia Muscle (2019).
Mourtzakis, M. et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 33, 997–1006 (2008).
Williams, B. A., Mandrekar, J., Mandrekar, S., Cha, S. & Furth, A. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes. (2006).
Goel, M. K., Khanna, P. & Kishore, J. Understanding survival analysis: Kaplan–Meier estimate. Int. J. Ayurveda Res. 1, 274–278 (2010).
McGlory, C. et al. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins. Leukot. Essent. Fatty Acids 90, 199–206 (2014).
Felton, C. V., Stevenson, J. C. & Godsland, I. F. Erythrocyte-derived measures of membrane lipid composition in healthy men: Associations with arachidonic acid at low to moderate but not high insulin sensitivity. Metabolism 53, 571–577 (2004).
Brown, J. C. et al. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I-III colorectal cancer: A population-based cohort study (C-SCANS). J. Cachexia. Sarcopenia Muscle 9, 664–672 (2018).
Shachar, S. S., Williams, G. R., Muss, H. B. & Nishijima, T. F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 57, 58–67 (2016).
Kansal, S., Bhatnagar, A. & Agnihotri, N. Fish oil suppresses cell growth and metastatic potential by regulating PTEN and NF-κB signaling in colorectal cancer. PLoS ONE 9, e84627 (2014).
Turk, H. F. & Chapkin, R. S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins. Leukot. Essent. Fatty Acids 88, 43–47 (2013).
Yang, T. et al. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells in vitro. J. Nutr. Biochem. 24, 744–753 (2013).
Sakai, M. et al. Arachidonic acid and cancer risk: A systematic review of observational studies. BMC Cancer 12, 606 (2012).
Korotkova, M. & Lundberg, I. E. The skeletal muscle arachidonic acid cascade in health and inflammatory disease. Nat. Rev. Rheumatol. 10, 295–303 (2014).
Trappe, T. A., Fluckey, J. D., White, F., Lambert, C. P. & Evans, W. J. Skeletal muscle PGF2α and PGE2 in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J. Clin. Endocrinol. Metab. 86, 5067–5070 (2001).
Trappe, T. A. et al. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am. J. Physiol. Metab. 282, E551–E556 (2002).
You, J.-S., Park, M.-N. & Lee, Y.-S. Dietary fish oil inhibits the early stage of recovery of atrophied soleus muscle in rats via Akt–p70s6k signaling and PGF2α. J. Nutr. Biochem. 21, 929–934 (2010).
Kamolrat, T. & Gray, S. R. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 432, 593–598 (2013).
Simopoulos, A. P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8, 128 (2016).
Simopoulos, A. P. Evolutionary aspects of diet and essential fatty acids. in Fatty Acids and Lipids—New Findings Vol. 88, 18–27 (KARGER, 2000).
Hutton, J. L. et al. Dietary patterns in patients with advanced cancer: Implications for anorexia-cachexia therapy. Am. J. Clin. Nutr. 84, 1163–1170 (2006).
Prado, C. M. M. et al. Dietary patterns of patients: With advanced lung or colorectal cancer. Can. J. Diet. Pract. Res. 73, e298–e303 (2012).
Amézaga, J. et al. Altered red blood cell membrane fatty acid profile in cancer patients. Nutrients 10, 1–13 (2018).
Tunes-da-Silva, G. & Klein, J. P. Cutpoint selection for discretizing a continuous covariate for generalized estimating equations. Comput. Stat. Data Anal. 55, 226–235 (2011).
Petrelli, F. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol. 3, 211–219 (2017).
Acknowledgements
Funding for this project is from the Canadian Institutes of Health Research (CIHR) operating Grants awarded to V.C.M and V.E.B. A.S.B. is the holder of the Alberta Innovates Technology Futures (AITF) award at the University of Alberta, Canada.
Funding
This work was supported by Canadian Institutes of Health Research (CIHR).
Author information
Authors and Affiliations
Contributions
A.B. performed the experiments, conducted the analysis and drafted the manuscript; A.A. contributed to the data collection; I.M.R.-S. performed the fatty acid analysis; A.D.-H. assisted with analysis; R.K., D.B., O.B, and T.M. provided access to patients and biopsies; C.P. contributed to concept; M.T.C. contributed to concept and data interpretation; V.B. contributed to interpretation; V.C.M. supervised the project. All authors contributed to the manuscript revisions, and have reviewed and approved the final submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhullar, A.S., Rivas-Serna, I.M., Anoveros-Barrera, A. et al. Depletion of essential fatty acids in muscle is associated with shorter survival of cancer patients undergoing surgery-preliminary report. Sci Rep 11, 23006 (2021). https://doi.org/10.1038/s41598-021-02269-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02269-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.