Abstract
Knowledge of energy expenditure informs conservation managers for long term plans for endangered species health and habitat suitability. We measured field metabolic rate (FMR) of free-roaming giant pandas in large enclosures in a nature reserve using the doubly labeled water method. Giant pandas in zoo like enclosures had a similar FMR (14,182 kJ/day) to giant pandas in larger field enclosures (13,280 kJ/day). In winter, giant pandas raised their metabolic rates when living at − 2.4 °C (36,108 kJ/day) indicating that they were below their thermal neutral zone. The lower critical temperature for thermoregulation was about 8.0 °C and the upper critical temperature was about 28 °C. Giant panda FMRs were somewhat lower than active metabolic rates of sloth bears, lower than FMRs of grizzly bears and polar bears and 69 and 81% of predicted values based on a regression of FMR versus body mass of mammals. That is probably due to their lower levels of activity since other bears actively forage for food over a larger home range and pandas often sit in a patch of bamboo and eat bamboo for hours at a time. The low metabolic rates of giant pandas in summer, their inability to acquire fat stores to hibernate in winter, and their ability to raise their metabolic rate to thermoregulate in winter are energetic adaptations related to eating a diet composed almost exclusively of bamboo. Differences in FMR of giant pandas between our study and previous studies (one similar and one lower) appear to be due to differences in activity of the giant pandas in those studies.
Similar content being viewed by others
Introduction
Giant pandas (Ailuropoda melanoleuca) are an icon of conservation and the flagship species for conservation in China. Because of their protection, numerous less well-known animals and plants are also protected in giant panda reserves1. It is of great concern that for such an important species so little is known about its basic physiology and ecology. To improve the conservation of giant pandas and better design their nature reserves we need more understanding of the limiting factors that control their distribution. Energy exchange is one of the most ubiquitous and influential relationships between an animal and its environment2. Factors such as radiation (sunlight, skylight, and radiant heat), air temperature, wind, and humidity affect the energy flow to and from an animal. In turn, the energy flow between an animal and the environment affects the metabolism and body temperature of the animal. A result of this dynamic is that animals typically live in the environment most suited to their physiological and energy requirements for energy balance2. Those individuals that can obtain and process energy with the greatest efficiency while balancing that task against other factors affecting their survival and reproduction will have the greatest genetic fitness3. Therefore, understanding the energy exchange of a giant panda will help us to better predict its success in nature.
Giant pandas are primarily herbivorous bears in the Family Ursidae. Other bears include the American black bear (Ursus americanus) an omnivore that eats primarily vegetation, but also insects, honey and meat when available; the Asiatic black bear (Ursus thibetanus) an omnivore with a similar diet; the sloth bear (Ursus ursinus) that eats termites; the sun bear (Ursus malayanus) that is an omnivore; the polar bear (Ursus maritimus) that is a carnivore, the brown or grizzly bear (Ursus arctos) that is an omnivore eating primarily vegetation but also hunting animals, catching fish (salmon) and eating carrion; and the Andean or spectacled bear (Tremarctos ornatus) that is primarily a herbivore. The giant panda is similar in size (males 100–160 kg and females 70–125 kg) to the Asiatic black bear (males 110–150 kg and females 65–90 kg), the sloth bear (males 80–145 kg and females 55–105 kg) and Andean bear (males 100–200 kg and females 35–82 kg). It is smaller than the American black bear (males 115–270 kg and females 92–170 kg), grizzly bear (males 180–400 kg and females 130–180 kg) and polar bear (males 350–700 kg and females 150–300 kg)4. Most bears, including giant pandas, are animals of the forest but before the arrival of Europeans, grizzly bears also inhabited the Great Plains of North America and polar bears are animals of the Arctic. All of these bears have a thick coat of insulation especially the grizzly and polar bears. It is possible that the habitat and unique diet of giant pandas may have led to unique specializations in energy expenditure.
Initially metabolic studies on bears focused on their reduced metabolism during hibernation5,6,7,8,9,10. Resting metabolic rates have been measured for sloth bears11, American black bears10, polar bears7,12,13, and grizzly bears12. In addition to the studies on giant pandas, field metabolic rates (FMR) have also been measured for polar bears12,13 and active metabolic rates have been reported for sloth bears11 and grizzly bears12. By comparing the metabolic rates of giant pandas in a natural environment with those measured in other bears we can assess whether giant pandas have a lower metabolic rate than expected based on allometric predictions.
Zhang et al.14 used a mechanistic model based on biophysical interactions to explore likely panda response to habitat alteration and climate change considering physiological, behavioral and ecological factors. Their analysis was limited by uncertainty about the metabolic rate of the giant panda. Fei et al.15. reported that the resting metabolic rate (RMR) of adult and sub-adult giant pandas measured with flow through respirometry in the laboratory was 0.150 ml/g/h, which was about 6 to 44% below the values for other mammals of similar size. The RMR of the giant pandas was 6.0 to 44.3% below those predicted by a regression line for carnivores/ungulates/pangolins (Fereuungulata) from Sieg et al.16. Nie et al.17 reported that giant pandas in captivity and the wild had FMR measured with doubly labeled water (DLW) as low as 1/3 the predicted value for mammals of their size and lower than the RMR of sleeping giant pandas in the laboratory reported by Fei et al.15 The FMR (also called daily energy expenditure DEE) is the average energy expended each day by an animal, including all of its physiological processes while it is active, feeding, breeding, and moving around in its environment18,19,20,21,22,23,24,25,26,27,28. However, Fei et al. reported that FMR was 0.284 ml/g/h, four times higher than for the captive animals in Nie et al. and 3.5 times higher than the rate for their wild animals. So, there is a conundrum that needs to be resolved. Are giant pandas low energy specialists or do they have a metabolism similar to that of other mammals of their size?
The Chengdu Research Base of Giant Panda Breeding is developing a release program to reintroduce captive bred giant pandas (Ailuropoda melanoleuca) into the wild. For this program we adapted an assisted soft release method, based largely on the soft release approach of Benjamin Kilham29 in New Hampshire, USA. This method links an investigator with a giant panda cub and provides an opportunity to collect more data on the physiology, behavior and ecology of pandas living in wild conditions via direct observations and measurements of undisturbed animals. Because of this relationship between the investigator and the giant panda we were able to inject DLW and withdraw blood samples on undisturbed animals that were fully awake. The release program pandas move from a standard zoo enclosure to large, naturally forested enclosures in a nature reserve where they have to forage for natural and provisioned bamboo, and finally into the wild habitat of the nature reserve. Our observations to date indicate that these pandas increase their activity to forage on natural growing bamboo. Also, giant pandas in large enclosures are more vigilant and regularly climb trees to avoid potential danger, increasing their activity compared to the highly habituated giant pandas in a zoo. Giant pandas released into the wild quickly adjust to the natural habitat and move about from one bamboo patch to another (personal observations).
It is now important to determine the FMR of giant pandas under these natural conditions in order to predict the number of giant pandas that can be released into various nature reserves. We have already measured the metabolic rates of giant pandas in captivity by flow through respirometry and DLW15. Those animals had metabolic rates measured by flow through respirometry similar to, but lower than similar sized mammals such as the tiger (Panthera tigris), lion (Panthera leo), cow (Bos taurus), and eland (Taurotragus oryx)16. Giant panda FMR in zoo enclosures were lower than some similarly sized mammals such as deer (Odocoileus hemionus and Cervus elaphus), and oryx (Oryx leucoryx), but higher than that of reindeer (Rangifer tarandus)26.
The objective of this study was to measure the metabolic rates of giant pandas in zoo enclosures and in large enclosures in nature reserves to compare the FMR of giant pandas under captive and more naturalistic conditions in order to answer the basic question: What is the FMR of a giant panda when living in naturalistic conditions?
Methods
Giant pandas
Giant pandas in this study were from the reintroduction program of the Chengdu Research Base of Giant Panda Breeding (Panda Base). We measured FMR of five pandas (2 pandas chosen for reintroduction and 3 control pandas) in enclosures at different study sites. The control pandas lived at the Panda Base and the other two pandas lived at Panda Valley and the Daxiangling Nature Reserve. In Panda Valley, a research facility in Dujiangyan, the release pandas ate the same species of harvested bamboo (stem, shoots, and leaves) as the control pandas in Panda Base. However, the release pandas also ate Phyllostachys bissetii bamboo that grew naturally inside a learning enclosure at Panda Valley. At Daxiangling Nature Reserve, the release giant pandas ate natural arrow bamboo (Bashania faberi) inside the learning enclosure. We also provided apples for the released giant pandas when we conducted blood sampling or body health checks. No other food was provided to the release giant pandas. The control captive pandas lived in a zoo enclosure and ate bamboo, bamboo shoots, apples, and “panda cake,” a biscuit made of a mixture of grains with vitamins at Panda Base. We measured FMR twice on each release panda at Panda Valley and Daxiangling Nature Reserve and once on each control panda at Panda Base zoo enclosure (Table 1).
Chengdu panda base
Panda Base in Chengdu, Sichuan Province, was established in 1987 as a rescue and breeding facility for giant pandas. A nonprofit organization open to the general public, the main functions of the Panda Base were giant panda breeding, research, conservation, education, educational tourism, and giant panda reintroduction. Pandas lived in a 0.77 ha enclosure and ate bamboo provided by the husbandry staff. The elevation of the Panda Base was 524 m. The mean annual temperature was 16 °C and the annual rainfall was 1000 mm.
Panda Valley
Panda Valley was located in Dujiangyan, a city west of Chengdu. It was a semi-wild facility of the Panda Base. We built a 3.8-hectare semi-wild learning enclosure for reintroduction pandas. Pandas in the experiment spent their time outside in the learning enclosure. The elevation of Panda Valley was 815 m. The mean annual temperature was 15.2 °C and the annual rainfall was 1200 mm. Pandas ate natural growing bamboo (Phyllostachys bissetii) and bamboo brought into the enclosure to supplement natural growing bamboo.
Daxiangling nature reserve
Daxiangling Nature Reserve was in the western part of the Sichuan Basin. The highest peak was 3552 m and elevation ranged from 1500 to 3553 m30. The climate was humid, annual rainfall was typically 1300–2000 mm, and the mean annual temperature was 16 °C31. The reintroduction giant pandas lived in natural enclosures of 13.3 and 49.4 ha at an elevation of 2456–2495 m. Pandas ate naturally growing bamboo.
Sample collection, analysis, and calculation
To measure the FMR on wild pandas in the past, researchers used anesthetics to sedate the panda before the blood collections or injections17. We were concerned that sedation may lead to death32 as well as alter our results in the experiment33,34. In addition, while sedation of pandas under the supervision of trained veterinary staff is relatively safe, there is always a risk of morbidity or mortality, or disturbance of natural behaviors and physiological processes of the animal for hours or days afterward35,36,37. This risk is compounded in the field setting. Thus, to avoid sedation risks, we built a strong bond with our pandas at a young age. Even when our reintroduction pandas were released into the field, we could still touch and weigh them without using anesthetics or other restraint methods. We trained our experimental giant pandas to voluntarily grab a small pole with their front paw so that we could take a blood sample without anesthesia (Fig. 1). The DLW method based on the Fei et al.15 study was used to measure the metabolic rate. First, we took a 1 ml background blood sample. Then, we injected the panda with 8.1–12.1 g (Table 1) of DLW depending on the mass and time needed for the decay curve (Sigma-Aldrich deuterium oxide-18 (99% D 75% O28) mixed with physiological saline. Fei et al.15 determined that the equilibration time for giant panda was 5 h, so we took another 1 ml blood sample after 5 h. Finally, we took a 1 ml blood sample after 4 days. We sealed all blood samples in microcapillary glass tubes using an alcohol burner, placed them in a bigger PCV tube with cotton to protect them, and stored them in a freezer at – 40 °C. We sent the blood sample to the Laboratory of Isotope Geology at the Chengdu University of Technology for further analysis. We used the two-sample technique38 to calculate the CO2 production after 4 days. We used 0.9 as RQ to predict oxygen consumption because the giant pandas ate primarily carbohydrates (bamboo) but may have eaten some protein as well. The body mass versus FMR equation of Nagy et al.39 was used to predict FMR of a studied giant panda.
We used 24-gauge Cu-Co thermocouples (+/− 0.05 °C) to measure the air temperature in the study site. For the statistical analysis, we reported the results as mean ± SD.
The data were confounded by the small sample size and the fact that giant pandas varied in mass, lived at different altitudes and two were sampled twice. There were not enough data (only 7 data points) to do a 3 Way ANCOVA (mass, altitude, temperature) to test for the effect of those factors. We did do a regression analysis for both temperature and mass but neither was statistically significant because of the small sample size. We did not have an activity budget for the giant pandas at Daxiangling because they were usually hidden in bamboo thickets and we could not observe them 24 h a day. We compared the RMR and FMR of giant pandas to those of bears and other large mammals by constructing regression equations using Excel and comparing to the predicted FMR regression of Nagy (see “Discussion”).
Animal use and care approval
This study was approved by the Institutional Animal Care and Use Committees of the Chengdu Research Base of Giant Panda Breeding (2019019) and Drexel University. All experiments were performed in accordance with the relevant guidelines of those committees. This study is reported in accordance with ARRIVE guidelines.
Results
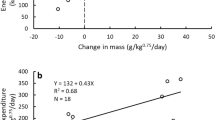
We measured the FMR using DLW in seven experiments on five individuals in three different seasons (Table 2). The highest FMR on a per unit basis was 0.89 ml O2/g/h for a panda at Daxiangling Nature Reserve in winter. The lowest FMR was 0.17 ml O2/g/h for a captive panda at Panda Base. The average FMR was 0.47 ml O2/g/h (SD = 0.26, range = 0.17–0.89 ml O2/g/h). The average FMR was 20,189 kJ/day (SD = 12,341, range = 6521–44,170 kJ/day). The highest FMR was from a release panda in winter (44,170 kJ/day) at the Daxiangling Nature Reserve. That panda also had the highest FMR on a per g basis. The lowest FMR was from the youngest captive panda (6521 kJ/day). That panda had the lowest FMR on a per g basis. Mass appeared to affect the FMR of giant pandas (Fig. 2a) but the regression of FMR on mass was not statistically significant due to the small sample size and the confounding effect of ambient temperature. Ambient temperature appeared to have a large effect on FMR (Fig. 2b) but a linear regression was not significant because of small sample size and the residuals were uninformative in determining the effect of ambient temperature on FMR. The FMR of captive pandas at Panda Base (524 m) at 8.8 to 14.9 °C (mean = 14,182 kJ/day) was similar to the FMR of release giant pandas in small and large natural enclosures in summer (815 m and 2475 m) at 14.0 and 18.2 °C (mean = 13,280 kJ/day). Giant pandas exposed to − 2.4 °C in a large enclosure at Daxiangling Nature Reserve in winter (21 January 2020) had the highest metabolic rates (0.82 and 0.89 ml O2/h/g) and highest FMR (28,045 and 44,170 kJ/day). The metabolic rate of one panda in winter was more than twice as high as its metabolic rate in summer and that of another panda was four times higher than its metabolic rate in autumn (Table 2, Fig. 2b).
Relationship of field metabolic rates (FMR) of giant pandas measured with DLW to mass (a) and ambient temperature (b). We did the experiments at Research Base of Giant Panda Breeding in Chengdu, Sichuan Province, China (PB), at the Panda Valley facility of Panda Base in Dujiangyan, Sichuan Province, China (PV) and at the Daxiangling Nature Reserve, Sichuan Province, China (DXL). Elevated FMR of two pandas at DXL occurred at low ambient temperature as seen in 2b.
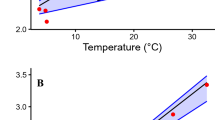
When we plotted the RMR reported by Fei et al.15 and the FMR measured in this study and by Fei et al.15 we were able to determine the thermal neutral zone of the giant panda. The thermal neutral zone is the range of temperatures in which a homeotherm maintains a constant metabolic rate40 by regulating its body temperature by insulation and vasomotor response alone. It appeared that the thermal neutral zone for the giant panda was between 8 °C and 28 °C (Fig. 3).
The thermal neutral zone of the giant panda as determined from field metabolic rates (FMR) measured in this study and resting metabolic rates (RMR) and FMR measured by Fei. et al.15. The lower critical temperature is 8 °C and the upper critical temperatrure is 28 °C. The horizontal red line represents the predicted thermal neutral zone of the giant panda (see arrows). Field metabolic rates only give an approximate indication of the thermal neutral zone because the giant pandas are active and not resting.
Discussion
Measurement of FMR of giant pandas revealed energetic adaptations in summer and winter. Giant pandas at Panda Base, Panda Valley, and Daxiangling Nature Reserve at 8.8 to 18.2 °C had FMRs (mean = 0.314 ml O2/g/h) similar to those reported by Fei et al.15 for giant pandas at Panda Base at 8.3 to 25.9 °C (mean = 0.349 ml O2/g/h)). The effect of temperature was striking in our study. Two giant pandas at − 2.4 °C had FMR of 0.821 and 0.890 ml O2/g/h, several times higher than the same pandas at 14.0 °C and 18.2 °C in autumn and summer. Clearly, giant pandas were below their thermal neutral zone at freezing temperatures and had to raise their metabolic rate to thermoregulate under those conditions. Thus giant pandas have a similar thermoregulatory capacity for cold as a polar bear cub, Arctic ground squirrel (Citellus parryii)41,domestic rabbit42, and red panda (Ailurus fulgens)43 and less thermoregulatory capacity than a snowshoe hare (Lepus americanus), and Arctic fox (Vulpes (Alopex) lagopus)40,41, all much smaller mammals. Bears, relatives of giant pandas, go into torpor or hibernation when faced with low winter temperatures. Their body temperature drops to 31 °C to 36 °C and metabolism, fueled by body fat, decreases by 30–50%43. Giant pandas cannot store up enough body fat to hibernate and thus are active all winter eating bamboo44.
Our FMRs of giant pandas were similar to those measured by Fei et al.15. They found the average daily energy expenditure (FMR) of 7 captive giant pandas (body mass averaged 125 kg) was 21,592 kJ/day (SD = 13,323, range = 9401 kJ/day to 47,716 kJ/day), which is lower than those of some similar-sized mammals26 but higher than Nie et al.17 reported. Nie et al.17 found average FMR for 5 captive pandas (body mass average 91.1 kg) was 4579.92 ± 1887.05 kJ/day (± SEM) and for 3 wild pandas (body mass average 92.6 kg) was 6246.13 ± 2173.56 kJ/day. The FMR of our free-roaming giant pandas in large wild enclosures (13,280 kJ/day) was higher than the FMR reported for adult wild-caught pandas (6,246.13 kJ/day, SD = 2173.56) by Nie et al.17 despite the fact that they weighed less. Our results are generally consistent with those of Fei et al.15 and indicate that the metabolic rates of giant pandas under natural conditions are higher than those reported by Nie et al.17. It is not clear to us why the giant pandas studied by Nie et al. had much lower metabolic rates than those studied here and by Fei et al. One possibility is that there were large differences in activity between the pandas in our study and Fei’s study as compared to those in the Nei study. To determine if giant pandas have exceptionally low FMR it is necessary to compare the FMR of the giant panda to those of other bears.
Resting metabolic rates of giant pandas measured with flow-through respirometry15 were higher than that of sloth bears11, similar to that of black bears10, but 63% of that predicted from a regression equation we calculated for large mammals (Fig. 4). Sloth bears had a RMR 48% of that predicted by that regression line while black bears had a RMR about as predicted. Polar bears had a RMR as predicted by that equation while grizzly bears had a much higher RMR than predicted12,45 (Table 3, Fig. 4). The FMRs of our pandas at 8.8 to 18 °C were lower than the active metabolic rate of sloth bears11 and 69% of the predicted FMR for mammals of that size calculated using the equation of Nagy et al.39. Fei’s giant pandas had a FMR 81% of that predicted by the Nagy et al. regression. (Table 4, Fig. 4). Both polar bears and grizzly bears had much higher FMR than those predicted by the Nagy et al. regression. The regression equation that we calculated for FMR based on data from large mammals produced a much steeper regression line (blue line in Fig. 4) than the regression line produced by the Nagy et al. equation (dashed green line in Fig. 4). That was because the largest mammal in the Nagy equation was about 100 kg and mammals we included in our regression (Table 4) included the large grizzly and polar bears (166 and 182 kg) and walruses (1310 kg). There are probably higher total energy costs for movement and activity in the large mammals than accounted for by the regression equation primarily based on smaller mammals although large mammals spend less energy per unit mass to move a given distance45. More data are needed for the FMR of large mammals to clarify these relationships.
Relationship of metabolic rate and body mass in giant pandas and other bears. Metabolic rate includes field metabolic rate (FMR), resting metabolic rate (RMR), and active metabolic rate. The orange regression line for RMR of large mammals was calculated using the data in Table 3 and included the resting metabolic rates of 20 large mammals from Sieg et al.16 and average resting metabolic rates of grizzly bears and polar bears from Pagano et al.12, sloth bears from McNab11, and black bears from Toien10. It did not include the RMR of the giant panda. The regression equation is (RMR) = 106.41 (masskg) + 671.77 (r2 = 0.524, p = 0.00), where y is RMR and x is body mass. The green dashed line is the regression line from Nagy et al.39 (FMR = 4.82 (massg)0.73). The blue regression line for FMR of large mammals included the FMR of 10 large mammals and active metabolic rates and FMR of bears from the Table 4. It did not include the FMR of giant pandas. The regression equation is (FMR) = 292.66 (masskg) + 585.35 (r2 = 0.99, p = 0.00) where y is FMR and x is body mass.
The FMRs of giant pandas measured in our study were similar to the FMRs (ml O2/g/h) of polar bears measured with DLW although FMR expressed as KJ/day was much higher in polar bears since they were much larger than the giant pandas in our study13. In addition, polar bears were living at much colder temperatures in nature than our giant pandas. Pagano46 found that polar bears have higher energy expenditure in spring and that the spring ice conditions affect the prey availability of polar bears. Therefore, giant pandas and sloth bears had lower overall energy costs than polar bears and grizzly bears because they traveled much smaller distances than those bears and spent less energy on thermoregulation. The giant panda had a high FMR in winter at − 2.4 C because it was out of its thermal neutral zone. Optimal foraging theory predicts that animals will minimize their energy expenditure to obtain maximum food when foraging47. So, the release giant pandas in our study like sloth bears could be adapting their seasonal foraging by managing their energy expenditure as first suggested by Schaller et al.44. It would be interesting to measure the FMR of the American black bear since it has a low RMR but is an active forager and the Andean bear because it is a herbivore with greater home ranges than giant panda4. Meanwhile, we conclude that giant pandas do not have exceptionally low metabolic rates for bears, but like sloth bears they are low metabolic rate bears, with a somewhat reduced FMR due to their less energy intensive life style.
Our results on field metabolic rate (FMR) of giant pandas in captive enclosures and large enclosures in the Daxiangling Nature Reserve indicate that giant pandas must raise their metabolic rates to survive under cold conditions in winter. Giant pandas are not polar bears and have a much higher critical temperature. Previous statements that giant pandas are mountain animals acclimated to cold temperatures are perhaps overstated48,49. Schaller et al.44 noted that giant pandas in the Wolong Nature Reserve lived at higher altitudes because local people were farming the valleys and lower slopes. It is now clear that the habitat of the wild giant panda has been moved upward to higher elevations because of human disturbance50,51,52. Giant pandas live in only a small fraction of their historic range in areas that are remote and could not be easily farmed53. It is fortunate that giant pandas can survive in the mountains, but our results indicate that it is not their preferred thermal habitat.
Giant pandas have higher metabolic requirements at the montane elevations of the Daxiangling Nature Reserve (2500 m). That is not unexpected since other species also have higher metabolic requirements at high altitude. For example, deer mice (Peromyscus maniculatus) have a 75% greater mean FMR at high altitude (3800 m) than low altitude (1230 m)54. Fei et al.15 discussed the relationship between energy expenditure and natural bamboo resources and found that there is enough bamboo to fulfill the energy needs for wild giant pandas in an area such as Daxiangling. Our results support their analysis. Based on our FMR measurements and the fact that giant pandas eat about 20 kg of bamboo a day15, there is enough bamboo in 1 km2 of Daxiangling Nature Reserve to support 22 giant pandas. Since the density of giant pandas in the wild is much lower than that and their home range is between 3 and 6 km2, there must be other factors that restrict the number of giant pandas in a given area. In any case there is more than enough bamboo in nature reserves to support much larger populations of giant pandas than currently live there. Reintroduction efforts can proceed in releasing ever more giant pandas into reserves in order to repopulate natural areas with the certainty that there is a sufficient supply of bamboo to support those animals.
Preferably, nature reserves should be large and diverse in habitat and elevation so that more moderate climates are included, and giant pandas can descend the mountains during winter to find warmer microclimates. Conservation of giant pandas will require maintaining their genetic diversity in the wild in a natural biological community53. Moving towards that goal, reintroduction efforts should concentrate on releasing giant pandas into reserves such as Daxiangling that provide a large range of elevations (1500–3500 m) and thus microclimates for those pandas.
Data availability
All data are presented in this manuscript. For more information on the experiments contact JRS (Spotiljr@drexel.edu).
References
Li, B. V. & Pimm, S. L. China’s endemic vertebrates sheltering under the protective umbrella of the giant panda. Conserv. Biol. 30, 329–339 (2016).
Porter, W. P. & Gates, D. M. Thermodynamic equilibria of animals with environment. Ecol. Monogr. 39, 227–244 (1969).
Dunham, A. E., Grant, B. W. & Overall, K. L. Interfaces between biophysical and physiological ecology and the population ecology of terrestrial vertebrate ectotherms. Physiol. Zool. 62, 335–355 (1989).
Nowak, R. M. Walker’s Mammals of the World Vol. II (Johns Hopkins University Press, 1991).
Nelson, R. A., Wahner, H. W., Jones, J. D., Ellefson, R. D. & Zollman, P. E. Metabolism of bears before, during, and after winter sleep. Am. J. Physiol. 224, 491–496 (1973).
Best, R. C. Thermoregulation in resting and active polar bears. J. Comp. Physiol. 146, 63–73 (1982).
Watts, P. D., Øritsland, N. A. & Hurst, R. J. Standard metabolic rate of polar bears under simulated denning conditions. Physiol. Zool. 60, 687–691 (1987).
Watts, P. & Cuyler, C. Metabolism of the black bear under simulated denning conditions. Acta Physiol. Scand. 134, 149–152 (1988).
Watts, P. D. & Jonkel, C. Energetic cost of winter dormancy in grizzly bear. J. Wildl. Manag. 52, 654–656 (1988).
Tøien, Ø. et al. Hibernation in black bears: Independence of metabolic suppression from body temperature. Science 331, 906–909 (2011).
McNab, B. K. Rate of metabolism in the termite-eating sloth bear (Ursus ursinus). J. Mammal. 73, 168–172 (1992).
Pagano, A. M. et al. Energetic costs of locomotion in bears: is plantigrade locomotion energetically economical?. J. Exp. Biol. 221, jeb175372 (2018).
Pagano, A. M. & Williams, T. M. Estimating the energy expenditure of free-ranging polar bears using tri-axial accelerometers: A validation with doubly labeled water. Ecol. Evol. 9, 4210–4219 (2019).
Zhang, Y., Mathewson, P. D., Zhang, Q., Porter, W. P. & Ran, J. An ecophysiological perspective on likely giant panda habitat responses to climate change. Glob. Change Biol. 24, 1804–1816 (2018).
Fei, Y. et al. Metabolic rates of giant pandas inform conservation strategies. Sci. Rep. 6, 27248. https://doi.org/10.1038/srep27248 (2016).
Sieg, A. E. et al. Mammalian metabolic allometry: Do intraspecific variation, phylogeny, and regression models matter?. Am. Nat. 174, 720–733 (2009).
Nie, Y. et al. Exceptionally low daily energy expenditure in the bamboo-eating giant panda. Science 349, 171–174 (2015).
Acquarone, M., Born, E. W. & Speakman, J. R. Field metabolic rates of walrus (Odobenus rosmarus) measured by the doubly labeled water method. Aquat. Mamm. 32, 363–369 (2006).
Nagy, K. & Montgomery, G. Field metabolic rate, water flux, and food consumption in three-toed sloths (Bradypus variegatus). J. Mammal. 61, 465–472 (1980).
Mautz, W. & Nagy, K. Ontogenetic changes in diet, field metabolic rate, and water flux in the herbivorous lizard Dipsosaurus dorsalis. Physiol. Zool. 60, 640–658 (1987).
Anava, A., Kam, M., Shkolnik, A. & Degen, A. Effect of group size on field metabolic rate of Arabian babblers provisioning nestlings. Condor 103, 376–380 (2001).
Fyhn, M. et al. Individual variation in field metabolic rate of kittiwakes (Rissa tridactyla) during the chick-rearing period. Physiol. Biochem. Zool. 74, 343–355 (2001).
Møller, A. P. Relative longevity and field metabolic rate in birds. J. Evol. Biol. 21, 1379–1386 (2008).
Riek, A. Relationship between field metabolic rate and body weight in mammals: Effect of the study. J. Zool. 276, 187–194 (2008).
Sparling, C. E., Thompson, D., Fedak, M. A., Gallon, S. L. & Speakman, J. R. Estimating field metabolic rates of pinnipeds: Doubly labelled water gets the seal of approval. Funct. Ecol. 22, 245–254 (2008).
Hudson, L. N., Isaac, N. J. & Reuman, D. C. The relationship between body mass and field metabolic rate among individual birds and mammals. J. Anim. Ecol. 82, 1009–1020 (2013).
Munn, A. J. et al. Field metabolic rate, movement distance, and grazing pressures by western grey kangaroos (Macropus fuliginosus melanops) and Merino sheep (Ovis aries) in semi-arid Australia. Mamm. Biol. 81, 423–430 (2016).
Drack, S. et al. Field metabolic rate and the cost of ranging of the red-tailed sportive lemur (Lepilemur ruficaudatus) in New Directions in Lemur Studies (eds. Rakotosamimanana, B., Rasamimanana H., Ganzhorn, J. U., & Goodman S. M.) 83–91 (1999).
Kilham, B. & Gray, E. Among the Bears: Raising Orphan Cubs in the Wild (Henry Holt, 2002).
Xu, W., Ouyang, Z., Jiang, Z., Zheng, H. & Liu, J. Assessment of giant panda habitat in the Daxiangling Mountain Range, Sichuan, China. Biodivers. Sci. 14, 223 (2006).
Zhao, C. et al. Relationship between human disturbance and endangered giant panda Ailuropoda melanoleuca habitat use in the Daxiangling Mountains. Oryx 51, 146–152 (2017).
Wysowski, D. K. & Pollock, M. L. Reports of death with use of propofol (Diprivan) for nonprocedural (long-term) sedation and literature review. J. Am. Soc. Anesthesiol. 105, 1047–1051 (2006).
Mistraletti, G., Donatelli, F. & Carli, F. Metabolic and endocrine effects of sleep deprivation. Essent. Psychopharmacol. 6, 312–317 (2005).
Champagne, C. D., Houser, D. S., Costa, D. P. & Crocker, D. E. The effects of handling and anesthetic agents on the stress response and carbohydrate metabolism in Northern elephant seals. PLoS ONE 7, e38442. https://doi.org/10.1371/journal.pone.0038442 (2012).
Fahlman, Å. Anaesthesia of wild carnivores and primates. Licentiate Thesis (Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005).
Arnemo, J. M. et al. Risk of capture-related mortality in large free-ranging mammals: experiences from Scandinavia. Wildl. Biol. 12, 109–113 (2006).
West, G., Heard, D. & Caulkett, N. Zoo Animal and Wildlife Immobilization and Anesthesia 2nd edn. (John Wiley & Sons, 2014).
Speakman, J. R. Doubly Labelled Water: Theory and Practice (Springer, 1997).
Nagy, K. A., Girard, I. A. & Brown, T. K. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 19, 247–277 (1999).
Prosser, C. L. & Brown, F. A. Jr. Comparative Animal Physiology, Environmental and Metabolic Animal Physiology 260–261 (W. B. Saunders, 1991).
Scholander, P. F., Hock, R., Walters, V., Johnson, F. & Irving, L. Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 99, 237–258 (1950).
Hart, J. S. Rodents in Comparative Physiology of Thermoregulation, Volume II Mammals (ed Whittow, G. C.) 1–149 (Academic Press, 1971).
McNab, B. K. The Physiological Ecology of Vertebrates: A View From Energetics (Cornell University Press, 2002).
Schaller, G. B., Hu, J. C., Pan, W. S. & Zhu, J. Giant Pandas of Wolong (The University of Chicago Press, 1985).
Taylor, C., Heglund, N. & Maloiy, G. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J. exp. Biol. 97, 1–21 (1982).
Pagano, A. M. Polar bear (Ursus maritimus) behavior and energetics: New metrics for examining the physiological impact of a changing Arctic environment. Ph.D. Dissertation (University of California Santa Cruz, CA, 2018).
Pyke, G. H., Pulliam, H. R. & Charnov, E. L. Optimal foraging: A selective review of theory and tests. Q. Rev. Biol. 52, 137–154 (1977).
Hu, J. C. Research on the Giant Panda (Shanghai Publishing House of Science and Technology, 2001).
Liu, G., Guan, T., Dai, Q., Li, H. & Gong, M. Impacts of temperature on giant panda habitat in the north Minshan Mountains. Ecol. Evol. 6, 987–996 (2016).
Hull, V. et al. Impact of livestock on giant pandas and their habitat. J. Nat. Conserv. 22, 256–264 (2014).
Hull, V. et al. Habitat use and selection by giant pandas. PLoS ONE 11(9), e0162266 (2016).
Li, B. V., Pimm, S. L., Li, S., Zhao, L. & Luo, C. Free-ranging livestock threaten the long-term survival of giant pandas. Biol. Cons. 216, 18–25 (2017).
Pan, W. et al. A Chance for Lasting Survival: Ecology and Behavior of Wild Giant Pandas (Smithsonian Institution Press, 2014).
Hayes, J. P. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at lowand high altitudes. Physiol. Zool. 62, 732–744 (1989).
Bi, W. Physiological ecology of soft-release giant pandas (Ailuropoda melanoleuca). PhD Dissertation. (Drexel University, Philadelphia, PA, 2020)
Acknowledgements
This research was supported by the Chengdu Giant Panda Breeding Research Foundation (CPF2017-20), the Global Cause Foundation, the Atlanta Zoo, The Memphis Zoo, the Betz Chair of Environmental Science at Drexel University and the Shrey Chair of Biology at Purdue University at Fort Wayne. Sheri Yi and John Spotila played a critical role in developing and carrying out this project. We thank all of the giant panda staff at the Chengdu Research Base of Giant Panda Breeding for their help and cooperation, especially Chao Chen, Feifei Feng, Jiabin Liu, Yanshan Zhou, Rui Ma, Jiang Gu, Xiang Yu, Fei Xue, Zusheng Li, Long Zhang, Guanwei Lan, Wenjun Huang, Li Luo, Xin Chen, Xiangming Huang, and Kongju Wu. Michael P. O’Connor and Susan Kilham assisted in design of the study and analysis of the results. This study was approved by the Chengdu Research Base of Giant Panda Breeding (2019019) and the Institutional Animal Care and Use Committee of Drexel University.
Author information
Authors and Affiliations
Contributions
W.B., J.R.S., R.H., J.O., M.V., F.V.P., D.Q. and Z.Z. conceived and designed the experiments. W.B. and M.V. performed the experiments. G.Y. analyzed the D.L.W. W.B., J.R.S., J.O., F.W., and F.V.P. analyzed the data. F.W. prepared the figures. D.Q. and Z.Z. contributed materials. All authors discussed the results and contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bi, W., Hou, R., Owens, J.R. et al. Field metabolic rates of giant pandas reveal energetic adaptations. Sci Rep 11, 22391 (2021). https://doi.org/10.1038/s41598-021-01872-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01872-5
This article is cited by
-
Ursids evolved dietary diversity without major alterations in metabolic rates
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.