Abstract
The genetic heterogeneity of sensorineural hearing loss (SNHL) is a major hurdle to the detection of disease-causing variants. We aimed to identify underlying causal genes associated with mid-frequency hearing loss (HL), which contributes to less than about 1% of SNHL cases, by whole exome sequencing (WES). Thirty families segregating mid-frequency SNHL, in whom biallelic GJB2 mutations had been previously excluded, were selected from among 851 families in our DNA repository of SNHL. DNA samples from the probands were subjected to WES analysis and searched for candidate variants associated with SNHL. We were able to identify the genetic aetiology in six probands (20%). In total, we found three pathogenic and three likely pathogenic variants in four genes (COL4A5, OTOGL, TECTA, TMPRSS3). One more proband was a compound heterozygote for a pathogenic variant and a variant of uncertain significance (VUS) in MYO15A gene. To date, MYO15A and TMPRSS3 have not yet been described in association with mid-frequency SNHL. In eight additional probands, eight candidate VUS variants were detected in five genes (DIAPH1, MYO7A, TECTA, TMC1, TSPEAR). Seven of these 16 variants have not yet been published or mentioned in the available databases. The most prevalent gene was TECTA, identified in 23% of all tested families. Furthermore, we confirmed the hypothesis that a substantive portion of cases with this conspicuous audiogram shape is a consequence of a genetic disorder.
Similar content being viewed by others
Introduction
Hearing loss (HL) is the most common sensory deficit, affecting 466 million people (5% of the world’s population) and with an incidence of 1.45/1000 newborns1,2. At least half of congenital deafness cases is due to genetic factors, the vast majority of which is monogenic. In syndromic HL, a specific phenotype may narrow the selection of candidate genes for targeted genetic analysis. However, in the case of non-syndromic hearing impairment, such preselection of candidate genes is very limited. Non-syndromic hereditary HL is also characterized by extreme genetic heterogeneity. More than 6000 mutations in more than 150 genes have been causally implicated in deafness3. This underscores the importance of the use of massively parallel sequencing for genetic diagnostics of HL.
Pure tone audiometry (0.25–8 kHz) is the gold standard for measuring hearing in older children and adults4. It allows determination of the degree of hearing impairment, assigns it to a specific type of audiometric curve and enables characterization of the distinct shapes of this curve (basocochlear, mediocochlear, pancochlear, apicocochlear, ski slope, etc.). The shape of the audiometric curve may also be indicative of certain diseases which commonly manifest by HL, e.g. the Carhart notch in otosclerosis5, the low-frequency HL found initially in Ménière's disease6 or in children with cytomegalovirus infection7,8.
Based on a detailed analysis of audiograms and the patient’s age, it is sometimes possible to predict the probable genetic cause of hearing impairment in patients with non-syndromic HL9. The high-frequency (basocochlear, descending) and pancochlear (flat) types of the audiometric curve dominate in clinical practice. On the other hand, audiograms characteristic for mid-frequency (mediocochlear, cookie-bite or U-shaped) and low-frequency (apicocochlear or ascending) hearing impairment are rare. Mid-frequency HL accounts for only about 0.7–1% of all SNHL cases10,11,12, and the majority of this HL is believed to have genetic aetiology13.
The aim of our study was to identify causative gene variants associated with mid-frequency hearing loss.
Results
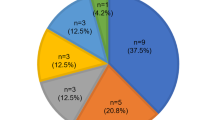
Using WES, we analyzed 30 families with mid-frequency HL, which represented 4.95% of all GJB2 unrelated SNHL cases in our cohort. We established the genetic aetiology of HL in six families (20%) and we assumed the cause of HL based on the detected candidate VUS variants in an additional eight families (27%). A compound heterozygote state of a pathogenic and a VUS variant was observed in one family (Table 1). Sixteen variants were found in total, seven of which are novel, i.e. they are not yet present in available deafness databases or the published literature. All detected variants occurred in genes known to be associated with HL (Table 2).
Dominant variants
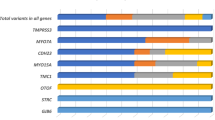
The most prevalent gene was TECTA (NM_005422.4) with five autosomal dominant missense variants (p.Cys650Arg, p.Gly1858Glu, p.Thr1866Met, p.Arg1890Cys, and p.Tyr1942Cys) and one frameshift variant (p.Lys2150Asnfs*9) detected in the heterozygous form in seven families. The pathogenic p.Arg1890Cys variant occurred in two families (FAM571, FAM623) (Fig. 1) and caused mild to moderately-severe HL. Over the years, the audiogram had a tendency to flatten in some of the affected family members. Another pathogenic variant, p.Thr1866Met, and the VUS p.Gly1858Glu variant were identified in families FAM622 and FAM642, respectively. Both families had several family members suffering from HL. They were not available for genetic testing, but the pedigrees suggest the presence of the autosomal dominant pattern of inheritance. In three families (FAM090, FAM200, and FAM417) only the proband’s DNA sample and reliable clinical data were available.
Dominant variants (all belonging to the VUS category) in other genes were detected in three families (Fig. 2). In family FAM806 we identified a novel p.Gly1197Asp variant in the DIAPH1 gene (NM_005219.5) in several family members. Hearing impairment in this family manifested in the age ranging from 2 to 15 years. A novel p.Pro650Leu variant in the TMC1 (NM_138691.3) gene was identified in an adult patient who self-reported HL onset by the age of 30 years. His twelve-year-old daughter, who also carries the p.Pro650Leu variant, still has normal hearing but may develop HL in the future, similarly as the proband. The last dominant p.Arg1338His variant in the MYO7A gene (NM_000260.4) was found in family FAM411, with late HL onset.
Recessive variants
We detected six recessive variants in genes MYO15A, OTOGL, TMPRSS3 and TSPEAR in four families (27%) (Fig. 2). Three families (FAM253, FAM834 and FAM837) were characterized by sporadic HL. Familial HL occurred in FAM174, with two affected siblings. We identified a pathogenic and VUS variant in the MYO15A gene (NM_016239.4) in family FAM837, which was characterized with early-onset HL. Due to rapid HL progression, the proband received a cochlear implant at the age of 9 years with excellent early outcomes (a free field pure tone average 38.75 dB at 10 months post-implantation). One pathogenic variant and one likely pathogenic variant in the OTOGL gene (NM_173591.5) were detected in the proband D1315 (family FAM834), who had congenital HL. In both families, it would be appropriate to investigate the genotype of the parents to exclude the cis form of the variants in the probands.
The two remaining families carrying homozygous variants are of Roma ethnicity. In family FAM253, with perilingual HL, we detected the known p.Arg106Cys variant in the TMPRSS3 gene (NM_024022.3). From a clinical point of view, the proband’s hearing gradually deteriorated to deafness between the 4th and 14th year of life. In the last family, FAM174, we detected VUS variant p.Glu624* in the TSPEAR gene (NM_144991.3). The proband’s sister (D1032) is also affected by HL but interestingly was found to only be a heterozygous carrier of the p.Glu624* variant. The HL phenotype was similar in both siblings.
X-linked
In family FAM618 we identified a novel variant p.Gly472Glu in the COL4A5 gene (NM_000495.5) in two affected siblings (D815, D817), both of whom showed a clinical picture of Alport syndrome. In addition to HL, they had both suffered from microscopic haematuria since childhood as well as symptoms of chronic glomerulonephritis. In subject D817, Alport syndrome was confirmed by kidney biopsy and the condition has progressively led to end-stage kidney failure with the need for haemodialysis.
Discussion
Mutations in GJB2 have been reported as the most frequent cause of hereditary HL14,15. For this reason, we first analyzed the GJB2 gene by Sanger sequencing in all 851 probands. Using this method, we determined the genetic aetiology in 245 (29%) of the probands, who were then excluded from further analyses.
In the next step, the remaining probands were selected based on the clinical phenotype. Although we focused on a relatively uniform group of patients from the audiological point of view (mid-frequency HL), the number of potentially involved genes was large (> 226). Moreover, the spectrum of the genes responsible for this particular form of HL is mostly unknown. It is not practical to analyze each gene separately in such a case. Therefore, next generation sequencing (NGS) allowing the analysis of many genes simultaneously seemed to be the best solution16,17.
Using WES, we identified the genetic aetiology in six of the 30 probands (20%) with mid-frequency HL. Additionally, novel candidate variants (VUS) were found in eight probands. A compound heterozygous pathogenic and a VUS variant were observed in one proband. The detected variants were found in nine genes (COL4A5, DIAPH1, MYO7A, MYO15A, OTOGL, TECTA, TMC1, TMPRSS3, TSPEAR) harbouring four pathogenic variants, three likely pathogenic variants and nine VUS variants associated with hearing impairment. Seven of these variants have not yet been described in the literature or have not yet been submitted to deafness databases (Table 2). Five of the identified genes (MYO7A, MYO15A, TMC1, TMPRSS3 and TSPEAR) have not yet been reported as a cause of mid-frequency SNHL. Other genes which are known to cause non-syndromic mid-frequency HL but were not detected in our cohort include: CCDC5018, COL11A119, COL11A220,21, EYA422, KCNQ423, OTOA24 and POU4F325. Among these genes, we were able to identify three distinct groups based on the site of their expression and their function18,26,27,28,29.
Glycoproteins expressed in the tectorial membrane
The tectorial membrane (TM) of the cochlea is a ribbon-like strip of extracellular matrix that contacts the tips of the sensory hair bundles of the outer hair cells. Sound induces movement of these hair cells relative to the TM, deflects the stereocilia, and leads to fluctuations in hair-cell membrane potential, transducing sound into electrical signals30,31.
The TECTA gene encodes a protein called alpha-tectorin. Mutations in TECTA account for 2–4% of all autosomal dominant non-syndromic SNHL characterized by mild prelingual HL from a clinical point of view15,32,33,34. TECTA contains several extracellular matrix (ECM)-interacting domains, including a nidogen-like domain and von Willebrand factor type D domains (vWFDs), which may allow binding to collagens and other glycoproteins, and a zona pellucida (ZP) domain important for the multimerization and stability of the protein. Missense mutations in different Tecta domains are known to cause different phenotypes with distinct changes in the structure of the tectorial membrane. Mutations in the zona pellucida domain cause mid-frequency HL with a typical U-shaped or shallow U-shaped audiogram, whereas mutations in the zonadhesin-like domain comprising vWFD1, vWFD2 and trypsin inhibitor-like (TIL) repeats lead to high-frequency HL35,36,37. Our results are in agreement with the findings of previous studies: four of our variants (p.Gly1858Glu, p.Thr1866Met, p.Arg1890Cys and p.Tyr1942Cys) located in the ZP domain were identified in probands with a U-shaped audiogram. However, the p.Cys650Arg variant identified in the cysteine-rich TIL1 domain is responsible for mild HL, which became clinically manifest at the age of 16 years. The audiometric curve has a shallow U-shape profile. Similarly, Yasukawa et al.37 identified the likely pathogenic p.Cys606Gly variant in the TIL1 domain in a proband with mid-frequency HL. Variant p.Lys2150Asnfs*9 is located in the last exon and causes replacement of the last six amino acids and extension of the glycosylphosphatidylinositol (GPI) anchorage of TECTA protein by two amino acids. It has been shown that GPI-dependent release of TECTA is required for elongation and bundled organization of collagen fibrils on the apical surface of inner supporting cells during formation of the TM38. Therefore, any change in the conformation or length of the GPI anchorage may impair this process and lead to a clinical phenotype. However, further study of these mechanisms is required. In our study, 10% of all probands with a U-shaped audiogram carried pathogenic variants in TECTA, and when we also take into account all the identified VUS variants, the theoretical contribution of TECTA to hearing impairment in our cohort may be as high as 47%. This is more than the published prevalence of TECTA in patients with U-shaped audiograms, for example in Japan, where 6% of patients with U-shaped audiograms could be attributed to TECTA36. The difference may be explained by high variability in the prevalence of causal deafness variants and genes among different ethnic populations as a well-known phenomenon in hereditary HL39.
The OTOGL gene encodes an otogelin-like protein, which has structural similarities to the epithelial-secreted mucin protein family40. Its mutations lead to non-syndromic prelingual HL, which is mild to moderate and stable, with autosomal recessive inheritance41. The 2,343-amino acid protein contains an N-terminal signal peptide, four paired von Willebrand factor (VWF) and cysteine-rich (C8) domains, four trypsin inhibitor-like (TIL) domains, and a C-terminal cystine knot motif40. We identified two heterozygous variants in proband D1315. The first is the known pathogenic nonsense variant p.Gln520* in vWFD242. The second likely pathogenic variant, c.4032_4054+30del, has not yet been published. It is located at the interface of the 34th exon and 34th intron and, according to HSF and MaxEnt, it can cause an alteration of splicing due to activation of several cryptic acceptor and donor sites. However, we cannot rule out exon skipping, which may result in a more significant shortening of the protein sequence located between domains vWFD3 and vWFD4. The exon skipping does not lead to a frameshift, and therefore, according to Abou Tayoun et al.43, only the PVS1_strong classification criterion can be applied. On the other hand, vWFD domains containing the multimerization consensus site CGLC are essential for the multimer assembly of proteins expressed in the tectorial membrane (like α-tectorin, otogelin or otogelin-like protein) to form a filament and higher order structures. Although little is known about these domains in OTOGL, mutations in the vWFDs of TECTA have been clearly connected with hearing impairment37,44. Moreover, the shape of the audiometric curve observed in our proband is almost identical to the audiogram obtained from the compound heterozygous patient carrying mutations p.Gln520* and p.Arg925* reported by Bonnet et al.42. The vestibular hypofunction observed in patients by Oonk et al.45 was not diagnosed in our proband.
Collagens expressed in the cochlea
The collagen superfamily comprises 28 types (I–XXVIII) in vertebrates46. Collagens are fibrous structural proteins involved in the construction of skin, cartilage, bone, eye, and other tissues47. All collagen molecules are comprised of one, two, or three different types of α-chain subunits tightly wrapped into a triple helix. A single missense mutation of a glycine to another residue in the triple helix can result in almost 40 genetic diseases46. Collagens encoded by genes COL2A1, COL4A3, COL4A4, COL4A5, COL4A6, COL9A1, COL9A3, COL11A1 and COL11A2 are essential for proper auditory function. Mutations in these genes are linked to non-syndromic or syndromic HL (Marshall syndrome, fibrochondrogenesis, Alport syndrome, Stickler syndrome)19,48. In this study, we present a novel variant in the COL4A5 gene. Variants in this gene are associated with X-linked Alport syndrome49. In 80% of all cases, Alport syndrome is caused by mutations in the COL4A5 gene50. The disorder shows considerable heterogeneity in the age of onset of end-stage renal disease and the occurrence of deafness among particular families51. Most patients have mid-frequency HL, although high frequency and flat SNHL curves may be present, as well. SNHL in males may first manifest around the age of 10 years and usually gets progressively worse49,52. In family FAM618 we identified the variant p.Gly472Glu, which interrupts the Gly-Val-Lys triplet in the α-chain and results in disruption of the triple helix’s function, changing the scaffolding and flexibility of collagen IV46. This results in an Alport syndrome phenotype in both siblings (D815, D817).
Genes expressed in hair cells
Myosins play an important role in the cellular organization of the cochlea. In general, myosins are motor proteins, and based on their variable C-terminal binding domains, they are included in conventional myosins (class II) and unconventional myosins (classes I and III to XV)53. Unconventional myosins IA, IIIA, VI, VIIA and XVA occur in the cochlea. MYO7A and MYO15A, encoding unconventional myosins, are expressed in the outer and inner hair cells of the Organ of Corti54,55.
MYO15A is known to be the third-most mutated gene causing severe to profound ARNSHL, after GJB2 and SLC26A4 mutations. Mutations in this gene cause both pre- and post-lingual forms of progressive HL41,56. Our patient D1321 clinically manifests profound non-syndromic HL. Interestingly, she passed the neonatal hearing screening, and her HL became apparent at the age of 1.5 years. We identified two variants, c.5693G>A and c.8050T>C, the latter of which was previously described in trans with other pathogenic variants57,58, whereas c.5693G>A variant was submitted only to the ClinVar archive and to the Deafness Variation Database (https://deafnessvariationdatabase.org/)59. It is located in the myosin motor domain of the MYO15A protein. This domain consists of ATP- and actin-binding sites, which can generate force and move actin filaments; therefore, motor domain dysfunction results in shorter stereocilia with an ectopic staircase structure of stereocilia associated with a severe deafness phenotype60.
The MYO7A tail contains a pair of myosintail homology 4-protein 4.1, ezrin, radixin, moesin (MyTH4-FERM) tandems separated by an SH3 domain. Mutations in the head and tail regions of MYO7A lead to defects in mechanotransduction and result in Usher syndrome 1B (USH1B), an autosomal recessive disorder with sensory impairment61. In addition to syndromic conditions, mutations in MYO7A can cause dominant (DFNA11)62 and recessive (DFNB2)63 non-syndromic HL. The p.Arg1338His variant detected in FAM411 is located in the FERM 1 domain of the tail of the myosin VIIA protein. The nonsense variants p.Arg1338AlafsTer61, p.Gln1336Ter or p.Glu1337SerfsTer62, linked to Usher syndrome, were identified close to our identified p.Arg1338His variant. VUS missense variants p.Arg1338Ser or p.Arg1338Cys with unclear clinical phenotype were also identified (https://deafnessvariationdatabase.org/). Our proband had HL onset at the age of 22 years with mild progression of hearing impairment and a U-shape audiogram. Moreover, no retinal abnormalities were recorded, indicating the dominant non-syndromic HL.
Transmembrane channel-like protein 1 is a protein encoded by the TMC1 gene. The predicted structure of TMC1 shows a dimeric channel with 10 transmembrane domains (S1–S10) and intracellular amino- and carboxyl-termini in each monomer. Transmembrane helices S4–S7 form a groove that lines the pore of sensory transduction channels64. The transmembrane channels are proteins necessary for hair cell mechanotransduction65. Dominant mutations in TMC1 cause the late-onset progressive HL phenotype, whereas ARSNHL cases are linked to congenital severe-to-profound HL66,67. We identified a missense variant, c.1949C>T, which is predicted to substitute a conserved proline at position 650 for leucine, which may influence the correct folding or assembly of TMC1 into multimers. It is located in the helical S9 domain. Two pathogenic autosomal recessive variants were previously identified in close proximity to our variant: p.Met654Val68 and p.Ser647Pro69. However, according to the phenotype observed in FAM416, we cannot rule out the dominant character of inheritance of the p.Pro650Leu variant or a yet undetected causal variant in a different gene. One young family member, D1337, showed still normal hearing, although she carried the same variant as her father, thus supporting the possibility of late-onset HL.
The TMPRSS3 gene encodes a member of the serine protease family. Transmembrane Serine Protease 3 is linked to hair cell sterocilia mechanics and to actin network formation, which supports cell motility and integrity. Apart from hair cells, the TMPRSS3 gene is also expressed in the inner and outer pillar cells and the Deiters cells, stria vascularis or ganglion spirale70,71. This gene was identified by its association with childhood onset of an autosomal recessive deafness. The hearing impairment in families with mutations in the TMPRSS3 gene has a prelingual to postlingual onset. A common feature of patients with TMPRSS3 mutations is progressive HL beginning in high frequencies and often leading to partial or complete deafness. However, Sasamori et al.72 reported a case of progressive mid- to low-frequency sensorineural HL associated with mutation of the TMPRSS3 gene. We identified a likely pathogenic variant, p.Arg106Cys, which is located within exon 4 and causes the negatively charged residue arginine to change to a neutral hydrophobic cysteine at position 106. This change can result in the loss of hydrogen bonds and/or disturb the correct folding of TMPRSS3. It has been observed in the compound heterozygote state with p.(Phe13Serfs∗12) and p.Ala306Thr, where it cosegregated with prelingual profound hearing impairment or postlingual milder hearing impairment, respectively70. The homozygous form observed in proband D298 has not previously been observed. Although Gao et al.70 predicted a very subtle or no effect on auditory function of homozygous p.Arg106Cys, we have shown that it is associated with moderate to severe perilingual progressive HL with a typical U-shape audiogram.
The DIAPH1 gene encodes human protein DIA1, a formin that elongates unbranched actin73,74. The correct polymerization of actin is critical for the formation and elongation of stereocilia on the apical surface of cochlear hair cells75. Expression of DIAPH1 in the Organ of Corti has been proven in the inner pillar hair cells as well as at the base of the outer hair cells and in the outer pillar cells76. DIA1 consists of the GTPase-binding domain (GBD), a partially overlapping N-terminal diaphanous inhibitory domain (DID), formin homology (FH) domains FH1 and FH2, and the C-terminal DAD. It is held inactive in the resting state through an autoinhibitory intramolecular interaction between the DID and the DAD, which is regulated by Rho family GTPases74. In this study, we identified the VUS variant p.Gly1197Asp which, according to the Human Splicing Finder, has no significant impact on splicing signals. It is located in close proximity to the MDxLLxxL recognition site (1199-1206aa), which presents the central consensus motif of the DAD domain and is essential for binding to the DID domain. We hypothesize that our missense c.3590G>A variant results in a change from a small, uncharged amino acid glycine to a larger, charged residue aspartate. This may have a profound effect on the conformation of the amphipathic helix of the DAD domain and subsequently impair interaction with the acidic groove of the DID. Disruption of the autoinhibitory intramolecular DID-DAD interaction and consequent activation of DIA1 may explain the DFNA1 pathology74. DIAPH1 mutations have been associated with initially mild, low-frequency SNHL, characterized by progressive deafness starting in childhood73. In contrast, some described variants showed hearing impairment beginning in the high-frequency range and progressing to deafness involving all frequencies74. Audiograms of our proband and family members showed a typical U-shape. Autosomal dominant deafness caused by mutations in the DIAPH1 gene can be associated with thrombocytopenia76,77. However, in the affected family members D1248, D1249 and D1250 no thrombocytopenia was confirmed suggesting non-syndromic HL. The only pathological finding identified by differential blood count analysis was neutropenia in the family member D1250.
The TSPEAR gene encodes the thrombospondin-type laminin G domain and EAR repeats protein (TSPEAR), which was detected at the basal region of the stereocilia of hair cells. Mutations in TSPEAR cause non-syndromic SNHL with prelingual onset78. We identified the nonsense variant p.Glu624* in exone 12 resulting in a loss of the EAR7 repeat. Seven epilepsy-associated repeats (EARs) of TSPEAR are predicted to form a beta-propeller structure, function as part of a ligand-binding domain and mediate protein–protein interactions79. Delmaghani et al.78 identified in 3 affected brothers from a consanguineous Iranian family segregating autosomal recessive nonsyndromic sensorineural deafness a homozygous frame-shift mutation (c.1726G>T+c.1728delC) that was predicted to result in termination of translation in the EAR6 repeat, connected with defective secretion of the mutant protein and congenital profound sensorineural deafness. As both affected siblings in our family FAM174 segregated a similar HL phenotype, but only one of them carried the homozygous variant in TSPEAR, we may assume that in addition to the identified p.Glu624* variant in the TSPEAR gene a second, yet unknown gene may contribute to HL in this family.
In general, autosomal recessive variants are mainly responsible for prelingual forms of severe to profound hearing impairment, whereas autosomal dominant variants cause HL that usually appears later and is often mild or moderate. Exceptions comprising postlingual HL include recessive variants in the TMPRSS3 gene; prelingual include dominant variants in the TECTA gene32.
The precise data on the prevalence of pathogenic variants in the genes described above in the general and hearing impaired populations in Slovakia are not yet available. Most of the variants were found in single families, although pathogenic variants in TMPRSS3 were also identified in two probands with typical ski-slope audiograms (unpubl. data).
Conclusions
This study confirms the genetic background in at least 20% of patients with mid-frequency SNHL. Two of the identified genes (TMPRSS3 and MYO15A) are reported in association with this audiogram configuration for the first time. Moreover, we attempted to narrow the spectrum of candidate genes in this specific and rare SNHL form to 3 distinct groups based on the site of their expression and their function in the cochlea. In our opinion, Sanger sequencing of TECTA or WES are currently two cost-effective options for DNA analysis of mid-frequency SNHL.
Methods
Patients recruitment
Patients with mid-frequency HL were selected from among 851 families (1074 hearing impaired individuals) with bilateral SNHL with onset before the age of 60 years whose DNA samples and clinical data were collected throughout the whole Slovak Republic over the last decade. The subjects were referred from major ENT departments in Bratislava or recruited at boarding schools for hearing-disabled children and in selected isolated Roma communities. Peripheral blood and/or buccal swabs were taken for DNA isolation. The GJB2 gene was analyzed in all probands first to exclude the most frequent cause of hereditary HL. Only non-GJB2 associated cases (71% of all probands) were considered for further analyses. All individuals or their legal representatives (in subjects under 18 years of age) signed informed consent to genetic testing and the study was approved by the Ethics Committee of University Hospital in Bratislava.
Mediocochlear HL was defined by the highest pure tone thresholds in mid-frequencies (1–4 kHz). In typical cases, the difference between the worst mid-frequency threshold and the high- and low-frequency thresholds was at least 20 dB. However, individual variability of the audiometric curve shapes was relatively common among the patients and sometimes also between the two ears of the same individual. Borderline cases with shallow curves were included only if the mid-frequency HL was confirmed to be reproducible during the audiometric follow-up. Preschool children and subjects in whom reliable audiometric data were not available were excluded from the study. Based on the criteria mentioned above, we selected 30 probands (27 of Caucasian and 3 of Roma ethnicity) with mid-frequency HL of yet unknown aetiology.
Whole exome sequencing
Probands meeting the inclusion criteria were selected for WES analysis. Genomic DNA was extracted from peripheral blood using standard procedures. WES was performed by service providers: BGI, Hong Kong, and Theragen, Republic of Korea. A DNA library was prepared using the BGI 59M Human Exome kit (probands D527, D753, D815, D821 and D822) and the SureSelect XT V6 kit (all remaining probands), respectively. Sequencing data were processed by the provider’s standard bioinformatics pipeline, which encompassed base calling and alignment of generated reads to the GRCh38 reference genome without the unplaced or alternate loci. Variant calling was routinely performed in-house using The Genetic Analysis Tool Kit (GATK) HaplotypeCaller, combined with the GATK UnifiedGenotyper in the case of BGI samples80.
Aligned reads and called variants were obtained in standard bioinformatics formats and subjected to the following bioinformatics pipeline. Variants were decomposed and normalized using vt81. Variant effect predictor82 was used to annotate the variants with respect to their potential effects on genes and transcripts and to add scores from in silico prediction algorithms PolyPhen and SIFT83. Finally, the Gemini framework84 was used for submitting variants into a newly created SQLite database and for annotating variants with additional data from genome annotation databases, e.g. the Single Nucleotide Polymorphism Database (dbSNP)85, Encyclopedia of DNA Elements (ENCODE) (The ENCODE Project Consortium), ClinVar86, 1000 Genomes (The 1000 Genomes Project Consortium), the Exome Sequencing Project (ESP) (http://evs.gs.washington.edu/EVS/), Exome Aggregation Consortium (ExAC) and the Genome Aggregation Database gnomAD87. Afterwards, variant prioritization and filtering were carried out by removing those variants with an MAF ≥ 0.01 (based on maximal MAF in all used population databases), and the resulting variant set was first narrowed down by removing variants not lying inside the regions of 226 genes with known association with SNHL. The set of evaluated SNHL genes was based mainly on the webpage https://hereditaryhearingloss.org/. In the case that no candidate variants were found among the virtual panel of 226 genes, the whole exome was evaluated. Candidate variants were clinically classified according to the American College of Medical Genetics and Genomics (ACMG) guidelines88 adapted by Oza et al.89, with modification for the PVS1 criterion according to Abou Tayoun et al.43. The VUS variants were selected as candidates only if they belonged to previously reported deafness genes. The limited number of available family members needed for cosegregation analysis makes it difficult to classify new gene variants among novel genes. Moreover, the further classification of VUS variant in unknown genes would be inconclusive without functional studies.
Sanger sequencing
Sanger sequencing was performed to verify variants identified by WES and to determine co-segregation of the candidate variants with HL in all participating family members. The primers were designed using the program Primer Blast90. The resulting PCR products were sequenced using the Big Dye Terminator v3.1 kit and analyzed with the ABI3500 Genetic Analyzer. Sequence chromatograms were analyzed by Sequence Scanner Software 2.0 (available from http://www.thermofisher.com/).
All methods were performed in accordance with the relevant guidelines and regulations. Copy Number Variants (CNV) were not investigated. Some of the unsolved families could be due to CNVs.
References
Kral, A. & O’Donoghue, G. M. Profound deafness in childhood. N. Engl. J. Med. 363, 1438–1450. https://doi.org/10.1056/NEJMra0911225 (2010).
Schmucker, C., Kapp, P., Motschall, E., Loehler, J. & Meerpohl, J. J. Prevalence of hearing loss and use of hearing aids among children and adolescents in Germany: a systematic review. BMC Public Health 19, 1277. https://doi.org/10.1186/s12889-019-7602-7 (2019).
Sloan-Heggen, C. M. et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Hum. Genet. 135, 441–450. https://doi.org/10.1007/s00439-016-1648-8 (2016).
Barbour, D. L. et al. Online machine learning audiometry. Ear Hear. 40, 918–926. https://doi.org/10.1097/AUD.0000000000000669 (2019).
Sabbe, A., Verhaert, N., Joossen, I., Lammens, A. & Debruyne, F. Otosclerosis: shift in bone conduction after stapedotomy. B-ENT 11, 183–189 (2015).
Ghazavi, H. & Asadpour, L. Hearing LOSS in Meniere’s disease. Am. J. Otolaryngol. 38, 367. https://doi.org/10.1016/j.amjoto.2017.01.025 (2017).
McCollister, F. P. et al. Hearing loss and congenital symptomatic cytomegalovirus infection: a case report of multidisciplinary longitudinal assessment and intervention. J. Am. Acad. Audiol. 7, 57–62 (1996).
Misono, S. et al. Congenital cytomegalovirus infection in pediatric hearing loss. Arch. Otolaryngol. Head Neck Surg. 137, 47–53. https://doi.org/10.1001/archoto.2010.235 (2011).
Taylor, K. R. et al. AudioGene: predicting hearing loss genotypes from phenotypes to guide genetic screening. Hum. Mutat. 34, 539–545. https://doi.org/10.1002/humu.22268 (2013).
Shah, R. K., Blevins, N. H. & Karmody, C. S. Mid-frequency sensorineural hearing loss: aetiology and prognosis. J. Laryngol. Otol. 119, 529–533. https://doi.org/10.1258/0022215054352216 (2005).
Demeester, K. et al. Audiometric shape and presbycusis. Int. J. Audiol. 48, 222–232. https://doi.org/10.1080/14992020802441799 (2009).
Birkenbeuel, J. et al. Characteristics of mid-frequency sensorineural hearing loss progression. Otol. Neurotol. 40, e497–e502. https://doi.org/10.1097/MAO.0000000000002232 (2019).
Wong, L. & Bance, M. Are all cookie-bite audiograms hereditary hearing loss?. J. Otolaryngol. 33, 390–392. https://doi.org/10.2310/7070.2004.00390 (2004).
Taniguchi, M. et al. Carrier frequency of the GJB2 mutations that cause hereditary hearing loss in the Japanese population. J. Hum. Genet. 60, 613–617. https://doi.org/10.1038/jhg.2015.82 (2015).
Vona, B., Doll, J., Hofrichter, M. A. H. & Haaf, T. Non-syndromic hearing loss: clinical and diagnostic challenges. Med. Gen. 32(2), 117–129. https://doi.org/10.1515/medgen-2020-2022 (2020).
Choi, M. et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc. Natl. Acad. Sci. USA 106, 19096–19101. https://doi.org/10.1073/pnas.0910672106 (2009).
Yan, D., Tekin, M., Blanton, S. H. & Liu, X. Z. Next-generation sequencing in genetic hearing loss. Genet. Test Mol. Biomark. 17, 581–587. https://doi.org/10.1089/gtmb.2012.0464 (2013).
Modamio-Hoybjor, S. et al. A mutation in CCDC50, a gene encoding an effector of epidermal growth factor-mediated cell signaling, causes progressive hearing loss. Am. J. Hum. Genet. 80, 1076–1089. https://doi.org/10.1086/518311 (2007).
Booth, K. T. et al. Splice-altering variant in COL11A1 as a cause of nonsyndromic hearing loss DFNA37. Genet. Med. 21, 948–954. https://doi.org/10.1038/s41436-018-0285-0 (2019).
McGuirt, W. T. et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nat. Genet. 23, 413–419. https://doi.org/10.1038/70516 (1999).
Chen, W. et al. Mutation of COL11A2 causes autosomal recessive non-syndromic hearing loss at the DFNB53 locus. J. Med. Genet. 42, e61. https://doi.org/10.1136/jmg.2005.032615 (2005).
van Beelen, E. et al. Audiometric characteristics of a Dutch DFNA10 family with mid-frequency hearing impairment. Ear Hear. 37, 103–111. https://doi.org/10.1097/AUD.0000000000000217 (2016).
Jung, J. et al. Whole-exome sequencing identifies two novel mutations in KCNQ4 in individuals with nonsyndromic hearing loss. Sci. Rep. 8, 16659. https://doi.org/10.1038/s41598-018-34876-9 (2018).
Sugiyama, K. et al. Mid-frequency hearing loss is characteristic clinical feature of OTOA-associated hearing loss. Genes (Basel). https://doi.org/10.3390/genes10090715 (2019).
Kitano, T. et al. POU4F3 mutation screening in Japanese hearing loss patients: Massively parallel DNA sequencing-based analysis identified novel variants associated with autosomal dominant hearing loss. PLoS ONE 12, e0177636. https://doi.org/10.1371/journal.pone.0177636 (2017).
Kharkovets, T. et al. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 25, 642–652. https://doi.org/10.1038/sj.emboj.7600951 (2006).
Wang, L. et al. Eya4 regulation of Na+/K+-ATPase is required for sensory system development in zebrafish. Development 135, 3425–3434. https://doi.org/10.1242/dev.012237 (2008).
Kim, B. J. et al. Clarification of glycosylphosphatidylinositol anchorage of OTOANCORIN and human OTOA variants associated with deafness. Hum. Mutat. 40, 525–531. https://doi.org/10.1002/humu.23719 (2019).
Xu, F., Yan, W. & Cheng, Y. Pou4f3 gene mutation promotes autophagy and apoptosis of cochlear hair cells in cisplatin-induced deafness mice. Arch. Biochem. Biophys. 680, 108224. https://doi.org/10.1016/j.abb.2019.108224 (2020).
Goodyear, R. J. & Richardson, G. P. Extracellular matrices associated with the apical surfaces of sensory epithelia in the inner ear: molecular and structural diversity. J. Neurobiol. 53, 212–227. https://doi.org/10.1002/neu.10097 (2002).
Richardson, G. P., Lukashkin, A. N. & Russell, I. J. The tectorial membrane: one slice of a complex cochlear sandwich. Curr. Opin. Otolaryngol. Head Neck Surg. 16, 458–464. https://doi.org/10.1097/MOO.0b013e32830e20c4 (2008).
Shearer, A. E., Hildebrand, M. S. & Smith, R. J. H. In GeneReviews((R)) (eds M. P. Adam et al.) (1993).
Hildebrand, M. S. et al. DFNA8/12 caused by TECTA mutations is the most identified subtype of nonsyndromic autosomal dominant hearing loss. Hum. Mutat. 32, 825–834. https://doi.org/10.1002/humu.21512 (2011).
Moteki, H. et al. TECTA mutations in Japanese with mid-frequency hearing loss affected by zona pellucida domain protein secretion. J. Hum. Genet. 57, 587–592. https://doi.org/10.1038/jhg.2012.73 (2012).
Legan, P. K. et al. Three deaf mice: mouse models for TECTA-based human hereditary deafness reveal domain-specific structural phenotypes in the tectorial membrane. Hum. Mol. Genet. 23, 2551–2568. https://doi.org/10.1093/hmg/ddt646 (2014).
Yamamoto, N. et al. Prevalence of TECTA mutation in patients with mid-frequency sensorineural hearing loss. Orphanet. J. Rare Dis. 12, 157. https://doi.org/10.1186/s13023-017-0708-z (2017).
Yasukawa, R. et al. The prevalence and clinical characteristics of TECTA-associated autosomal dominant hearing loss. Genes (Basel). https://doi.org/10.3390/genes10100744 (2019).
Kim, D. K. et al. The release of surface-anchored alpha-tectorin, an apical extracellular matrix protein, mediates tectorial membrane organization. Sci. Adv. https://doi.org/10.1126/sciadv.aay6300 (2019).
Masindova, I. et al. MARVELD2 (DFNB49) mutations in the hearing impaired Central European Roma population–prevalence, clinical impact and the common origin. PLoS ONE 10, e0124232. https://doi.org/10.1371/journal.pone.0124232 (2015).
Yariz, K. O. et al. Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. Am. J. Hum. Genet. 91, 872–882. https://doi.org/10.1016/j.ajhg.2012.09.011 (2012).
Oonk, A. M., Huygen, P. L., Kunst, H. P., Kremer, H. & Pennings, R. J. Features of autosomal recessive non-syndromic hearing impairment: a review to serve as a reference. Clin. Otolaryngol. 41, 487–497. https://doi.org/10.1111/coa.12567 (2016).
Bonnet, C. et al. Biallelic nonsense mutations in the otogelin-like gene (OTOGL) in a child affected by mild to moderate hearing impairment. Gene 527, 537–540. https://doi.org/10.1016/j.gene.2013.06.044 (2013).
Abou Tayoun, A. N. et al. Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum. Mutat. 39, 1517–1524. https://doi.org/10.1002/humu.23626 (2018).
Pfister, M. et al. A genotype-phenotype correlation with gender-effect for hearing impairment caused by TECTA mutations. Cell Physiol. Biochem. 14, 369–376. https://doi.org/10.1159/000080347 (2004).
Oonk, A. M. et al. Similar phenotypes caused by mutations in OTOG and OTOGL. Ear Hear. 35, e84-91. https://doi.org/10.1097/AUD.0000000000000008 (2014).
Fidler, A. L., Boudko, S. P., Rokas, A. & Hudson, B. G. The triple helix of collagens—an ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. https://doi.org/10.1242/jcs.203950 (2018).
Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978. https://doi.org/10.1101/cshperspect.a004978 (2011).
Shpargel, K. B., Makishima, T. & Griffith, A. J. Col11a1 and Col11a2 mRNA expression in the developing mouse cochlea: implications for the correlation of hearing loss phenotype with mutant type XI collagen genotype. Acta Otolaryngol. 124, 242–248. https://doi.org/10.1080/00016480410016162 (2004).
Zhang, X. et al. X-linked Alport syndrome: pathogenic variant features and further auditory genotype-phenotype correlations in males. Orphanet. J. Rare Dis. 13, 229. https://doi.org/10.1186/s13023-018-0974-4 (2018).
Watson, S., Padala, S. A. & Bush, J. S. Alport Syndrome. in StatPearls, https://www.ncbi.nlm.nih.gov/pubmed/29262041 (2020).
Gross, O., Netzer, K. O., Lambrecht, R., Seibold, S. & Weber, M. Meta-analysis of genotype-phenotype correlation in X-linked Alport syndrome: impact on clinical counselling. Nephrol. Dial Transplant 17, 1218–1227. https://doi.org/10.1093/ndt/17.7.1218 (2002).
Sirimanna, K. S., France, E. & Stephens, S. D. Alport’s syndrome: can carriers be identified by audiometry?. Clin. Otolaryngol. Allied Sci. 20, 158–163. https://doi.org/10.1111/j.1365-2273.1995.tb00035.x (1995).
Mermall, V., Post, P. L. & Mooseker, M. S. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279, 527–533. https://doi.org/10.1126/science.279.5350.527 (1998).
Redowicz, M. J. Myosins and deafness. J. Muscle Res. Cell Motil. 20, 241–248. https://doi.org/10.1023/a:1005403725521 (1999).
Duman, D. & Tekin, M. Autosomal recessive nonsyndromic deafness genes: a review. Front. Biosci. (Landmark Ed) 17, 2213–2236. https://doi.org/10.2741/4046 (2012).
Farjami, M. et al. The worldwide frequency of MYO15A gene mutations in patients with non-syndromic hearing loss: a meta-analysis. Iran J. Basic Med. Sci. 23, 841–848. https://doi.org/10.22038/IJBMS.2020.35977.8563 (2020).
Cabanillas, R. et al. Comprehensive genomic diagnosis of non-syndromic and syndromic hereditary hearing loss in Spanish patients. BMC Med. Genomics 11, 58. https://doi.org/10.1186/s12920-018-0375-5 (2018).
Hirsch, Y. et al. A synonymous variant in MYO15A enriched in the Ashkenazi Jewish population causes autosomal recessive hearing loss due to abnormal splicing. Eur. J. Hum. Genet. 29, 988–997. https://doi.org/10.1038/s41431-020-00790-w (2021).
Azaiez, H. et al. Genomic landscape and mutational signatures of deafness-associated genes. Am. J. Hum. Genet. 103, 484–497. https://doi.org/10.1016/j.ajhg.2018.08.006 (2018).
Zhang, J. et al. Genotype-phenotype correlation analysis of MYO15A variants in autosomal recessive non-syndromic hearing loss. BMC Med. Genet. 20, 60. https://doi.org/10.1186/s12881-019-0790-2 (2019).
Jacobson, S. G. et al. Retinal disease course in Usher syndrome 1B due to MYO7A mutations. Invest. Ophthalmol. Vis. Sci. 52, 7924–7936. https://doi.org/10.1167/iovs.11-8313 (2011).
Luijendijk, M. W. et al. Identification and molecular modelling of a mutation in the motor head domain of myosin VIIA in a family with autosomal dominant hearing impairment (DFNA11). Hum. Genet. 115, 149–156. https://doi.org/10.1007/s00439-004-1137-3 (2004).
Riazuddin, S. et al. Mutation spectrum of MYO7A and evaluation of a novel nonsyndromic deafness DFNB2 allele with residual function. Hum. Mutat. 29, 502–511. https://doi.org/10.1002/humu.20677 (2008).
Pan, B. et al. TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99, 736-753.e736. https://doi.org/10.1016/j.neuron.2018.07.033 (2018).
Maeda, R. et al. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc. Natl. Acad. Sci. USA 111, 12907–12912. https://doi.org/10.1073/pnas.1402152111 (2014).
Makishima, T., Kurima, K., Brewer, C. C. & Griffith, A. J. Early onset and rapid progression of dominant nonsyndromic DFNA36 hearing loss. Otol. Neurotol. 25, 714–719. https://doi.org/10.1097/00129492-200409000-00011 (2004).
Nakanishi, H., Kurima, K., Kawashima, Y. & Griffith, A. J. Mutations of TMC1 cause deafness by disrupting mechanoelectrical transduction. Auris Nasus Larynx 41, 399–408. https://doi.org/10.1016/j.anl.2014.04.001 (2014).
Kurima, K. et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat. Genet. 30, 277–284. https://doi.org/10.1038/ng842 (2002).
Brownstein, Z. et al. Targeted genomic capture and massively parallel sequencing to identify genes for hereditary hearing loss in Middle Eastern families. Genome Biol. 12, R89. https://doi.org/10.1186/gb-2011-12-9-r89 (2011).
Gao, X. et al. Novel mutations and mutation combinations of TMPRSS3 cause various phenotypes in one chinese family with autosomal recessive hearing impairment. Biomed. Res. Int. 2017, 4707315. https://doi.org/10.1155/2017/4707315 (2017).
Liu, W. et al. Expression of trans-membrane serine protease 3 (TMPRSS3) in the human organ of Corti. Cell Tissue Res. 372, 445–456. https://doi.org/10.1007/s00441-018-2793-2 (2018).
Sasamori, K. et al. A case of TMPRSS3 mutation with mid- to low-frequency hearing loss. Practica oto-rhino-laryngologica Suppl. 152, 12–13. https://doi.org/10.5631/jibirinsuppl.152.12 (2018).
Lynch, E. D. et al. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science 278, 1315–1318 (1997).
Ueyama, T. et al. Constitutive activation of DIA1 (DIAPH1) via C-terminal truncation causes human sensorineural hearing loss. EMBO Mol. Med. 8, 1310–1324. https://doi.org/10.15252/emmm.201606609 (2016).
Frolenkov, G. I., Belyantseva, I. A., Friedman, T. B. & Griffith, A. J. Genetic insights into the morphogenesis of inner ear hair cells. Nat. Rev. Genet. 5, 489–498. https://doi.org/10.1038/nrg1377 (2004).
Neuhaus, C. et al. Extension of the clinical and molecular phenotype of DIAPH1-associated autosomal dominant hearing loss (DFNA1). Clin. Genet. 91, 892–901. https://doi.org/10.1111/cge.12915 (2017).
Karki, N. R., Ajebo, G., Savage, N. & Kutlar, A. DIAPH1 mutation as a novel cause of autosomal dominant macrothrombocytopenia and hearing loss. Acta Haematol. 144, 91–94. https://doi.org/10.1159/000506727 (2021).
Delmaghani, S. et al. Defect in the gene encoding the EAR/EPTP domain-containing protein TSPEAR causes DFNB98 profound deafness. Hum. Mol. Genet. 21, 3835–3844. https://doi.org/10.1093/hmg/dds212 (2012).
Scheel, H., Tomiuk, S. & Hofmann, K. A common protein interaction domain links two recently identified epilepsy genes. Hum. Mol. Genet. 11, 1757–1762. https://doi.org/10.1093/hmg/11.15.1757 (2002).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498. https://doi.org/10.1038/ng.806 (2011).
Tan, A., Abecasis, G. R. & Kang, H. M. Unified representation of genetic variants. Bioinformatics 31, 2202–2204. https://doi.org/10.1093/bioinformatics/btv112 (2015).
McLaren, W. et al. The ensembl variant effect predictor. Genome Biol. 17, 122. https://doi.org/10.1186/s13059-016-0974-4 (2016).
Li, M. M. et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the association for molecular pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 19, 4–23. https://doi.org/10.1016/j.jmoldx.2016.10.002 (2017).
Paila, U., Chapman, B. A., Kirchner, R. & Quinlan, A. R. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput. Biol 9, e1003153. https://doi.org/10.1371/journal.pcbi.1003153 (2013).
Sherry, S. T. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311. https://doi.org/10.1093/nar/29.1.308 (2001).
Harrison, S. M. et al. Using ClinVar as a resource to support variant interpretation. Curr Protoc. Hum. Genet. https://doi.org/10.1002/0471142905.hg0816s89 (2016).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443. https://doi.org/10.1038/s41586-020-2308-7 (2020).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. https://doi.org/10.1038/gim.2015.30 (2015).
Oza, A. M. et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum. Mutat. 39, 1593–1613. https://doi.org/10.1002/humu.23630 (2018).
Ye, J. et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 13, 134. https://doi.org/10.1186/1471-2105-13-134 (2012).
Sagong, B. et al. Two novel missense mutations in the TECTA gene in Korean families with autosomal dominant nonsyndromic hearing loss. Ann. Clin. Lab. Sci. 40, 380–385 (2010).
Nam, G. S. et al. The TECTA mutation R1890C is identified as one of the causes of genetic hearing loss: a case report. BMC Med. Genet. 20, 57. https://doi.org/10.1186/s12881-019-0775-1 (2019).
Acknowledgements
We thank the patients and their families for their kind collaboration.
Funding
This research was funded by grants of the Slovak Research and Development Agency (APVV-15-0067, APVV-20-0236), Comenius University in Bratislava (UK/200/2020) and Integrated Infrastructure Operational Program, ITMS: 313011AFG5, co-financed by the European Regional Development Fund.
Author information
Authors and Affiliations
Contributions
L.V. and D.G. designed the study. Z.P., L.V. and S.B. wrote the manuscript. Z.P. prepared figures. L.V. performed the clinical investigations. Z.P., S.B., M.S., M.H. and M.K. performed the molecular-genetic investigations and analyzed the WES data. All authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pavlenkova, Z., Varga, L., Borecka, S. et al. Comprehensive molecular-genetic analysis of mid-frequency sensorineural hearing loss. Sci Rep 11, 22488 (2021). https://doi.org/10.1038/s41598-021-01876-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01876-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.