Abstract
We tried to identify the interaction between dietary quality indices and apolipoprotein B Ins/Del and EcoR1 polymorphisms on biochemical and anthropometric factors in patients with type 2 diabetes mellitus (T2DM). This cross-sectional study recruited 700 adults with T2DM in Tehran. The genotypes of Ins/Del and EcoR1 single nucleotide polymorphisms (SNP) were explored via polymerase chain reaction (PCR). Dietary quality index-international (DQI-I), healthy eating index-2015 (HEI-2015) and dietary phytochemical index (DPI) were calculated by semi-quantitative food frequency questionnaire (FFQ). In both crude and adjusted model for confounding factors, we observed significant interactions between DQI-I and Ins/Del SNP on leptin in and 8-iso-prostaglandin F2 α (8-iso-PGF2α), DPI and EcoR1 SNP on total cholesterol (TC) and between Ins/Del SNP and HEI-2015 on interleukin-18 (IL-18). Furthermore, in crude model there were close to meaningful interactions between EcoR1 SNP and DQI-I on total antioxidant capacity (TAC) and between EcoR1 SNP and HEI-2015 on serum leptin and superoxide dismutase (SOD) levels. Our finding indicated that the association between DQI-I, HEI-2015 and DPI with IL-18, TC, leptin and 8-iso-PGF2α in patients with T2DM might be dependent on Ins/Del and EcoR1 variants in ApoB gene.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is a pandemic disease associated to insulin resistance, beta-cell dysfunction and hyperglycemia1. Epidemiological studies reported that worldwide prevalence of diabetes mellitus (DM) was about 451 million in 2017 with most being T2DM2. Majority of T2DM patients present with biochemical abnormalities including elevated oxidative stress, inflammatory status, disturbed lipid profile3,4,5,6. High appetite and obesity is another common symptom in T2DM patients that is characterized by high BMI, high blood ghrelin and low blood leptin levels7.
There are important relationships between many of these abnormalities and further progression of T2DM disease and other adverse chronic conditions8. The causes of hyperglycemia, hyperglycemic-induced oxidative stress and inflammation and dyslipidemia and then T2DM development are multifactorial, and efforts to identify these causes are necessary for the development of beneficial preventive strategies. In addition to modifiable factors such as physical activity and dietary intake, T2DM is affected by non-modifiable factors such as genetic predisposition9. Furthermore, recent studies have indicated that genetic variation interact with potential environmental factors such as dietary patterns and intake of food components and hence influence development of T2DM10,11. The assessment of gene-diet interaction is called nutrigenetics.
The aim of nutrigenetics is understanding the concept of “personalized nutrition”, which targets to inhibit the progression of chronic diseases by creating dietary recommendations based on individual’s genetic predisposition12. Until now, genome-wide investigations have revealed the biological roles of several single-nucleotide polymorphisms (SNPs) in the pathogens of DM13.
Since Apo-lipoprotein B (Apo B) participates in the cellular cholesterol uptake from cholesterol-rich lipoproteins14, single-nucleotide polymorphism (SNP) in Apo B gene could predict changes in hypercholesterolemia and subsequent oxidative, inflammatory, lipid and anthropometrics states15,16. There has been high evidence by far for the Apo B ECOR1 and Ins/Del variants in the Apo B gene, with the E- and Del alleles being probably linked to increasing T2DM risk factors17,18. These SNPs have also been indicated to modify associations to dietary components intakes for T2DM -related phenotypes. For instance it has been demonstrated that, in people carrying Del allele in Apo B Ins/Del SNP, there is a higher probability of elevated blood low density lipoprotein (LDL), total cholesterol (TC) and waist to hip ratio (WHR) compared to subjects with Ins/Ins genotype. However, it was later found that low calorie diet and omega-3 poly-unsaturated fatty acids (W-3 PUFA) intake can significantly reduce LDL concentrations and waist circumference in Del allele carriers19,20,21,22. Moreover, homozygous E + E + genotypes have been observed to have lower probability of elevated BMI in response to a high calorie and saturated fat diet, compared to those with E − allele23. However, there have been significantly low nutrigenetics studies on the interaction between dietary patterns and ECOR1 and Ins/Del SNPs with regard to biochemical and anthropometric markers.
Evaluating dietary pattern by various validated indices is an effective way to demonstrate a people’s dietary status. Because, a single food group may not make significant effect on various aspects of health24. Dietary quality index-international (DQI-I) and healthy eating index (HEI-2015) are among popular dietary indicators developed to assess overall quality of the diet in different populations. Dietary phytochemical index (DPI) measures total dietary phytochemical content and ranks persons according to their intakes of phytochemical rich foods25. A number of studies have also suggested that adherence to healthy diet presented by high scores of DQI-I, HEI-2015 and DPI is negatively associated with lower level of T2DM risk factors26,27.
It is unclear if interactions of Apo B ECOR1 and Ins/Del SNPs with dietary intake affect cardiovascular risk factors levels in different populations. In this study, using Iranian subjects with T2DM, we aimed to evaluate if scores of DQI-I, HEI-2015 and DPI modifies associations of biochemical and anthropometric factors and Apo B ECOR1 and Ins/Del SNPs.
Methods
Study design and subjects
We conducted an observational cross-sectional study on patients with T2DM. Sample size calculating was through following formula:
N = (([(Z1 − α + Z1 − β) × √1 − r2]/r)2 + 2), When r = 0.15 β = 0.95 and α = 0.05, the N was equal to 694. Due to data availability, we recruited 700 participants. Subjects were recruited by multistage cluster random sampling method from individuals who visited diabetes referral centers in different regions of Tehran, Iran during June 2011 to October 2012. Subjects were eligible for participation if they were adult and had been previously diagnosed with T2DM based on the decision of the endocrinologist. We excluded pregnant and lactating women and patients who had a current or a previous 2 months history of malignancies, abnormalities in kidney, hepatic, thyroid and cardiovascular system, alcohol intake, dependency on cigarette or drug, consumption of anti-inflammatory medications or dietary supplements. Additionally, subjects with an ongoing insulin therapy were not allowed to enter this research. There was no source of bias in present research. All of the methods of this research were carried out in accordance with the Declaration of Helsinki28. All stages of this study had been approved by the Ethics Committee of Tehran University of Medical Sciences (Ethics number: IR.TUMS.VCR.REC.1395.15060). In addition, all eligible individuals signed a declaration on consent to participate after they were informed about aims and protocol of the study.
Assessment of sociodemographic, anthropometric and physical activity variables
Eligible participants were answered the questionnaires regarding their sociodemographic information such as age, job, medical history, duration and family history of T2DM, smoking and alcohol usage and consumption of lipid lowering medications. Assessment of physical activity was done using a validated questionnaire that was based on metabolic equivalent to task (MET-h/day) of daily activities29. Weight (kg) and height (m) were measured via Seca falcon scales (Seca, Germany) by standard protocols30. Waist circumference was measured at the level midway between the lower margin of the last palpable rib and the top of the iliac crest at the end of several consecutive natural breaths. BMI was calculated via the following formula: weight/height2 (kg/m2).
Assessment of healthy eating index (HEI-2015), dietary quality index international (DQI-I) and dietary phytochemical index (DPI)
The participant’s usual dietary intake on a daily, weekly, monthly or annual basis during last year was assessed through face-to-face interviews performed by trained nutritionists using a validated semi-quantitative food frequency questionnaire (FFQ) for Iranian population31. The detailed structures of HEI-2015, DQI-I and DPI have been numerously described elsewhere32,33,34. Healthy eating index-2015 (HEI-2015) is a new tool for prediction of the adherence level to 2015–2020 DGA. In brief, for total HEI-2015 score calculation, FFQ items were classified in to 13 food groups including (1) Total Vegetables, (2) Dairy, (3) Whole Fruits, (4) Total Fruits, (5) Total Protein Foods, (6) Greens and Beans, (7) Seafood and Plant Proteins, (8) Whole Grains, (9) Refined Grains, (10) Added Sugars, (11) Saturated Fats, (12) Fatty Acids and (13) Sodium. In this classification, there were similar score ranges (0–5) for all parameters except for number 1–6 food groups which had a score range between 1 and 10. Scores of 13 food parameters were added to obtain the final HEI-2015 score in which 0 and 100 were indicative of minimum and maximum adherence to 2015–2020 DGA respectively. In DQI-I score, variety component (total score: 0–20) composed of overall diversity between food groups (scores: 0–15) and diversity within protein food sources (scores: 0–5). In adequacy component (total score: 0–40), there were scores ranging from 0 to 5 for investigation of sufficient intakes of 8 parameters including fruits, grains, fiber, protein, iron, calcium and vitamin C. Moderation component (total score: 0–30) investigated restraint intake of 5 parameters (each of them had scores ranging from 0 to 6) including total fat, saturated fat, sodium, cholesterol and empty calorie food. Overall balance (total score: 0–10) was also according to the proper ratio of macronutrient intake including fat, carbohydrate and protein (score 0–6) and fatty acids intake including saturated fatty acids (SFA), PUFA and monounsaturated fatty acids (MUFA) (score 0–4). Scores of four aspects were summed up to obtain the total score of DQI-I, in which score 0 showed minimal diet quality, while score 100 was indicative of maximal diet quality. The DPI for each subject was calculated as: the total calorie of all phytochemical rich food components (including whole grains, fruits, vegetables, natural fruit and vegetable juices, soy products, tomato sauces, nuts, legumes, olive and olive oil) divided by total calorie intake.
Assessment of genetic and biochemical parameters
Blood sampling carried out for all subjects after overnight fasting (8–12 h). Afterwards, blood samples were undergone either centrifugation or PCR analysis. The detailed description of PCR, TaqMan assay method for evaluation of ApoB Ins/Del and EcoR1 SNPs have been published in our previous study35. The centrifugation was conducted for measurement of biochemical markers in the serum. Enzymatic method by commercially existing kits was used for determination of serum TC and triglyceride (TG) levels (Pars Azmoon, Iran), serum leptin (Bioassay Technology Co, China) and ghrelin (Bioassay Technology Co, Mediagnost, Germany) levels and serum IL-18, 8-iso-PGF2α and pentraxin-3 (PTX-3) levels (Shanghai Crystal Day Biotech Co., Ltd). Turbidimetry by a Roche Hitachi analyzer (Roche, Germany) was used for serum high density lipoprotein (HDL) and LDL levels determinations. Spectrophotometry procedure for serum TAC levels measurement was applied. Colorimetric procedure was used (Cayman Chemical Company, USA) for serum SOD levels measurements.
Statistical analysis
The Kolmogorov–Smirnov (K-S) test was used to decide whether variables in population had a normal distribution. Transformations to square or logarithmic were used for abnormal data. There were three genotypes of ApoB Ins/Del SNP including Ins/Ins, Ins/Del and Del/Del. The genotypes of Apo B EcoR1 SNP included E + E + , E + E − and E − E − . Independent t-test was applied for comparison of biochemical and anthropometric factors (BMI, WC, LDL, HDL, LDL/HDL, TC, TG, IL-18, TAC, PTX-3, SOD, iso-PGF2α, CRP, ghrelin and leptin) between Del allele carriers and subjects with Ins/Ins homozygous and between E − allele carriers and subjects with E + E + homozygous. HEI-2015, DQI-I and DPI scores were divided in to 3 equal intervals (tertile) for evaluating the adherence of subjects to indices. Analysis of covariance (ANCOVA) analysis test was carried out in order to compare the abovementioned study outcomes among groups of dietary indices.
Finally, interaction between ApoB Ins/Del SNP and dietary indices (DQI-I, HEI-2015 and DPI) as well as interaction between ApoB EcoR1 SNP and diet indices on the abovementioned variables were tested by using ANCOVA multivariate interaction models with and without adjusting for confounding factors (gender, age, smoking habits, and alcohol intake and physical activity). The results of interactions were presented as plots to visualize findings and help their illustrations.
In all stages of our research’s analysis, IBM SPSS (SPSS Inc., Chicago, IL, USA, version 21) was used, in which a P-value lower than 0.05 was assumed as significantly meaningful.
Results
Associations between DQI-I, HEI-2015 and DPI with biochemical and anthropometric parameters
Table 1 provides the findings of ANOVA test for comparing biochemical and anthropometric factors within the tertiles of dietary indices in recruited subjects. We observed significant differences in HDL (P = 0.01) and LDL/HDL (P = 0.04) levels between HEI-2015 tertiles. Our analysis did not display anymore significant or borderline significant difference in study’s variables within tertiles of DQI-I, HEI-2015 and DPI (P > 0.05).
Associations between ApoB Ins/Del and EcoR1 SNP and biochemical and anthropometric parameters
The comparison of risk factors of biochemical and anthropometric variables within genotypes of ApoB Ins/Del and EcoR1 SNPs are presented in Table 2. A significant greater level of HDL was observed in E − allele carriers than subjects with E + E + homozygous (P = 0.001). No significant association was discovered between other study outcomes and investigated polymorphisms (P > 0.05).
Interactions between Apo B Ins/Del and EcoR1 SNPs and dietary indices (DQI-I, HEI-2015 and DPI) on biochemical and anthropometric parameters
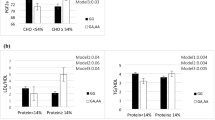
The results of the significant interactions are illustrated in Figs. 1, 2, 3, 4, 5, 6, and 7. Interaction between DQI-I score and Apo B Ins/Del SNP on serum 8-iso-PGF2α level was significant (Fig. 1). In fact, in people with Ins/Ins genotype, there was lower 8-iso-PGF2α concentration in tertile 2 of DQI-I score in comparison to tertile 1 and 3 of DQI-I score. However, in Del allele carriers, 8-iso-PGF2α level was significantly higher in tertile 2 of DQI-I score than tertile 1 and 3. This interaction was also significant after adjusting for confounding factors (gender, age, smoking habits, and alcohol intake and physical activity) (Fig. 1).
Moreover, there was significant interaction between DQI-I score and Apo B Ins/Del SNP on serum level of leptin was statically significant (Fig. 2). Based on Fig. 2, in Del allele carriers, leptin level was lower in tertile 2 than tertile 3 and 1. However, in Ins/Ins homozygous there was mildly lower leptin level in tertile 2 of DQI-I score compared to tertile 1 and 3. This interaction remained significant after adjusting for confounding factors (Fig. 2).
Findings of ANCOVA multivariate analysis also indicated a significant interaction between HEI-2015 score and Apo B Ins/Del SNP on serum IL-18 level in both crude and adjusted models (Fig. 3). In fact, in the interaction between HEI-2015 score and Apo B Ins/Del, level of IL-18 in patients with Ins/Ins genotype, was lower in tertile 1 and 3 than tertile 2 of HEI-2015 score. While in Del allele carriers, IL-18 concentration in tertile 1 and 3 of HEI-2015 was more than tertile 2 (Fig. 3).
Furthermore, the interaction test showed that in crude model, the interaction between ApoB EcoR1 SNP and DQI-I on serum TAC level was close to statistically significant (Fig. 4). As it is illustrated in Fig. 4, in E − allele carriers, there was increasing trend in TAC level from tertile 1 towards tertile 3 of HEI-2015. On the other hand, there was a mild higher TAC level in tertile 1 of HEI-2015 score than tertile 2 and 3 in people carrying E + E + genotype. This close to significant interaction disappeared after adjusting for confounding factors (Fig. 4).
In addition, in crude model, there were close to meaningful interactions between Apo B EcoR1 SNP and HEI-2015 score on serum leptin level in the participants which disappeared in adjusted model (Figs. 5). In Fig. 5, it can be observed that, there was a reducing trend in leptin level from tertile 1 towards tertile 3 of HEI-2015 in people carrying E − allele. However, this trend was milder and inversed in E + E + homozygous.
Furthermore, in crude model, there were borderline significant interaction between Apo B EcoR1 SNP and HEI-2015 score on SOD level (Fig. 6). In this interaction, while SOD concentration had a decreasing trend from tertile 1 towards tertile 3 of HEI-2015 in E − allele carriers, the level of SOD level increased from tertile 1 towards tertile 3 in participants with E + E + . This borderline significant interaction did not remain in adjusted model (Fig. 6).
Finally, we found a significant interaction between Apo B EcoR1 SNP and DPI score on serum TC level in both crude and adjusted models (Fig. 7). In fact, TC concentration had decreasing and increasing trends from tertile 1 towards tertile 3 of DPI score in E + E + homozygous and in E − allele carriers, respectively (Fig. 7).
ANCOVA multivariate interaction did not indicate any more significant or close to significant interactions between Apo B Ins/Del and EcoR1 SNPs and dietary quality indices (DQI-I, HEI-2015 and DPI) on study outcomes (Supplementary figures).
Discussion
The present study indicated a significant interaction between ApoB Ins/Del SNP and HEI-2015 on serum IL-18 level. In subjects with Ins/Ins genotype, there were higher IL-18 levels in tertile 2 than those in tertiles 1 and 3 of HEI-2015. While the trend of the IL-18 level seemed to be inversed in Del-allele carries (i.e. subjects had higher IL-18 in the tertiles 1 and 3 of HEI-2015 than tertile 2). In fact, when HEI-2015 score was moderate, Del allele carriers and Ins/Ins homozygous had lower and higher IL-18 level, respectively. It has been reported that high blood IL-18 levels rise CVDs risk36. No precious mechanism has been proved yet, but some possible explanation could be proposed for the interaction of the Apo B Ins/Del polymorphism and HEI-2015 on IL-18 level. However there are possible explanations. For example, HEI-2015 considers high whole carbohydrate intakes as a part of healthy dietary pattern. People with T2DM often care to avoid eating refined carbohydrates and consume whole carbohydrates, instead. Consequently, participants with Ins/Ins genotype in tertile 2 of HEI-2015 consumed more whole carbohydrates and therefore higher total carbohydrates than Ins/Ins homozygous in tertile 1. In T2DM, higher total carbohydrate intake is associated with elevated inflammatory state37.
In conclusion, in Ins/Ins genotype carriers, higher total carbohydrate intake could have caused lower IL-18 level in tertile 2 compared to tertile 1. On the other hand, HEI-2015 focuses on main elements of a healthy diet such as limited consumption of SFA and added sugar as well as high intake of fruits, vegetables and nuts which have inflammation reducing properties38,39. Therefore, in Ins/Ins homozygous, in tertile 3 of HEI-2015, greater intakes of anti-inflammatory and antioxidant food components might have overcome the higher carbohydrates intakes and caused lower IL-18 levels in tertile 3 in comparison to tertile 240.
As it was illustrated in Table 2, people with risk Del allele had more total cholesterol level than those with Ins/Ins genotype. There is currently evidence that Ins/Del SNP is related to deletion of 3 amino acids (Ala-Leu-Ala) from ApoB gene which alters the normal formation of recognition sit of ApoB for LDL recipient41. Del allele in this SNP may lead to dyslipidemia especially hypercholesterolemia42. It has been shown that people with hypercholesterolemia are more prone to inflammatory status reduction through diet or lifestyle interventions in comparison to people with healthy lipid profile43. Therefore, in Del-allele carriers, an increase in diet quality might have led to reduction in IL-18 level from tertile 1 towards tertile 2 of HEI-2015 score. On the other hand, persons with different kinds of hypercholesterolemia are more genetically predisposed to inflammatory status and premature CVDs events in response to high calorie intake than normal individuals44. Therefore, higher IL-18 in Del allele carriers in tertile 3 than tertile 2 and 1 of HEI-2015, might be due to the overcome of the adverse effect of greater calorie intakes on high healthy food components intake.
However, there are numerous factors that influence diet, which in turn can have an impact on various biomarkers. This calls for more investigations, and maybe alternative approaches, to determine the actual mechanism of these relationships.
According to our best knowledge to date, there is no research on the interaction between ApoB SNPs and dietary factors on IL-18 levels and other inflammatory cytokines. However, there are some interaction studies between lipid, SFA and cholesterol intakes and ApoB Ins/Del polymorphism on lipid profile that reported insignificant results on TC20,45. However, there was not significant interaction between Ins/Del and dietary indices on TC in our study. This inconsistency might be due to differences in study population and the interaction of other food parameters in dietary indices in this study with ApoB Ins/Del SNP. In our study, we also represented a significant interaction between DQI-I and Ins/Del variants on serum leptin concentration in both crude and adjusted models. It was observed that, serum leptin in individuals with Ins/Ins genotype was lower in tertile 3 and 1 of DQI-I than tertile 2. Although in those with Del allele, leptin level was not very different within DQI-I tertiles. A dietary pattern with high DQI-I and HEI-2015 scores is rich in MUFAs, PUFAs and fiber, poor in SFA and sugar and has a suitable proportion of total fat intake which can inhibit hypertriglyceridemia46,47,48. Low blood TG level can protect against leptin resistance via change in receptor signaling or metabolism of leptin49. Secondarily, we explored a borderline significant interaction between HEI-2015 and EcoR1 SNP on leptin level which disappeared after controlling of confounding factors. There was a reducing trend in leptin concentration from tertile 1 toward tertile 3 of HEI-2015 in E − allele carriers, while there was only a mild increasing trend in subjects with E + E + homozygous. EcoR1 SNP is related to the substitution of lysine for glutamic acid that alters the formation and tendency of recognition site for LDL receptor that might lead to hypercholesterolemia50. It appears that subjects with Del allele in Ins/Del SNP and E − allele in EcoR1 SNP are more beneficially responsive to healthier diet than those with Ins/Ins and E + E + homozygous. Leptin is an adipokine which plays important roles in energy homeostasis and satiety. But hyperleptinemia and leptin resistance might be predictive factors of CVDs risk51. To our knowledge, only a study by Rafiee et al. assessed the interaction between dietary components and ApoB SNP on blood leptin levels in patients with T2DM. In their study, in Del allele carriers with T2DM, higher intake of MUFA, PUFA, SFA and protein and lower intake of carbohydrates were related to lower serum leptin concentration. However there was not any significant difference in subjects with Ins/Ins genotype21. Another finding of our study was the significant interaction between ApoB EcoR1 polymorphism and DPI on serum TC concentrations in both crude and adjusted models. Based on our analysis, during tertiles of DPI in E + E + homozygous and E − allele carriers, TC levels had decreasing and increasing trends, respectively, indicating that phytochemical components may reduce cholesterol level in diabetic patients homozygous for E + allele through their anti-oxidative and anti-inflammatory characteristics25,32. Currently, there is no experimental or human study on the interaction between genetic profile and DPI on cardio metabolic parameters. However, some researched have demonstrated the reducing effect of DPI on inflammation, obesity and pre-diabetes25,52. Furthermore, the interaction between dietary patterns or components and ApoB SNPs on oxidative stress and anti-oxidative markers has not been investigated, yet. Present study indicated significant interaction between ApoB Ins/Del polymorphism and DQI-I on 8-iso-PGF2α in both crude and adjusted models. In fact, in Ins/Ins homozygous, moderate DQI-I score (tertile 2) was associated with benefits for oxidative stress shown by lower 8-iso-PGF2α levels compared to high (tertile 3) and low (tertile 1) DQI-I scores. On the other hand, there was increasing trend in 8-iso-PGF2α level from tertile 1 and 3 toward tertile 2 in Del allele carriers. Puchau B revealed a possible protective role of high DQI-I scores against oxidative stress in healthy subjects53. Therefore, in diabetic patients carrying Del allele, DQI-I appeared to have the strongest impact on reducing the serum 8-iso-PGF2α level, when it has the highest score due to high intake of antioxidant nutrients such as vitamin E, C, selenium, fiber54,55. While, it seems that only a moderate score of DQI-I is enough to reduce 8-iso-PGF2α level in subjects with Ins/Ins homozygous. Additionally, in subjects with Ins/Ins homozygous, high intakes of energy, refined grains and fruits in tertile 3 of DQI-I may have contributed to higher 8-iso-PGF2α compared to tertile 2. The change of tendency of ApoB to LDL recipients might explain variability in plasma 8-iso-PGF2α associations with DQI-I values between genotypes of Ins/Del polymorphism. In crude models, we also observed borderline significant interactions between ApoB EcoR1 SNP and DQI-I on TAC level and between ApoB EcoR1 SNP and HEI-2015 on SOD level. There was an elevating impact of high DQI-I scores in serum TAC in E − allele carriers. However, our results indicated that the response of SOD level as a main marker of antioxidant defense system to HEI-2015 was inversed and its concentrations decreased within HEI-2015 tertiles in E − allele carriers in crude model. But these borderline significant interactions on TAC and SOD disappeared after adjusting for confounding variables including BMI, age and smoking or alcohol uses. Findings of the relationship between HEI and oxidative stress are very limited. Two investigations have demonstrated that the HEI-2015 had a positive association with TAC and HEI-2010 had an insignificant association with SOD56,57. We further indicated that greater adherence to the HEI-2015 had favorable effects on serum HDL level and LDL/HDL ratio, respectively, which can be due to high intake of cardio protective micro-nutrients irrespective of differences in genotypes of ApoB SNPs52. In addition, serum HDL concentration was significantly higher in E − allele carriers in ApoB EcoR1 SNP than E + E + homozygous. It should be noted that, high HDL level in E + E + homozygous in the current study can’t be interpreted as a cardio protective factor because some studies have indicated that genetic mutations that increase blood HDL level do not necessarily protects against CVDs58.
In this study, lack of causal interpretation due to cross-sectional design and measuring of ApoB serum level due to limited budget must be taken into consideration as limitations. However, our research is unique, because for the first time, we found that in T2DM patients carrying Del allele in ApoB Ins/Del SNP, moderate DQI-I values might reduce 8-iso-PGF2α and leptin as well as moderate HEI-2015 scores may be accompanied with reduction of IL-18. Additionally, we suspect that higher DPI in E + E + genotype of ApoB EcoR1 is probably associated with lower serum TC in patients with T2DM. Further investigations are required to identify the exact underlying mechanisms of the observed interaction in this study.
Data availability
Data sharing not applicable.
References
Association, A. D. Diagnosis and classification of diabetes mellitus. Diabet. Care 33(Supplement 1), S62–S69 (2010).
Cho, N. et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabet. Res. Clin. Pract. 138, 271–281 (2018).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 114(12), 1752–1761 (2017).
Badawi, A. et al. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabet., Metab. Syndr. Obes.: Targets Ther. 3, 173 (2010).
Zhang, S., Zhang, Q., Zhang, L., Li, C. & Jiang, H. Expression of ghrelin and leptin during the development of type 2 diabetes mellitus in a rat model. Mol. Med. Rep. 7(1), 223–228 (2013).
Mooradian, A. D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 5(3), 150–159 (2009).
Zhang, S., Zhang, Q., Zhang, L., Li, C. & Jiang, H. Expression of ghrelin and leptin during the development of type 2 diabetes mellitus in a rat model. Mol. Med. Rep. 7(1), 223–228 (2012).
Lin, P. J., Kent, D. M., Winn, A., Cohen, J. T. & Neumann, P. J. Multiple chronic conditions in type 2 diabetes mellitus: Prevalence and consequences. Am. J. Manag. Care 21(1), e23–e34 (2015).
Murea, M., Ma, L. & Freedman, B. I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud.: RDS 9(1), 6–22 (2012).
Dietrich, S. et al. Gene-lifestyle interaction on risk of type 2 diabetes: A systematic review. Obes. Rev.: Off. J. Int. Assoc. Stud. Obes. 20(11), 1557–1571 (2019).
Karimi, E. et al. A personalised diet study: The interaction between ApoA2− 265T> C polymorphism and dietary inflammatory index on oxidative and inflammatory markers and lipid profile in patients with type 2 diabetes mellitus: A cross-sectional study. Int. J. Clin. Pract. 75, e14178 (2021).
Mullins, V. A., Bresette, W., Johnstone, L., Hallmark, B. & Chilton, F. H. Genomics in personalized nutrition: Can you “eat for your genes”?. Nutrients 12(10), 3118 (2020).
Ali, O. Genetics of type 2 diabetes. World J. Diabet. 4(4), 114 (2013).
Blackhart, B. et al. Structure of the human apolipoprotein B gene. J. Biol. Chem. 261(33), 15364–15367 (1986).
Catapano, A. L., Pirillo, A. & Norata, G. D. Vascular inflammation and low-density lipoproteins: Is cholesterol the link? A lesson from the clinical trials. Br. J. Pharmacol. 174(22), 3973–3985 (2017).
Zhang, X. et al. Genetic polymorphism of APOB is associated with diabetes mellitus in sickle cell disease. Hum. Genet. 134(8), 895–904 (2015).
Vimaleswaran, K. S. et al. The APOB insertion/deletion polymorphism (rs17240441) influences postprandial lipaemia in healthy adults. Nutr. Metab. 12(1), 7 (2015).
Gajra, B. et al. Influence of polymorphisms for apolipoprotein B (ins/del, XbaI, EcoRI) and apolipoprotein E on serum lipids and apolipoproteins in a Javanese population. Genet. Epidemiol. 11(1), 19–27 (1994).
Hixson, J., McMahan, C., McGill, H. & Strong, J. Apo B insertion/deletion polymorphisms are associated with atherosclerosis in young black but not young white males. Pathobiological determinants of atherosclerosis in youth (PDAY) research group. Arterioscler. Thromb.: J. Vasc. Biol. 12(9), 1023–1029 (1992).
Jemaa, R., Mebazaa, A. & Fumeron, F. Apolipoprotein B signal peptide polymorphism and plasma LDL-cholesterol response to low-calorie diet. Int. J. Obes. 28(7), 902–905 (2004).
Rafiee, M. et al. The interaction between apolipoprotein b insertion/deletion polymorphism and macronutrient intake on lipid profile and serum leptin and ghrelin levels in type 2 diabetes mellitus patients. Eur. J. Nutr. 58(3), 1055–1065 (2019).
Renges, H., Wile, D., McKeigue, P., Marmot, M. & Humphries, S. Apolipoprotein B gene polymorphisms are associated with lipid levels in men of South Asian descent. Atherosclerosis 91(3), 267–275 (1991).
Nikbazm, R., Sotoudeh, G., Eshraghian, M. & Koohdani, F. Oral: Association of the Apo Becori polymorphism and saturated fatty acids intake and energy intake with body mass index in patients with type-2 diabetes mellitus. Nutr. Food Sci. Res. 1(1), 50 (2014).
Jacobs, D. R. Jr., Gross, M. D. & Tapsell, L. C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 89(5), 1543s-s1548 (2009).
Vincent, H. K., Bourguignon, C. M. & Taylor, A. G. Relationship of the dietary phytochemical index to weight gain, oxidative stress and inflammation in overweight young adults. J. Hum. Nutr. Diet. 23(1), 20–29 (2010).
Shin, M. K., Kim, Y. S., Kim, J. H., Kim, S. H. & Kim, Y. Dietary patterns and their associations with the diet quality index-international (DQI-I) in Korean women with gestational diabetes mellitus. Clin. Nutr. Res. 4(4), 216–224 (2015).
Hu, E. A., Steffen, L. M., Coresh, J., Appel, L. J. & Rebholz, C. M. Adherence to the healthy eating index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J. Nutr. 150(2), 312–321 (2020).
Association, W. M. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20), 2191–2194 (2013).
Klishadi, R., Khosravi, A., Famouri, F., Sadeghi, M. & Shirani, S. Assessment of physical activity of adolescents in Isfahan. J. Shahrekord Univ. Med. Sci. 3(2), 27–33 (2001).
Doaei, S. et al. The association between self-efficacy in weight management with anthropometric indices in male adolescents. J. North Khorasan Univ. Med. Sci. 10(2), 1–6 (2018).
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 13(5), 654–662 (2010).
McCarty, M. F. Proposal for a dietary “phytochemical index”. Med. Hypotheses 63(5), 813–817 (2004).
Kim, S., Haines, P. S., Siega-Riz, A. M. & Popkin, B. M. The Diet Quality index-international (DQI-I) provides an effective tool for cross-national comparison of diet quality as illustrated by China and the United States. J. Nutr. 133(11), 3476–3484 (2003).
Krebs-Smith, S. M. et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. 118(9), 1591–1602 (2018).
Rafiee, M. et al. Dietary ω-3 polyunsaturated fatty acid intake modulates impact of insertion/deletion polymorphism of ApoB gene on obesity risk in type 2 diabetic patients. Nutr. (Burbank, Los Angel. County, Calif.) 32(10), 1110–1115 (2016).
Everett, B. M., Bansal, S., Rifai, N., Buring, J. E. & Ridker, P. M. Interleukin-18 and the risk of future cardiovascular disease among initially healthy women. Atherosclerosis 202(1), 282–288 (2009).
Alzahrani, A. H. et al. Effects of a self-prepared carbohydrate-reduced high-protein diet on cardiovascular disease risk markers in patients with type 2 diabetes. Nutrients 13(5), 1694 (2021).
Calder, P. C. et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 101(Suppl 1), S1-45 (2009).
Giugliano, D., Ceriello, A. & Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 48(4), 677–685 (2006).
Millar, S. R., Navarro, P., Harrington, J. M., Perry, I. J. & Phillips, C. M. Dietary quality determined by the healthy eating index-2015 and biomarkers of chronic low-grade inflammation: A cross-sectional analysis in middle-to-older aged adults. Nutrients 13(1), 222 (2021).
Boerwinkle, E. & Chan, L. A three codon insertion/deletion polymorphism in the signal peptide region of the human apolipoprotein B (APOB) gene directly typed by the polymerase chain reaction. Nucl. Acids Res. 17(10), 4003 (1989).
Machado, M. O., Hirata, M. H., Bertolami, M. C. & Hirata, R. D. Apo B gene haplotype is associated with lipid profile of higher risk for coronary heart disease in Caucasian Brazilian men. J. Clin. Lab. Anal. 15(1), 19–24 (2001).
Rantala, M., Rantala, T. T., Savolainen, M. J., Friedlander, Y. & Kesäniemi, Y. A. Apolipoprotein B gene polymorphisms and serum lipids: Meta-analysis of the role of genetic variation in responsiveness to diet. Am. J. Clin. Nutr. 71(3), 713–724 (2000).
Mannu, G. S., Zaman, M. J., Gupta, A., Rehman, H. U. & Myint, P. K. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr. Cardiol. Rev. 9(1), 2–14 (2013).
Pajukanta, P. E. et al. The effects of the apolipoprotein B signal peptide (ins/del) and XbaI polymorphisms on plasma lipid responses to dietary change. Atherosclerosis 122(1), 1–10 (1996).
Julibert, A. et al. Total and subtypes of dietary fat intake and its association with components of the metabolic syndrome in a Mediterranean population at high cardiovascular risk. Nutrients 11(7), 1493 (2019).
Hannon, B. A. et al. Dietary fiber is independently related to blood triglycerides among adults with overweight and obesity. Curr. Dev. Nutr. 3(2), nzy094 (2019).
Kell, K. P., Cardel, M. I., Bohan Brown, M. M. & Fernández, J. R. Added sugars in the diet are positively associated with diastolic blood pressure and triglycerides in children. Am. J. Clin. Nutr. 100(1), 46–52 (2014).
Vasselli, J. R., Scarpace, P. J., Harris, R. B. & Banks, W. A. Dietary Components in the Development of Leptin Resistance (Oxford University Press, 2013).
Sharma, R., Mahajan, M., Singh, B., Singh, G. & Singh, P. Role of the APOB gene polymorphism (c. 12669G> A, p. Gln4154Lys) in coronary artery disease in the Indian Punjabi population. Balkan J. Med. Genet. 14(2), 35–40 (2011).
Patel, S. B., Reams, G. P., Spear, R. M., Freeman, R. H. & Villarreal, D. Leptin: Linking obesity, the metabolic syndrome, and cardiovascular disease. Curr. Hypertens. Rep. 10(2), 131–137 (2008).
Davoudi-Kiakalayeh, A., Mohammadi, R., Pourfathollah, A. A., Siery, Z. & Davoudi-Kiakalayeh, S. Alloimmunization in thalassemia patients: New insight for healthcare. Int. J. Prev. Med. 8, 101 (2017).
Puchau, B., Zulet, M., Echávarri, A., Hermsdorff, H. H. & Alfredo, M. Dietary total antioxidant capacity: A novel indicator of diet quality in healthy young adults. J. Am. Coll. Nutr. 28, 648–656 (2009).
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 4(8), 118 (2010).
Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 59(1), 43–49 (2011).
Zanella, P., August, P., Alves, F., Matte, C. & de Souza, C. G. Association of healthy eating index and oxidative stress in adolescent volleyball athletes and non-athletes. Nutrition 60, 230 (2019).
Heidari, N. et al. The association between healthy eating index and serum antioxidant indices in patients with different degrees of cataract compared to healthy subjects: A case-control study. Nutr. Food Sci. 51, 426–438 (2020).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet (Lond., Engl.) 380(9841), 572–580 (2012).
Acknowledgements
The authors would like to thank Tehran University of Medical Sciences for fund provision (Grant No: 20414) as well as the participants in this investigation.
Funding
Tehran University of Medical Sciences provided fund for this investigation (Grant No: 20414).
Author information
Authors and Affiliations
Contributions
E.K. Conceptualized; Investigated and Wrote the Original draft. G.S. Supervised; Validated and provided Resources. M.R. Performed Formal analysis and Supervision and Wrote the review & editing. F.K. Conceptualized, Supervised; provided Funding and Project performed administration. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karimi, E., Sotoudeh, G., Rafiee, M. et al. Dietary quality indices modify the effects of apolipoprotein B polymorphisms on biochemical and anthropometric factors in type 2 diabetes mellitus. Sci Rep 11, 22395 (2021). https://doi.org/10.1038/s41598-021-01884-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01884-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.