Abstract
This study presents the morphology of calcar in adult Delphacidae based on representatives of the genera Ugyops Guérin-Meneville, 1834, Notuchus Fennah, 1969 (Ugyopini), Asiraca Latreille, 1798 (Asiracini), Kelisia Fieber, 1866, (Kelisini), Stenocranus Fieber, 1866 (Stenocranini), Chloriona Fieber, 1866, Megadelphax Wagner, 1963, Muellerianella Wagner, 1963, Javesella Fennah, 1963, Conomelus Fieber, 1866, Euconomelus Haupt, 1929, Hyledelphax Vilbaste, 1968, Stiroma Fieber, 1866, Struebingianella Wagner, 1963 and Xanthodelphax Wagner, 1963 (Delphacini). We used SEM electron microscopy, to define seven types of calcar structure (Types 1, 2, 5, 6, 7, 8, and 9) based on combinations of characters including shape, number of teeth and differentiation of sensory structures in species from fifteen genera. Additionally, two other types (Types 3 and 4) were determined based on the calcar descriptions from previous studies. Similarities and differences in calcar structure and function were discussed and emerging relationships between planthopper species and their particular habitats were indicated.
Similar content being viewed by others
Introduction
The planthopper family Delphacidae Leach, 1815 (Hemiptera, Fulgoromorpha: Fulgoroidea) is a relatively large and widely distributed group of opophagous insects feeding on plants, mainly on phloem. Delphacids comprise 2217 species in 427 genera and six subfamilies worldwide1,2. These planthoppers are associated mostly with monocotyledonous plants, especially grasses and sedges3,4,5. Some species also feed on horsetails and even mosses6,7, often living in wet—or at least mesic—situations8,9; endemic delphacids in Hawai’i have evolved to feeding onto many dicotyledons taxa10.
The economic importance of planthoppers is well documented11. The family contains at least 55 species that feed on economic plants including major pests of five of the top ten major world food crops, such as rice, sugarcane, maize, taro, and cereals. For example, Nilaparvata lugens (Stål, 1854), Sogatella furcifera (Horváth, 1899) and Laodelphax striatellus (Fallén, 1826) cause serious damage to rice paddies in south-eastern Asia12,13,14,15.
Delphacidae are mostly small or medium-sized insects, with most species between 2 and 4 mm in size (ranging from ~ 1.5 to ~ 10.0 mm); their compact bodies with usually short head and distinct keels are mainly cylindrical in cross-section. The presence of a movable calcar—the spur is the most charismatic and diagnostic character of the family, by which delphacids are easily distinguished from other planthoppers and similar insect families (viz. the other planthoppers and cicadomorphans). The calcar is located at the tip of the metatibia and base of the metatarsus, and it is articulated with the hind tibia16,17. Other distinctive characters include: basitmetarsomere and midmetarsomere with a row of apical teeth; ovipositors enormously long dividing the sternites right to the base of the abdomen; the tegmina membranous or somewhat thickened (especially when brachypterous), and held tent-like; they may be clear or patterned. Dimorphism is often expressed with adults either long-winged (macropterous, or dispersal forms, with fully developed flying wings) or short-winged (brachypterous, adult wings not functional for flying) within a single population of a species9,18. The calcar and its features are used to differentiate major groups within the family.
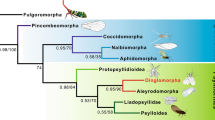
The classification and internal subdivisions of Delphacidae have been subjects of disputes. The division into two subfamilies (Asiracinae Motschulsky, 1863 and Delphacinae Leach, 1815) proposed by Muir19,20, followed by Metcalf21, subdivided Delphacinae into three tribes: Alohini Muir, 1915, Tropidocephalini Muir, 1915 and Delphacini Leach, 1815 (= Areopini Metcalf, 1938). Nevertheless, other opinions were also presented. Haupt22 suggested division into 4 subfamilies (Asiracinae, Tropidocephalinae Muir, 1915, Criomorphinae Kirkaldy, 1910 (= Megamelinae Haupt, 1929 see Nast23), and Delphacinae Leach, 1815). Later, Wagner24 proposed a division with subfamilies: Asiracinae, Kelisiinae Wagner, 1963, Jassidaeinae Wagner, 1963, Stirominae Wagner, 1963, Achorotilinae Wagner, 1963, Delphacinae, Chlorioninae Wagner, 1963, Stenocraninae Wagner, 1963, and Megamelinae. Vilbaste25 recognized the subfamily Saccharosydninae Vilbaste, 1968. Finally, Fennah26 divided the Asiracinae into two tribes (Asiracini Motschulsky, 1863 and Ugyopini Fennah, 1979). Those proposals were critically tested and verified with a detailed morphological cladistic analysis by Asche27. He distinguished 5 clades (subfamilies) within Delphacidae: paraphyletic Asiracinae and monophyletic ones: Kelisiinae, Stenocraninae, Plesiodelphacinae Asche, 198527 and Delphacinae; later, he added a small subfamily Vizcayinae Asche, 1990 (as the sixth clade) into the family28. Similar subdivisions were presented by Emeljanov29 after morphological studies on larval stages, with proposed subfamilies Ugyopinae Fennah, 1979 (with tribes Neopunanini Emeljanov, 1995, Eodelphacini Emeljanov, 1995 and Ugyopini), Asiracinae (with tribes Tetrastreini Emeljanov, 1995, Platysystatini Emeljanov, 1995, Asiracini and Idiosystatini Asche, 1985b) and Delphacinae (with 7 tribes: Vizcayini Asche, 1990, Kelisiini Wagner, 1963, Stenocranini Wagner, 1963, Plesiodelphacini Asche, 1985, Tropidocephalini Muir, 1915, Saccharosydnini Vilbaste, 1968 and Delphacini Leach, 1815). Later, Emeljanov30 recognized Kelisiinae, Sternocraninae, Saccharosydninae, and Tropidocephalinae as subfamilies. Hamilton31, following the general scheme of Emeljanov29, proposed to treat Kelisiini as a subtribe of Stenocranini, and Saccharosydnini (Delphacinae) as a subtribe of Tropidocephalini.
The first advances based on molecular data based on 504 bp of cytochrome oxidase I32 and 352 bp of 12S rDNA33 employed limited taxonomic and data sampling but provided some insight into higher level relationships within Delphacidae. Later, Urban et al.4 tested the phylogenetic proposals of Delphacidae based on DNA nucleotide sequence data from four genetic loci (18S rDNA, 28S rDNA, wingless and cytochrome oxidase I) and 132 coded morphological characters. The received topology generally supported higher classifications of Delphacidae proposed by Asche, Emeljanov and Hamilton, and suggested a rapid diversification of the Delphacini associated with host shifts to, and within, Poaceae, and specifically from C3 to C4 grasses4. More molecular studies explored the phylogeny of the delphacid subfamily Delphacinae based on nuclear ribosomal and mitochondrial DNA sequences of four genetic loci (16S rDNA, 28S rDNA, COI and Cyt b)5,34. Recently, mitogenomes of some Delphacidae were sequenced and used to reconstruct the phylogeny35,36,37,38. Additional morphological data on the ovipositor were presented by Wallner & Bartlett39.
The family Delphacidae is commonly accepted as a monophyletic unit, based on unique synapomorphy (the presence of a metatibial calcar). The origin of the family remains doubtful; several previous studies based on either morphological16,27,40,41 or molecular1,4,42,43,44 evidence suggested that Delphacidae might have arisen from within the planthopper family Cixiidae—resulting in the paraphyletic status of the latter.
The sister group of Delphacidae—the Cixiidae Spinola, 1839 is known in the fossil record from the Lower Cretaceous, Barremian45,46, while the oldest undoubted fossil Delphacidae are much younger, from the Middle Eocene Baltic amber47 and then from Miocene deposits of Russia, and Miocene fossil resins48,49. Fossils from early Eocene deposits of Green River50,51,52,53, Late Eocene/Early Oligocene of Russian Far East53 and Late Oligocene of Rott54 cannot be unequivocally ascribed to Delphacidae.
The calcar is an ancient feature, which occurs on legs of many insects and differs from setae in being multicellular in origin; in some species it has been lost or modified over evolutionary time to suit the adaptive needs of different groups55,56. The size and shape of this structure is highly variable, and it seems to have been put to a wide variety of uses (like the blades on a Swiss army knife). However, calcar’s exact function remains unclear because only few studieshave dealt with related structures57,58,59,60,61. This reservation also applies to a specialized spur of Delphacidae—the calcar. It is generally believed that the calcar is used to assist in jumping; however, it is morphologically disparate within delphacid subfamilies. The features of calcar were previously studied under light microscopy, and the results were presented by Metcalfe62, Wilson & McPherson63, Asche27, Liang64 and Bartlett & Webb65. The reassessment of calcar characters can provide additional data giving insights into the differentiation and morphological disparity of Delphacidae subfamilies; potentially it can indicate adaptation to the structure and surface of their host plants. Additionally, the knowledge of calcar structures will be of great importance for interpreting morphological data available from fossils and tracing evolutionary changes. Therefore, we examined the calcar using SEM to describe new features and possible adaptive characters for moving on different host-plant surfaces.
Material and methods
The studied materials come from the collections of the Upper Silesian Museum in Bytom (USMB) (Notuchus and Ugyops), the collection of the Zoology Research Team, University of Silesia in Katowice (DZUS) (species of several different genera e.g., Asiraca, Kelisia, Chloriona, Megadelphax, Conomelus, Euconomelus, Muellerianella, Hyledelphax, Stiroma, Struebingianella, Xanthodelphax) and the collection of the Laboratory of Terrestrial Invertebrates (LTIB)—State Scientific and Production Amalgamation The Scientific and Practical Center for Bioresources, National Academy of Sciences of Belarus (mainly Stenocranus and Javesella).
SEM examinations were conducted in the Laboratory of Scanning Microscopy of the Institute Biology, Biotechmology and Environmental Protection, the University of Silesia in Katowice. The dry material (20 species and 80 specimens) was cleaned in an ultrasonic cleaner for several seconds. Then the specimens were subsequently dehydrated in an ascending ethanol series (30, 50, 70, 80, 96, and 100%, for 10 min in each concentration with three 100% ethanol changes) and were air-dried at room temperature for 12 h. The samples were mounted on aluminium stubs with double-sided adhesive carbon tape and sputter-coated in a Pelco SC-6 sputter coater (Ted Pella Inc., Redding, CA, USA) with a thin film of gold. After processing, samples were imaged by the Phenom XL scanning electron microscope. For taking images, an eucentric sample holder was used to allow the sample to freely move, including rotate and tilt, to show all surfaces. The calcar usually protrudes from the tibia, so samples could be rotated and imaged for all elements in detail in SEM.
As previously noted, the metatibial apical movable spur of the Delphacidae, i.e., the calcar, is defined as a special structure usually flattened, foliaceous, and bearing a row of black-tipped teeth on the posterior (trailing) margin or spine-like shaped and not toothed9. The historical background of the calcar studies and descriptions are presented in Table S1. Terms of surface sculpturing have been used according to a web available glossary66.
Taxa examined:
Asiracinae: Asiracini—Asiraca clavicornis Latreille, 1802;
Asiracinae: Ugyopini—Notuchus linnavuorii Gębicki & Walczak, 2021; Ugyops inermis Distant, 1920; Ugyops nemestrinus Fennah, 1969; Ugyops taranis Fennah, 1964;
Kelisiinae—Kelisia praecox Haupt, 1935;
Delphacinae: Delphacini—Chloriona smaragdula (Stål, 1853); Conomelus anceps (Germar, 1821); Euconomelus lepidus (Boheman, 1847); Hyledelphax elegantulus (Boheman, 1847); Javesella (Javesella) pellucida (Fabricius, 1794); Megadelphax sordidula (Stål, 1853) Muellerianella brevipennis (Boheman, 1847); Stiroma affinis Fieber, 1866; Struebingianella lugubrina (Boheman, 1847); Xanthodelphax straminea (Stål, 1858); Stenocraninae: Stenocranus fuscovittatus (Stål, 1858); Stenocranus major (Kirschbaum, 1868).
Results
The present study distinguished nine types of the calcar structure based on the combination of characters of its shape, number of teeth and differentiation of sensory structures. The calcar (clc) (Fig. 1a–f) as a whole is a movable structure, with a membranous connection (ms) at the apex of metatibia, under the metatibial crown (mtt) of a few teeth (apical row of teeth). The sensory structures were identified as sensilla trichoidea (St)—larger setae, hair-like, and sensilla chaetica (Sch)—shorter and stouter setae. Both sensilla belong to a group of the mechanosensilla, which detect most of the tactile sensations perceived by the insect. Sensilla trichoidea (St) are characteristic in a more flexible stem as opposed to sensilla chaetica, which are provided with a stiffer, slightly grooved and acutely terminating stem. Both types of sensilla are embedded in flexible sockets (s), with a flexible membrane (m) surrounding the hair base. The distinguished types of calcar are listed below and in Table 1.
Calcar types in Delphacidae, (a) Ugyops taranis, membranous connection (ms) of calcar (clc), (b) Asiraca clavicornis, narrow spike calcar (clc) with sensilla trichoidea (St), (c) Ugyops taranis, triangular calcar with sensilla trichoidea (St) and chaetica(Sch), (d) Kelisia praecox, calcar (clc) with several large teeth (t) with sensilla trichoidea (St), (e) Chloriona smaragdula, calcar (clc) with numerous smaller teeth (t) and with sensilla trichoidea (St), (f) Stiroma affinis, calcar (clc) with numerous sensilla trichoidea (St), m flexible membrane, ms membranous connection of the calcar, mtt metatibial apical row of teeth, s socket.
Type 1: calcar awl-shaped (subulate), elongated, not flattened
The calcar (clc) is similar to a long and narrow awl with convex surfaces, bearing several sensilla trichoidea. This type is present in species of the subfamily Asiracinae. In Asiraca clavicornis (Asiracini), the calcar is in the form of an elongated awl, about twice as long as metatibial apical teeth (apical row in the form of an open crown-like wreath). The apex has slightly costate sculpturing. The calcar is rounded in cross-section. Several irregularly arranged sensilla trichoidea (St) are located on the outer side (Fig. 2a,b).
Type 2: calcar subulate, angular
In the representatives of the tribe Asiracine, the Ugyopini’s calcar is strongly spinose, subtriangular in cross-section, about three times as long as teeth of apical row; calcar internal (adplantar) side and dorsal (explantar) edges are delineated by rows of long sensilla trichoidea and stout sensilla chaetica. In Ugyops taranis (Fig. 2c), three rows of short and stout sensilla chaetica are visible. In Ugyops nemestrinus (Fig. 2d) and Ugyops inermis (Fig. 2e), there are two rows of long sensilla trichoidea (h) and one row of short stout sensilla chaetica (Sch) on the surface. The stout sensilla chaetica resemble sharp teeth. In Notuchus linnavuorii (Fig. 2f), the calcar is more subulate with external (explantar) surface convex and internal (adplantar) surface slightly concave; external (explantar) surface covered with shortened sensilla trichoidea (St). Inthree mentioned Ugyops species, the costate sculpturing of the calcar apex is deeper than that in Asiraca clavicornis.
Type 3: calcar subulate,sparsely dentate
In Vizcaya Muir, 1917, and Neovizcaya Liang, 2002, the calcar is elongated, terete in cross-section, but a distinct row of 8–12 large teeth is present at the adplantar margin (Table S1). Bases of sensilla chaetica are placed above the bases of these teeth. A few sensilla trichoidea are irregularly interspersed between the bases of teeth (64: Fig. 18).
Type 4: calcar cultrate, with large sparse teeth
Superficially similar to Type 3, the calcar is elongate, cultrate, oval in cross-section, convex on the adplantar and explantar sides, with a row of 4–8 large conical teeth along the inner margin; two rows of a few scarcely dispersed setae (sensilla trichoidea) along the outer margin, sensilla chaetica as short setae placed at the base of each internal tooth (19,27,67, viz.27, Figs. 294–305) (Table S1). Muir19 used this pattern of calcar to define tribe Alohini Muir, 1915 (in this paper comprising mostly endemic to Hawai’i and Pacific Islands genera: Aloha Kirkaldy, 1904, Dictyophorodelphax Swezey, 1907, Leialoha Kirkaldy, 1910, Nesodryas Kirkaldy, 1908 (subgenera Nesodryas and Nesothoe Kirkaldy, 1908), Nesorestias Kirkaldy, 1908, Nesosydne Kirkaldy, 1907, Proterosydne Kirkaldy, 1907); later Asche27 (p.310, see also Asche10: 369–370) synonymized Alohini under Delphacini.
Type 5: calcar tectiform, sparsely dentate
Here, the edges (ventrad adplantar and dorsad adplantar) of calcar sloping downwards on two sides from a raised external, explantar margin. The ventral adplantar margin with a variable number (more than 8) of large distinct immovable conical teeth (t) (from 9 to 11 teeth, including the apical one), with subbasal, long sensilla trichoidea (St) (Fig. 3a–f). The dorsal adplantar margin is smooth and bent to the ventral side. Adplantar surface of calcar concave, in the form of a wide and shallow groove (Fig. 4a–f). The size and shape of calcar differ in the studied species.
In Kelisia praecox (Fig. 3a,b) (Kelisiinae) the ventral adplantar margin bears 11 long and thin conical teeth (including the apical tooth) and on the surface of each tooth there is one or two sensilla trichoidea (St). The remainder of calcar surface with a sparse sensilla (St).
In Conomelus anceps (Delphacinae: Delphacini) the ventral adplantar margin bears 9 short and stout teeth (Fig. 3c,d) which are shorter than those in Kelisia praecox. The surface of each tooth possesses one sensilla trichoidea (St). The remainder of calcar surface has a sparse sensilla (St) (Fig. 3c,d).
Type 6: calcar tectiform, densely dentate
The calcar is subtriangular to sickle-shaped in cross-section, dorsal edge less noticeable. The ventral adplantar margin bears more than a dozen small teeth, covered with more or less dense mechanosensilla (St). The adplantar side of calcar lacks sensilla.
In Megamelus notulus (Germar, 1830) (Delphacinae: Delphacini) the adplantar margin bears 19 small teeth (Fig. 3e,f). The surface of each tooth possesses two rows of the sensilla trichoidea (St) above the teeth. The remainder of the calcar surface has a sparse mechnosensilla (St).
In Euconomelus lepidus (Delphacinae: Delphacini) (Fig. 5a,b) the adplantar margin bears about 20 small teeth. The surface of each tooth and remainder of the adplantar slope are covered by abundant mechnosensilla (St), forming a brush hiding the teeth at the adplantar slope of calcar. The calcar adplantar surface bears no sensilla (Fig. 5b).
In Muellerianella brevipennis (Delphacinae: Delphacini) (Fig. 5c–f the adplantar margin bears 15 small teeth. The surfaces of each tooth and adplantar slope are covered by abundant mechanosensilla (St); however, the tips of the teeth are free of sensilla. These sensilla form a brush on the calcar edge. The inner surface of calcar bears no sensilla (Fig. 5d,f).
In Javesella pellucida (Fig. 6a–c) and Laodelphax striatellus (Fig. 6 d) (Delphacinae: Delphacini) the adplantar margin bears 20–24 small teeth (t) and several rows of the sensilla trichoidea (St).
Type 7: calcar tectiform, densely denticulate
Similarly to Type 4, it is subtriangular to sickle-shaped in cross-section. The ventral adplantar margin bears 20 or more small teeth, and rows of densely packed mechanosensilla (St). The adplantar side of calcar lacks sensilla.
In Megadelphax sordidula (Delphacinae: Delphacini) (Fig. 7), the 3rd instar’s calcar is short, wide, with 7 teeth; the 4th instar’s adplantar margin of calcar is more elongated and bears 9 teeth and several sensilla chaetica (Fig. 7a,b). The calcar is distinctly elongated in adults, with numerous small teeth (30) and a wide row of the sensilla trichoidea at the adplantar margin (Fig. 7c–f).
In Xanthodelphax straminea (Fig. 8a–c) and Struebingianella lugubrina (Fig. 8d–f) (Delphacinae: Delphacini) the adplantar margin of calcar bears 20 teeth and abundant sensilla trichoidea.
In Chloriona smaragdula (Delphacinae: Delphacini) (Fig. 9a–d), the calcar is strongly elongated, and the adplantar margin bears a row of about 40 small, densely packed teeth (t). A few rows of sensilla are located along the adplantar margin above the teeth (Fig. 9b).
Type 8: calcar densely denticulate with bristly mechanosensilla
This type seems to be further modified than Types 4 and 5, triangular to sickle-shaped in cross-section, with the ventral adplantar margin elongated teeth, and rows of densely packed mechanosensilla and with apex diminutive.
In Stenocranus fuscovittatus (Stenocraninae) (Fig. 10a–f), the calcar adplantar margin presents a row of stout teeth (14), but in Stenocranus major (Fig. 11a–d) the adplantar margin bears 20 teeth. Specific features of this type includes deep separation of each tooth on the adplantar margin and location of the brush of sensilla trichoidea along with the teeth.
Type 9: calcar tectiform toothless (edentate)
Here the calcar is flattened, sickle-like in cross-section, but the explantar (dorsad) edge is obsolete; the edplantar edge is toothless and covered with several rows of sensilla trichoidea.
In Stiroma affinis (Fig. 12a,b,d) and Hyledelphax elegantulus (Fig. 12c) (Delphacinae: Delphacini) the calcar is toothless (edentate). Its edge is covered with several rows of sensilla trichoidea. The proximal and median portions ofcalcar are significantly wider than the distal, tapering portion.
Discussion
Although a charismatic feature of Delphacidae, the calcar has not been previously the subject of detailed comparative and morpho-functional studies. The delphacid tibial calcar has been widely used in taxonomic treatments of members of this family from Muir19 to present times9,68,69. The features of calcar studied under light microscopy were presented by Metcalfe62, Wilson & McPherson63,70, Asche27, Liang64 and Bartlett & Webb65. The pioneering papers with the use of Scanning Electron Microscopy to study the calcar were presented by Mora et al.71 and Liang64. However, since then, no further attention has been directed to studies of calcar structure. The calcar is present also in the nymphs, since the 1st instar; however it is different in size, shape and armature from those in the imagines25,63,70,72,73. A preliminary attempt to trace evolutionary changes of the calcar was presented by Muir19. Later, Wilson & McPherson63 and Asche27 discussed the features of calcar in the evolutionary aspect. A general tendency involves a change from a subulate calcar to a tectiform one (Table 1), which is variously armed and flattened.
These opinions could be confirmed by analysing the data in recent phylogenetic studies of Delphacidae4,5 and our results. In the present study, in SEM, the calcar differs between various tribes of Asiracinae. Type 1 calcar present in Asiracini (Asiracinae) seems to be the most plesiomorphic condition, movable, an awl-shaped and long spine, not different except size and movability from apical teeth. A slightly modified Type 1calcar, more quadrangular in cross-section is mentioned in the genera Notuchoides Donaldson, 1988 and Kiambrama Donaldson, 198874. The same type of calcar is observed in other Asiracini genera Copicerus Swartz, 1802 and Elaphodelphax Fennah, 194927,75.
A little more modified type, subular and with sensilla chaetica and sensilla trichoidea organized in rows (Type 2) is present in Ugyopini (Asiracinae). Within this tribe, in species of the genus Ugyops the calcar is elongate, subular and angulate with sensilla chaetica and sensilla trichoidea arranged in rows. Within this model variabilities are observed in particular species: a row of the sensilla chaetica and two rows of long sensilla trichoidea in U. nemestrinus and U. inermis as well as three rows of short and stout sensilla chaetica in U. taranis. In other known species of Ugyops (Table S1), the calcar presents the same model (Type 2). However, the calcar in examined Notuchus linnavuorii (Ugyopini) is slightly different from the species of Ugyops and more similar to Asiraca (Type 1). It was mentioned in previous works76,77, as short and subulate. This observation is confirmed here. Interestingly, in subterranean species of Asiracini of the genus Notuchus viz. Notuchus kaori Hoch & Asche, 2006 and Notuchus ninguae Hoch & Asche, 2006 the calcar is strongly diminished and vestigial78.
Little can be said about details of the structure of the calcar in other Asiracinae. It seems to be Type 1 in Idiosystatini, Neopunanini, Platysystatini and Tetrasteirini Emeljanov 198527,79,80,81. In Eodelphacini, the calcar represents Type 282.
A few fossil Asiracinae have been reported. In Serafinana perperunae Gębicki & Szwedo, 2000 from the Eocene Baltic amber (Ugyopini), the calcar is subulate, long, with setae arranged in rows as in Type 247 and similar to that in Ugyopini of Dominican amber (Fig. 13). Solórzano-Kraemer49 briefly described another fossil from Miocene Mexican amber, placed in the genus Eucanyra Crawford, 1914 (synonym of Ugyops). The calcar in this fossil also seems to represent Type 2.
In the Vizcayinae (genera Vizcaya Muir, 1917 and Neovizcaya Liang, 2002) the calcar appears to represent Type 3—it is subulate, round in cross section with a row of teeth on the adplantar side (28: Figs. 35, 51,64: Figs. 18, 20, 31, 48, 51, 53).
A similar Type 3 calcar appears in Plesiodelphacinae: genera Burnilia Muir & Giffard, 1924 and Plesiodelphax Asche, 1985 (83: Figs. 284–285); the calcar is cultrate, convex on both sides and with a row of teeth on the adplantar surface83,84.
Type 4 calcar is present in the genera Aloha, Dictyophorodelphax, Leialoha, Nesodryas, Nesorestias and Nesosydne of Delphacinae: Delphacini (19,27: Figs. 294–301,67). Species of these genera inhabiting Hawaii and Marquesas seem to be more related to trees and shrubs of various plant families, mostly dicotyledonous, probably using a greater diversity of microhabitats available85,86 and having adapted to them. Asche10 postulated that this type of calcar evolved independently, possibly to enhance walking on particular surfaces (e.g., on woody substrates). Interestingly, ‘alohine’ calcars were mentioned also in Burnilia (Plesiodelphacinae)84 but earlier Asche83 described the calcar in Plesiodelphacinae as “kelisioid” rather than “alohinid”. An “alohinoid” calcar was also reported for the genus Sparnia Stål, 1862 (Delphacini), by Asche & Emeljanov87.
Within the Kelisiinae (genera Anakelisia Wagner, 1963 and Kelisia), the calcar represents Type 5, with a subtriangular cross-section, a slightly concave adplantar surface and a distinct row of long, conical teeth on the adplantar margin. As observed in Kelisia praecox and Anakelisia fasciata (Kirschbaum, 1868), each tooth bears one or two mechanosensilla. The remainder of calcar surface bears sparse mechanosensilla.
In the Stenocraninae, at least these with known detailed structures of calcar, it is Type 8. This model is observed in Stenocranus major (Kirschbaum, 1868), S. longipennis (Curtis, 1837), S. pacificus Kirkaldy, 1907, Afrotropical Embolophora monoceros Stål, 1855, Stenokelisia angusta Ribaut, 1934, East Palearctic Terauchiana (Terauchiana) singularis Matsumura, 191527,88. This type is also present in New World genera Frameus Bartlett, 2010, Kelisicranus Bartlett, 2006, Obtusicranus Bartlett, 2006, Tanycranus Bartlett, 201089,90,91. It is not clearly evident from available sources, but Type 8 is probably present also in the genus Preterkelisia Yang, 198992,93. Almost nothing is known on the calcar in the Stenocraninae genus Proterosydne, except it, is “… solid, elongate, narrow, with 8 spines” (94: 131), suggesting it could represent Type 5 or Type 8.
Within the representatives of subfamily Delphacinae the calcar diversity is the largest, with Types 5, 6, 7 and 9 distributed among various taxa. In the Saccharosydnini various types are reported, e.g., various species of the genus Saccharosydne Kirkaldy, 1907 present Type 7 (with 18–25 teeth), Lacertinella Rossi Batiz et Remes Lenicov, 2012 presents Type 6 (with 14–20 teeth), Neomalaxa Muir, 1918 Type 6 (with 14–16 small teeth) and also in the genus Pseudomacrocorupha Muir, 1930 Type 6 is present (with 14 teeth)27,30,93,95,96. Based on the available data, representative of the tribe Tropidocephalini probably present Type 9 calcar (toothless tectiform) e.g., Malaxa Melichar, 1914, and Jassidaeus Fieber, 1866 (27:Figs. 289–290), Lamaxa Bartlett & Kennedy, 2018, Xalama Bartlett & Kennedy, 201897. According to Bartlett et al.91, Tropidocephalini can be recognized by the calcar being solid and triangular in cross-section and lacking teeth (although a terminal tooth is often present). This type of calcar was also reported in other genera, e.g., Tropidocephala Stål, 1853, Columbiana Muir, 1919, Jassidaeus, Purohita Distant, 190668,69,91,98,99.
The highest variability of calcar is known among Delphacini, e.g., Type 9 is present in Paranectopia Ding et Tian, 1981 originally described in Tropidocephalini, but moved to Delphacini5. Type 9 is also known in the genera Achorotile Fieber, 1866, Astatometopon Campodonico, 2017, Eurybregma Scott, 1875, Hyledelphax Vilbaste, 1968, Nataliana Muir, 1926, Stiroma Fieber, 186627. Type 5 is present e.g., in the genera Conomelus Fieber, 1866, Onidodelphax Yang, 1989, and species Formodelphax formodus Yang, 198993. The calcar attributed to Type 6 is present e.g., in genera Euconomelus Haupt, 1929, Isodelphax Fennah, 1963, Javesella Fennah, 1963, Laodelphax Fennah, 1963, Megamelus Fieber, 1866, Muellerianella Wagner, 1963, Syndelphax Fennah, 1963100. The calcar representing Type 7 is present in a wide spectrum of genera, e.g., Delphax Fabricius, 1798, Delphacodes Fieber, 1866, Ditropis Kirschbaum, 1868, Abbrosoga Caldwell, 1951, Bostaera Ball, 1902, Chloriona Fieber, 1866, Lepidelphax Lenicov & Walsh, 2013, Megadelphax Wagner, 1963, Megamelodes LeQuesne, 1960, Nilaparvata Distant, 1906, Nycheuma Fennah, 1964, Pseudaraeopus Kirkaldy, 1904, Sogatella Fennah, 1956, Struebingianella Wagner, 1963, Thymalops Fennah, 1965, Unkanodes Fennah, 1956, Xanthodelphax Wagner, 196327,69,101,102.
The exact function of calcar and its mode in Delphacidae remains unresolved. The reasons for the disparity of calcar structures are not well recognized. It is generally believed that the calcar is used to assist in jumping; however, observations and experiments have not confirmed it. The recent observations of Delphacidae jumping mechanisms focused on the thorax, its musculature and base of legs with no attention to the role or function of calcar at the process103,104. With the presence of several types of sensory hairs, the calcar seems to be involved in the process of jumping, but its variability could be related to the surfaces from which the jump is taken. It seems plausible that this diversity is somewhat related to the structure and properties of the surface on which planthopper is living. Delphacids are relatively host-specific, and most mainland species (92% of records) attack monocots; dicot feeding dominates (82% of records) only on oceanic islands3,10,67. The Asiracinae Ugyopini recorded on dicotyledones mainly feed on woody dicots (most probably secondarily) and on monocotyledon Arecaceae. The family Arecaceae comprises 240 genera and approximately 2700 species predominantly concentrated in tropical and subtropical regions105,106, with fossil record reaching Late Cretaceous107. Type 2 calcar, as in Ugyopini, could be, on the one hand, a conservative model and, on the other, an expression of adaptation and a long co-evolutionary history with their host plants. Type 1 calcar, is present in Asiracini; for these planthoppers, most monocot records is related to Cyperaceae, but some are recorded on dicots and even ferns (this is a definitively secondary adaptation to host plant). Cyperaceae crown groups appeared in the Late Cretaceous-Early Paleogene, but their diversification took place at the end of the Eocene108,109. Host plants of Vizcayinae remain unknown; Kelisiinae seem to be strictly associated with Cyperacae and Juncaceae, plants of extraordinary ecological importance, occupying a broad range of habitats from rain forests to tundra, as components of open habitats including many types of wetlands, temperate and tropical grasslands and savannas, especially in moist sites, or more shaded ones as understory of forests110,111. The evolutionary shifts in the history of these plants took place at the terminal Eocene-Early Oligocene, during a global cooling period and in the Miocene, during global warming, then cooling periods, resulting in rapid diversification111,112. Therefore it could be assumed that Kelisiinae retaining Type 5 of calcar shifted to these host plants simultaneously with or after their diversification and spreading. In Stenocraninae, the calcar of Type 8 is present, which could be related to adaptations to a broader array of host plants. Stenocraninae seem to be strongly associated with monocotyledons in modern fauna, with a clear dominance of Poaceae over Cyperaceae3,90. In Delphacinae, the variability of types of calcar is the highest (Table1); while in vast majority they feed on various Poaceae, shifts to other monocots or dicotyledons are known3,4. Also, modifications of calcar were reported in Delphacini taxa associated with water plants (e.g., waterlily), e.g. Megamelus davisi Van Duzee, 1897 with an exceptionally large, thin and leaflike calcar with about 20 small teeth, but allowing to place it to Type 7113,114,115,116. The shape of the calcar probably facilitates jumping from the water surface (personal communication, Ch. Bartlett). Delphacini seem to have experienced several host shifts but do not present a co-evolutionary pattern; representatives of this lineage are clearly ecological opportunists4. Therefore the variability and disparity of calcar and its pattern in this group is homeoplasous, not giving strong phylogenetic signal, but could be a good tool to understand the ecological history of the Delphacini and their temporal shifts and adaptations to host plants. Increased diversification within Delphacini may reflect a shift to grass-feeding, and host shifts within Poaceae, perhaps from grasses with C3–C4 photosynthetic pathways4, and different anatomical features of C3 and C4 grasses117. It must be noted that host plant associated diversification within Delphacidae was mediated by co-evolutionary relationships with endosymbiotic bacteria and fungi118,119; therefore, the natural selection and adaptation of these planthoppers took place at various planes.
The calcar is the most significant character defining Delphacidae but its evolutionary origin is not fully resolved. Basing on its postembryonic development, it is supposed that the calcar is derived from one of lateral apical teeth of the hind-tibia as present in Cixiidae29,63,120. Modern Cixiidae, as well as most of the known fossils, present some variability in the armature of metatibial apex; however, the elongate outer tooth of the external group often stands out, and the two next teeth of the same group are shorter; these three teeth form a medial group, and three external teeth form an opposed group. In the tribes Oecleini Muir, 1922 and Gelastocephalini Emeljanov, 2000 the teeth of external and medial groups are separated with an interspace—the row is interrupted by diastema121. Such a pattern could correspond to the condition present in Delphacidae ancestors. Emeljanov29 discussed the possible transformations of metatibial apical teeth proposed earlier by Asche27 and interpreted formation of calcar setation differently; he postulated four indistinct rows of setae as a plesiomorphic condition with further transformations due to metatopy. Recently Fu et al.122 indicated that the cloned full-length Ubx ortholog (NlUbx) activates the development of spines on the T3 tibia and basitarsus. The Hox gene Ultrabithorax (Ubx) plays pivotal roles in modifying specific morphological differences between T2 and T3 in various hemipterans122,123,124. However, more data and observations are needed to fully understand the developmental and evolutionary ways of calcar origination and formation.
The fossil record of Delphacidae is very scarce; Eocene Baltic amber inclusion Serafinana perperunae Gębicki & Szwedo, 2000, represents Ugyopini, Solórzano Kramer (2007) mentioned not formally described Ugyopini (identified as Eucanyra—synonym of Ugyops Guérin-Méneville, 1834) from Miocene Mexican amber; more Ugyopini were found as inclusions in Miocene Dominican amber (Fig. 13). The other Miocene taxon: Amagua fortis Cockerell, 1924 from Late Eocene/Early Oligocene of Amgu (Amagu) River, Sikhote-Alin, Russia is a fragmentary imprint of forewing and body, not to be placed in any subfamily or tribe; Chloriona stavropolitana Becker-Migdisova, 1964 from the Messinian, Miocene of Vishnevaya Balka, Northern Caucasus, Russia based on partly preserved tegmen, seems to be the oldest record of Delphacini. It is doubtful whether the Ypresian, Eocene, and Green River Formation fossils: Delphax senilis Scudder, 1870 and Delphax veterum Cockerell, 1921 should be assigned to Delphacidae and the original materials must be revised; Chattian, Oligocene fossil named ‘Delphax’ rhenana Statz, 1950 does not seem to represent Delphacidae48,50,52,53,54. The calcar from fossil resins is not different from the one present in modern Ugyopini. The taphonomic potential of Delphacidae for fossilisation as adpressions seems not to be high due to various extrinsic and intrinsic factors125,126; therefore, the inclusions in amber and other fossil resins remain an invaluable source of information.
Conclusion
The calcar of Delphacidae is their unique synapomorphy, defining the family as a whole. Its structure is highly variable, but the use and estimation of its phylogenetic and classification values seem challenging, as detailed knowledge of theof calcar structure and its function remains limited. On the other hand, the variability and disparity of calcar and its patterns observed in Delphacidae could be a good tool to understand the ecological history of these insects and their temporal shifts and adaptations to host plants. Being a charismatic character of the Delphacidae, the calcar still poses a number of problems to be addressed, e.g., its evolutionary origin, the developmental ways of formation, factors influencing its disparity, even the exact function. Here, we presented the first attempt to systematize calcar models and structures and evaluate its potential in morphological, evolutionary and ecological studies. We have also justified the need for restudying this morphological structure.
Abbreviations
- USMB:
-
Upper Silesian Museum in Bytom
- DZUS:
-
Department of Zoology, University of Silesia
- LTIB:
-
Laboratory of Terrestrial Invertebrates: State Scientific and Production Amalgamation The Scientific and Practical Center for Bioresources, National Academy of Sciences of Belarus
- mtt:
-
Metatibial apical row of teeth
- ms:
-
Membranous connection of the calcar
- clc:
-
Calcar
- s:
-
Socket
- m:
-
Flexible membrane
- t:
-
Calcar tooth
- St:
-
Sensillum trichoideum
- Sch:
-
Sensillum chaeticum
References
Urban, J. M. & Cryan, J. R. Evolution of the planthoppers (Insecta: Hemiptera: Fulgoroidea). Mol. Phylogenet. Evol. 42(2), 556–572. https://doi.org/10.1016/j.ympev.2006.08.009 (2007).
Bourgoin, T. FLOW (Fulgoromorpha Lists on The Web); A World Knowledge Base Dedicated to Fulgoromorpha. Version 8. Updated 11 June 2021. https://hemiptera-databases.org/flow/.
Wilson, S. W., Mitter, C., Denno, R. F. & Wilson, M. R. Evolutionary patterns of host plant use by delphacid planthoppers and their relatives. In Planthoppers: Their Ecology and Management (eds Denno, R. F. & Perfect, T. J.) 7–113 (Chapman & Hall, 1994).
Urban, J. M., Bartlett, C. R. & Cryan, J. R. Evolution of Delphacidae (Hemiptera: Fulgoroidea): Combined-evidence phylogenetics reveals importance of grass host shifts. Syst. Entomol. 35(4), 678–691. https://doi.org/10.1111/j.1365-3113.2010.00539.x (2010).
Huang, Y. X., Zheng, L. F., Bartlett, C. R. & Qin, D. Z. Resolving phylogenetic relationships of Delphacini and Tropidocephalini (Hemiptera: Delphacidae: Delphacinae) as inferred from four genetic loci. Sci. Rep. 7(3319), 1–10. https://doi.org/10.1038/s41598-017-03624-w (2017).
Wheeler, A. G. Jr. Bryophagy in the Auchenorrhyncha: Seasonal history and habits of a moss specialist, Javesella opaca (Beamer) (Fulgoroidea: Delphacidae). Proc. Entomol. Soc. Wash. 105(3), 599–610 (2003).
Glime, J. M. Terrestrial Insects: Hemimetabola: Hemiptera (Non-Heteroptera) and Thysanoptera. Chapter 12–7. In Bryophyte Ecology (ed. Glime, J. M.) Volume 2. (2017). Interactions. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. eBook last updated 19 July 2020. http://digitalcommons.mtu.edu/bryophyte-ecology2/.
Nickel, H. The Leafhoppers and Planthoppers of Germany (Hemiptera Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects 460 (Co-published by Pensoft Publishers Sofia-Moscow and Goecke & Evers, 2003).
Bartlett, C. R., O’Brien, L. B. & Wilson, S. W. A review of the planthoppers (Hemiptera: Fulgoroidea) of the United States. Mem Am Entomol Soc. 50, 1–287 (2014).
Asche, M. A review of the systematics of Hawaiian planthoppers (Hemiptera: Fulgoroidea). Pac. Sci. 51(4), 366–376 (1997).
Wilson, S. W. Keys to the families of Fulgoromorpha with emphasis on planthoppers of potential economic importance in the Southeastern United States (Hemiptera: Auchenorrhyncha). Fla. Entomol. 88(4), 464–481 (2005).
Zhu, J.-L. et al. Effect of rice sowing date on occurrence of small brown planthopper and epidemics of planthopper-transmitted rice stripe viral disease. Agric. Sci. China 8(3), 332–341 (2009).
Zhang, H.-M., Wang, H.-D., Jian, Y., Adams, M. J. & Chen, J.-P. Detection, occurrence, and survey of rice stripe and black-streaked dwarf diseases in Zhejiang Province China. Rice Sci. 20(6), 383–390 (2013).
Heong, J. C. & Escalada, M. M. (eds) Rice planthoppers: Ecology Management Socio Economics and Policy 1st edn, 231 (Springer-Verlag, 2015).
Zhou, C., Yang, H., Wang, Z., Long, G.-Y. & Jin, D.-C. Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 8, 8773. https://doi.org/10.1038/s41598-018-27062-4 (2018).
Asche, M. Preliminary thoughts on the phylogeny of Fulgoromorpha (Homoptera, Auchenorrhyncha). Proc. 6th Auchenorrhyncha Meeting, Turin, Italy. 7–11 September 1987 47–53 (Consiglio Nazionale delle Ricerche, 1988).
Emeljanov, A.F. Opyt postroeniya filogeneticheskogo dreva fulgoroidnykh tsikadovykh (Homoptera, Cicadina). Entomol. Obozr, 69, 353–356 (1990). Translated into English as: Emel’yanov, A.F. 1991. An attempt to construct a phylogenetic tree for planthoppers (Homoptera, Cicadina). Entomol. Rev. 70, 24–28 (1990).
Bartlett, C. R. et al. The diversity of the true hoppers (Hemiptera: Auchenorrhyncha). In Insect Biodiversity. Science and Society, 2 (eds Foottit, R. G. & Adler, P. H.) 501–590 (Wiley-Blackwell, 2018).
Muir, F. A. G. A contribution toward the taxonomy of the Delphacidae. Can. Entomol. 47, 208–212 (1915).
Muir, F. A. G. On the classification of the Fulgoroidea. Ann. Mag. Nat. Hist. 10(6), 461–478 (1930).
Metcalf, Z. P. General Catalogue of the Hemiptera. Fascicle IV, Fulgoroidea, Part 3, Araeopidae (Delphacidae) 552 (Northampton, Smith College, 1943).
Haupt, H. Neueinteilung der Homoptera-Cicadina nach phylogenetisch zu wertenden Merkmalen. Zool. Jahrb. Abt Syst. Okol. Geograph. Tiere 58, 173–286 (1929).
Nast, J. Palaearctic Auchenorrhyncha (Homoptera) An annotated check list 550 (Państwowe Wydawnictwo Naukowe [Polish Scientific Publishers], 1972).
Wagner, W. Dynamische Taxionomie, angewandt auf die Delphaciden Mitteleuropas. Mitt. Hamb. Zool. Mus. Inst. 60, 111–180 (1963).
Vilbaste, J. Preliminary key for the identification of the nymphs of North European Homoptera Cicadinea. Ann. Ent. Fenn. 34, 65–74 (1968).
Fennah, R.G. Tribal classification of Asiracine Delphacidae (Homoptera: Fulgoroidea). Entomologist’s Record I (V/79) (1979).
Asche, M. Zur Phylogenie der Delphacidae Leach, 1815 (Homoptera: Cicadina: Fulgoromorpha). Marburger Ent. Publ. 1, 1–398 (1985b); (volume 1), 2, 399–910 (volume 2) (1985b).
Asche, M. Vizcayinae, a new subfamily of Delphacidae with revision of Vizcaya Muir (Homoptera: Fulgoroidea): A significant phylogenetic link. Bishop Mus. Occas. Pap. 30, 154–187 (1990).
Emeljanov, A. F. K voprosu o sistemie i filogenii sem. Delphacidae (Homoptera, Cicadina) c uchetom lichinochnykh priznakov. Entomol. Obozr. 74 (4), 780–794 (1995). Translated in to English as: Yemel’yanov, A.F. 1996. On the question of the classification and phylogeny of the Delphacidae (Homoptera, Cicadina), with reference to larval characters. Entomol. Rev. 75, 134–150 (1996).
Anufriev, G. A. & Emeljanov, A. F. Suborder Cicadinea—(Auchenorrhyncha). In Keys to the insects of the Far East of the USSR, 2 (ed. Ler, P. A.) 12–495 (Nauka, 1988).
Hamilton, K. G. A. The planthopper genus Stenocranus in Canada: Implications for classification of Delphacidae (Hemiptera). Can. Entomol. 138(4), 493–503. https://doi.org/10.4039/n06-805 (2006).
Dijkstra, E., Rubio, J. M. & Post, R. J. Resolving relationships over a wide taxonomic range in Delphacidae (Homoptera) using the COI gene. Syst. Entomol. 28, 89–100. https://doi.org/10.1046/j.1365-3113.2003.00203.x (2003).
Dijkstra, E., Slotman, M. A. & Post, R. J. Resolution of phylogenetic relationships of the major subfamilies of the Delphacidae (Homoptera: Fulgoroidea) using the mitochondrial ribosomal DNA. Insect Sci. 13, 167–177. https://doi.org/10.1111/j.1744-7917.2006.00079.x (2006).
Song, N. & Liang, A.-P. A preliminary molecular phylogeny of planthoppers (Hemiptera: Fulgoroidea) based on nuclear and mitochondrial DNA sequences. PLoS ONE 8(3), e58400. https://doi.org/10.1371/journal.pone.0058400 (2013).
Huang, Y. X. & Qin, D. Z. The complete mitochondrial genome sequence of the corn planthopper, Peregrinus maidis (Hemiptera: Fulgoroidea). Mitochondrial DNA Part B 2, 783–784 (2017).
Huang, Y. X. & Qin, D. Z. Sequencing and analysis of the complete mitochondrial genome of Changeondelphax velitchkovskyi (Hemiptera: Fulgoroidea). Mitochondrial DNA Part B 3, 90–91 (2018).
Huang, Y. X. & Qin, D. Z. First mitogenome for the tribe Saccharosydnini (Hemiptera: Fulgoroidea: Delphacidae: Delphacinae) and the phylogeny of three predominant rice planthoppers. Eur. J. Entomol. 115, 242–248. https://doi.org/10.14411/eje.2018.023 (2018).
Huang, Y.-X., Ren, F. J., Bartlett, C. R., Wei, Y. S. & Qin, D. Z. Contribution to the mitogenome diversity in Delphacinae: Phylogenetic and ecological implications. Genomics 112(2), 1363–1370. https://doi.org/10.1016/j.ygeno.2019.08.005 (2020).
Wallner, A. M. & Bartlett, C. R. Comparative morphology of female gonapophyses IX in Delphacidae (Hemiptera: Auchenorrhyncha: Fulgoromorpha) with key to tribes. Zootaxa 4564(1), 137–172. https://doi.org/10.11646/zootaxa.4564.1.5 (2019).
Muir, F. A. G. On the classification of the Fulgoroidea (Homoptera). Proc. Hawaiian Ent. Soc. 5, 205–247 (1923).
Ceotto, P. & Bourgoin, T. Insights into the phylogenetic relationships within Cixiidae (Hemiptera: Fulgoromorpha): Cladistic analysis of a morphological dataset. Syst. Entomol. 33(3), 484–500. https://doi.org/10.1111/j.1365-3113.2008.00426.x (2008).
Bourgoin, T., Steffen-Campbell, J. D. & Campbell, B. C. Molecular phylogeny of fulgoromorpha (Insecta, Hemiptera, Archaeorrhyncha). The enigmatic Tettigometridae: Evolutionary affiliations and historical biogeography. Cladistics 13(3), 207–224. https://doi.org/10.1111/j.1096-0031.1997.tb00316.x (1997).
Ceotto, P., Kergoat, G. J., Rasplus, J. Y. & Bourgoin, T. Molecular phylogenetics of cixiid planthoppers (Hemiptera: Fulgoromorpha): New insights from combined analyses of mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 48(2), 667–678. https://doi.org/10.1016/j.ympev.2008.04.026 (2008).
Bucher, M. & Bourgoin, T. Which biases still hamper a solid planthopper phylogeny? 16thInternational Auchenorrhyncha Congress, 12th International Workshop on Leafhoppers and Planthoppers of Economic Significance, Cuc Phuong NP, Vietnam, July 2nd 8th, 2019. Program and Abstracts 52–53 (National Museum of Nature, 2019).
Fennah, R. G. The occurrence of a cixiine fulgoroid (Homoptera) in the Weald Clay. Ann. Mag. Nat. Hist. 4(39), 161–163. https://doi.org/10.1080/00222936108655796 (1961).
Fennah, R. G. A new genus and species of Cixiidae (Homoptera: Fulgoroidea) from Lower Cretaceous amber. J. Nat. Hist. 21(5), 1237–1240. https://doi.org/10.1080/00222938700770751 (1987).
Gębicki, C. & Szwedo, J. The first ugyopine planthopper Serafinana perperunae gen. and sp. n. from Eocene Baltic amber (Hemiptera, Fulgoroidea: Delphacidae). Pol.J. Entomol./Pol. Pismo Entomol. 69(4), 389–395 (2000).
Szwedo, J., Bourgoin, T. & Lefèbvre, F. Fossil Planthoppers (Hemiptera Fulgoromorpha) of the World. An Annotated Catalogue with Notes on Hemiptera Classification. 199 (Studio 1, 2004).
Solórzano Kraemer, M. M. Systematic, palaeoecology, and palaeobiogeography of the insect fauna from Mexican amber. Palaeontogr. Abt. A 282(1–6), 1–133. https://doi.org/10.1127/pala/282/2007/1 (2007).
Scudder, S. H. The first discovered traces of fossil insects in the American Tertiaries. Geol. Surv. Can. 3, 741–762 (1877).
Scudder, S. H. The fossil insects of North America (with notes on some European species). 2. The Tertiary insects of North America. U. S. Geol. Surv. 13, 1–734 (1890).
Cockerell, T. D. A. Some Eocene insects from Colorado and Wyoming. Proc. U. S. Natl. Mus. 59, 29–39 (1921).
Cockerell, T. D. A. Fossil insects in the United States National Museum. Proc. U. S. Natl. Mus. 64(13), 1–15 (1924).
Statz, G. Cicadariae (Zikaden) aus den Oberoligocanen Ablagerungen von Rott. Palaeontogr. Abt. A 98, 1–46 (1950).
Davies, R. G. Insect structure and function. In Outlines of Entomology 7th edn 7–96 (Chapman & Hall, 1988).
Gullan, P. J. & Cranston, P. S. The Insects: An Outline of Entomology 5th edn, 624 (Wiley-Blackwell, 2014).
Roth, L. M. & Willis, E. R. Tarsal structure and climbing ability of cockroaches. J. Exp. Zool. 119, 483–517. https://doi.org/10.1002/jez.1401190307 (1952).
Gladun, D. & Gorb, S. N. Insect walking techniques on thin stems. Arthropod Plant Interact. 1(2), 77–91. https://doi.org/10.1007/s11829-007-9007-2 (2007).
Spagna, J. C., Goldman, D. I., Lin, P.-C., Koditschek, D. E. & Full, R. J. Distributed mechanical feedback in arthropods and robots simplifies control of rapid running on challenging terrain. Bioinspir. Biomim. 2(1), 9–18. https://doi.org/10.1088/1748-3182/2/1/002 (2007).
Bußhardt, P., Kunze, D. & Gorb, S. Interlocking-based attachment during locomotion in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). Sci. Rep. 4, 6998. https://doi.org/10.1038/srep06998 (2014).
Beutel, R. G. et al. Distal leg structures of the Aculeata (Hymenoptera): A comparative evolutionary study of Sceliphron (Sphecidae) and Formica (Formicidae). J. Morphol. 281(7), 737–753. https://doi.org/10.1002/jmor.21133 (2020).
Metcalfe, J. R. Studies on the biology of the sugarcane pest Saccharosydne saccharivora Homoptera Delphacidae. Bull. Entomol. Res. 59 (3), 393–408. https://doi.org/10.1017/S0007485300003370 (1969[1968])
Wilson, S. W. & McPherson, J. E. Ontogeny of the tibial spur in Megamelus davisi (Homoptera: Delphacidae) and its bearing on delphacid classification. Great Lakes Entomol. 14(1), 49–50 (1981).
Liang, A.-P. New taxa of Vizcayinae (Hemiptera: Auchenorrhyncha: Delphacidae), including a remarkable new genus from China. J. Nat. Hist. 36, 601–616. https://doi.org/10.1080/00222930110062327 (2002).
Bartlett, C. R. & Webb, M. D. The planthopper genus Spartidelphax, a new segregate of Nearctic Delphacodes (Hemiptera, Delphacidae). ZooKeys 453, 19–36. https://doi.org/10.3897/zookeys.453.8369 (2014).
Zimmerman, E. C. Insects of Hawaii 4. Homoptera: Auchenorhyncha 268 (University Hawaii Press, 1948).
Ding, J. H. Homoptera Delphacidae Fauna Sinica, Insecta Vol. 45, 776 (Science Press, 2006).
Dellagiustina, W. Les Delphacidae de France et des Pays Limitrophes Faune de France, 100 Vol. 1, 400–432 (Fédération Française des Sociétés de Sciences Naturelles, 2019).
Wilson, S. W. & McPherson, J. E. Life history of Megamelus davisi with descriptions of immature stages. Ann. Entomol. Soc. Am. 74, 345–350. https://doi.org/10.1093/aesa/74.4.345 (1981).
Mora, R., Retana, A. & Espinoza, A. M. External morphology of Tagosodes orizicolus (Homoptera: Delphacidae) revealed by Scanning Electron Microscopy. Ann. Entomol. Soc. Am. 94(3), 438–448. https://doi.org/10.1603/0013-8746(2001)094[0438:EMOTOH]2.0.CO;2 (2001).
Wilson, S. W. Descriptions of the immature stages of Delphacodes bellicosa (Homoptera: Fulgoroidea: Delphacidae). Pan-Pac. Entomol. 61, 72–78 (1985).
Rossi Batiz, M. F. & de RemesLenicov, A. M. M. Description of the immature stages of the planthopper Lacertinella australis (Hemiptera: Delphacidae). J. Insect Sci. 14(113), 1–9. https://doi.org/10.1673/031.014.113 (2014).
Donaldson, J. F. Further studies on Asiracinae (Homoptera: Delphacidae) In Australia and New Caledonia. J. Austral. Entomol. Soc. 27, 133–141. https://doi.org/10.1111/j.1440-6055.1988.tb01161.x (1988).
Llano, C. A., Bartlett, C. R. & Guevara, G. First record of the subfamily Asiracinae and Copicerus irroratus (Hemiptera: Auchenorrhyncha: Delphacidae) in Colombia. Fla. Entomol. 99(1), 120–122. https://doi.org/10.1653/024.099.0123 (2016).
Donaldson, J. F. Revision of the genus Notuchus Fennah (Homoptera: Fulgoroidea: Delphacidae). J. Austral. Entomol. Soc. 18, 181–185 (1979).
Gębicki, C., Walczak, M., Krupa, P. & Kalandyk-Kołodziejczyk, M. Ugyopini of New Caledonia (Hemiptera: Fulgoromorpha: Delphacidae: Asiracinae) with a description of Notuchus linnavuorii sp. nov. Bonn Zool. Bul. 70(1), 97–113 (2021).
Hoch, H., Asche, M., Burwell, C., Monteith, G. M. & Wessel, A. Morphological alteration in response to endogeic habitat and ant association in two new planthopper species from New Caledonia (Hemiptera: Auchenorrhyncha: Fulgoromorpha: Delphacidae). J. Nat. Hist. 40(32), 1867–1886. https://doi.org/10.1080/00222930601046576 (2006).
Asche, M. Aufgliederung der Asiracinen-Gattung Punana Muir, 1913: Equasystatus gen. nov. aus Equador und Neopunana gen. nov. von den Karibischen Inseln (Homoptera: Auchenorrhyncha: Fulgoromorpha: Delphacidae). Marburger Ent. Publ. 1(8), 127–166 (1983).
Barringer, L. E. & Bartlett, C. R. A review of New World Asiracinae (Hemiptera: Auchenorrhyncha: Delphacidae) with five new taxa. Cicadina 12, 7–39 (2011).
Asche, M. & Webb, M. D. A remarkable new asiracine delphacid planthopper from Ecuador (Hemiptera, Fulgoroidea, Delphacidae). Dtsch. Entomol. Z. 60(2), 163–169. https://doi.org/10.1002/mmnd.201300022 (2013).
Chen, X.-S. & Hou, X.-H. Revision of the planthopper tribe, Eodelphacini, in China (Hemiptera, Fulgoromorpha, Delphacidae) with descriptions of one new genus and two new species. Fla. Entomol. 95(2), 362–374. https://doi.org/10.1653/024.095.0219 (2012).
Asche, M. A new subfamily, genus and species of Delphacidae from South America: Plesiodelphacinae subfam. nov., Plesiodelphax guayanus gen. et spec. nov. (Homoptera Fulgoroidea). Marburger Entomol. Publ. 1(10), 219–240 (1985).
Asche, M., Hayashi, M. & Fujinuma, S. Enigmatic distribution: First record of a hitherto New World planthopper taxon from Japan (Hemiptera, Fulgoroidea, Delphacidae, Plesiodelphacinae). Dtsch. Entomol. Z. 63(1), 75–88. https://doi.org/10.3897/dez.63.7178 (2016).
Denno, R. F. Influence of habitat structure on the abundance and diversity of planthoppers. In Planthoppers: Their Ecology and Management (eds Denno, R. F. & Perfect, T. J.) 140–159 (Chapman & Hall, 1994).
Denno, R. F. Life history variation in planthoppers. In Planthoppers: Their Ecology and Management (eds Denno, R. F. & Perfect, T. J.) 163–215 (Chapman & Hall, 1994).
Asche, M. & Emeljanov, A. F. Review of the Neotropical genus Sparnia Stål (Hemiptera, Fulgoroidea: Delphacidae). Entomol. Rev. 96(9), 1209–1233. https://doi.org/10.1134/S0013873816090062 (2016).
Susilo, F. X. et al. The white-bellied planthopper (Hemiptera: Delphacidae) infesting corn plants in South Lampung, Indonesia. J. Hama Penyakit Tumbuh. Trop. 17(1), 96–103. https://doi.org/10.23960/j.hptt.11796-103 (2017).
Bartlett, C. R. Two new genera and species of stenocranine planthoppers (Hemiptera: Delphacidae) from North America. Entomol. News 116(5), 291–303 (2006).
Bartlett, C. R. Diversity in New World Stenocranine planthoppers (Hemiptera: Delphacidae). Trans. Am. Entomol. Soc. 135 (4), 443–486. https://doi.org/10.3157/061.135.0407 (2010[2009]).
Bartlett, C. R. & contributors. Planthoppers of North America. https://sites.udel.edu/planthoppers/ 2020 (and updates), (accessed on [24.01.2021]).
Ding, J. H. & Kuoh, C. L. New species of Stenocranus from China (Homoptera: Delphacidae). Acta Zootaxon. Sin. 6(1), 74–84 (1981).
Yang, C. T. Delphacidae of Taiwan (II). Natl. Sci. Council Spec. Publ. 6, 1–334 (1989).
Kirkaldy, G.W. Leafhoppers – Supplement (Hemiptera). Bull. Div. Ent. Hawaiian Sug. Plant. Assoc. 3, 1–186 (1907a).
de Remes Lenicov, A. M. M. & Rossi Batiz, M. F. A new species of Saccharosydne Kirkaldy from Argentina (Hemiptera: Delphacidae). Neotrop. Entomol. 39(4), 584–589 (2010).
Rossi-Batiz, M. F. Taxonomía, Distribución y Biología de la Tribu Saccharosydnini (Insecta-Hemiptera-Fulgoromorpha). Universidad Nacional de La Plata, La Plata, Buenos Aires, Argentina. Doctoral Thesis, 183 pp. (2014).
Bartlett, C. R. & Kennedy, A. C. A review of New World Malaxa (Hemiptera: Fulgoroidea: Delphacidae). Zootaxa 441(3), 511–528. https://doi.org/10.11646/zootaxa.4441.3.5 (2018).
Mirza, R. P. The Delphacid Planth-Hoppers (Homoptera: Fulgoroidea, Delphacidae) of East and West Pakistan (A Part of Oriental Rerion). A Taxonomic, Ecological and Economic study. Department of Zoology, University of Karachi, Karachi, Doctoral thesis, x+239 pp. (1969).
Yang, J. T. & Yang, C. T. Delphacidae of Taiwan (I) Asiracinae and the tribe Tropidocephalini (Homoptera: Fulgoroidea). Nat. Taiwan Mus. Spec. Publ. 6, 64–76 (1986).
Fennah, R. G. New genera of Delphacidae (Homoptera: Fulgoroidea). Proc. R. Entomol. Soc Lond. (B) 32, 15–16 (1963).
Caldwell, J. S. & Martorell, L. F. Review of the auchenorynchous Homoptera of Puerto Rico. Part II. The Fulgoroidea except Kinnaridae. J. Agric. Univ. Puerto Rico 34(2), 133–269 (1951).
Sohail, K., Naveed, H., Qin, D. & Zhang, Y. Taxonomic study of the genus Unkanodes (Hemiptera, Fulgoroidea, Delphacidae) from Pakistan, with description of a new species. ZooKeys 995, 1–13. https://doi.org/10.3897/zookeys.995.48766 (2020).
Ogawa, N. & Yoshizawa, K. Morphological dissection of behavior: Thoracic musculature clarifies independent development of jumping mechanisms between sister groups, planthoppers and leafhoppers (Insecta: Hemiptera: Auchenorrhyncha). Org. Divers. Evol. 17(3), 521–530. https://doi.org/10.1007/s13127-017-0336-4 (2017).
Siwanowicz, I. & Burrows, M. Three dimensional reconstruction of energy stores for jumping in planthoppers and froghoppers from confocal laser scanning microscopy. Elife 6, e23824. https://doi.org/10.7554/eLife.23824 (2017).
Dransfield, J. et al. Genera Palmarum: The Evolution and Classification of Palms (Royal Botanic Gardens, 2008).
Govaerts, R., Dransfield, J., Zona, S.F., Hodel, D.R. & Henderson, A. World Checklist of Arecaceae. (Royal Botanic Gardens, 2021). http://wcsp.science.kew.org/.
Matsunaga, K. K. S. & Smith, S. Y. Fossil palm reading: Using fruits to reveal the deep roots of palm diversity. Am. J. Bot. 108(3), 1–23. https://doi.org/10.1002/ajb2.1616 (2021).
APG IV. Angiosperm Phylogeny Website, version 14. July 2017. last updated 06 February 2021. http://www.mobot.org/MOBOT/research/APweb/.
Magallón, S., Sánchez-Reyes, L. L. & Gómez-Acevedo, S. L. Thirty clues to the exceptional diversification of flowering plants. Ann. Bot. 123(3), 491–503. https://doi.org/10.1093/aob/mcy182 (2019).
Semmouri, I. et al. Phylogeny and systematics of Cyperaceae, the evolution and importance of embryo morphology. Bot. Rev. 85, 1–39. https://doi.org/10.1007/s12229-018-9202-0 (2019).
Riess, K. et al. The origin and diversification of the Entorrhizales: Deep evolutionary roots but recent speciation with a phylogenetic and phenotypic split between associates of the Cyperaceae and Juncaceae. Org. Divers. Evo. 19, 13–30. https://doi.org/10.1007/s13127-018-0384-4 (2019).
Bouchenak-Khelladi, Y., Muasya, A. M. & Linder, H. P. A revised evolutionary history of Poales: Origins and diversification. Bot. J. Linn. Soc. 175(1), 4–16. https://doi.org/10.1111/boj.12160 (2014).
de Remes Lenicov, A. M. M., Defea, B., Rusconi, J. & Cabrera Walsh, G. Studies on the immature stages of the planthopper Lepidelphax pistiae (Hemiptera: Delphacidae), a potential biocontrol agent for the aquatic weed Pistia stratiotes (Araceae) from Argentina. Austral. Entomol. 56, 384–391. https://doi.org/10.1111/aen.12248 (2017).
de Remes Lenicov, A. M. M. & Walsh, G. C. A new genus and species of Delphacini (Hemiptera: Fulgoromorpha: Delphacidae) associated with hydrophytic plants in Argentina. Fla. Entomol. 96(4), 1350–1358 (2013).
Beardsley, J. W. Notes on immigrant delphacid planthoppers in Hawaii (Homoptera: Fulgoroidea). Proc. Hawaii Entomol. Soc. 30, 121–129 (1990).
Sosa, A., de Remes Lenicov, A. M. M. & Mariani, R. Species of Megamelus (Hemiptera: Delphacidae) associated with pontederiaceae in South America. Ann. Entomol. Soc. Am. 100(6), 798–809. https://doi.org/10.1603/0013-8746(2007)100[798:SOMHDA]2.0.CO;2 (2007).
Ueno, O., Kawano, Y., Wakayama, M. & Takeda, T. Leaf vascular systems in C3 and C4 grasses: A two-dimensional analysis. Ann. Bot. 97(4), 611–621. https://doi.org/10.1093/aob/mcl010 (2006).
Müller, H. J. Die symbiose der Fulgoroiden (Homoptera-Cicadina). Zoologica 98, 1–220 (1940).
Douglas, A. E. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 14, 731–743. https://doi.org/10.1038/nrmicro.2016.151 (2016).
Asche, M. New phylogeny of Delphacidae and its implication in geographic distribution. Mitt. Schweiz. Entomol. Ges. 57(4), 407–409 (1984).
Emeljanov, A. F. Contribution to classification and phylogeny of the family Cixiidae (Hemiptera, Fulgoromorpha). Denisia 176, 103–112 (2002).
Fu, S. J. et al. Functional analysis of Ultrabithorax in the wing-dimorphic planthopper Nilaparvata lugens (Stål, 1854) (Hemiptera: Delphacidae). Gene 737(144446), 1–8. https://doi.org/10.1016/j.gene.2020.144446 (2020).
Angelini, D. R., Liu, P. Z., Hughes, C. L. & Kaufman, T. C. Hox gene function and interaction in the milkweed bug Oncopeltus fasciatus (Hemiptera). Dev. Biol. 287(2), 440–455. https://doi.org/10.1016/j.ydbio.2005.08.010 (2005).
Mahfooz, N., Turchyn, N., Mihajlovic, M., Hrycaj, S. & Popadić, A. Ubx regulates differential enlargement and diversification of insect hind legs. PLoS ONE 2(9), e866. https://doi.org/10.1371/journal.pone.0000866 (2007).
Behrensmeyer, A. K., Susan, M., Kidwell, S. M. & Castaldo, R. A. Taphonomy and paleobiology. Paleobiology 26(S4), 103–147. https://doi.org/10.1017/S0094837300026907 (2000).
Grupe, G. & Harbeck, M. Taphonomic and diagenetic processes. In Handbook of Paleoanthropology (eds Henke, W. & Tattersall, I.) 417–439 (Springer, 2015).
Acknowledgements
The authors would like to thank the University of Silesia in Poland for the access to the Laboratory of Scanning Microscopy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.M., M.W. and J.B., Methodology, D.M., M.W.and J.B, Investigation, D.M., M.W., J.B. and J.S., Data curation, M.W., J.B. and J.S., Writing—Original Draft Preparation, D.M., M.W., O.B., J.S. and J.B. Writing—Review & Editing, J.S. and J.B. Supervision, J.B. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markevich, D., Walczak, M., Borodin, O. et al. Morphological reassessment of the movable calcar of delphacid planthoppers (Hemiptera: Fulgoromorpha: Delphacidae). Sci Rep 11, 22294 (2021). https://doi.org/10.1038/s41598-021-01771-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01771-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.