Abstract
Cardiac alterations are frequently observed after acute neurological disorders. Takotsubo syndrome (TTS) represents an acute heart failure syndrome and is increasingly recognized as part of the spectrum of cardiac complications observed after neurological disorders. A systematic investigation of TTS patients with neurological disorders has not been conducted yet. The aim of the study was to expand insights regarding neurological disease entities triggering TTS and to investigate the clinical profile and outcomes of TTS patients after primary neurological disorders. The International Takotsubo Registry is an observational multicenter collaborative effort of 45 centers in 14 countries (ClinicalTrials.gov, identifier NCT01947621). All patients in the registry fulfilled International Takotsubo Diagnostic Criteria. For the present study, patients were included if complete information on acute neurological disorders were available. 2402 patients in whom complete information on acute neurological status were available were analyzed. In 161 patients (6.7%) an acute neurological disorder was identified as the preceding triggering factor. The most common neurological disorders were seizures, intracranial hemorrhage, and ischemic stroke. Time from neurological symptoms to TTS diagnosis was ≤ 2 days in 87.3% of cases. TTS patients with neurological disorders were younger, had a lower female predominance, fewer cardiac symptoms, lower left ventricular ejection fraction, and higher levels of cardiac biomarkers. TTS patients with neurological disorders had a 3.2-fold increased odds of in-hospital mortality compared to TTS patients without neurological disorders. In this large-scale study, 1 out of 15 TTS patients had an acute neurological condition as the underlying triggering factor. Our data emphasize that a wide spectrum of neurological diseases ranging from benign to life-threatening encompass TTS. The high rates of adverse events highlight the need for clinical awareness.
Similar content being viewed by others
Introduction
Patients with acute neurological disorders are susceptible to experience cardiac complications such as acute myocardial infarction, heart failure, arrhythmias, or cardiac arrest1,2,3,4,5,6. Takotsubo syndrome (TTS) is an emerging cardiovascular condition and represents an acute heart failure syndrome, which prototypically affects elderly women after a preceding triggering event7. Due to left ventricular recovery within weeks after the acute event it was widely anticipated that TTS is a self-limiting and rather benign cardiac condition. However, recent studies demonstrated that TTS is accompanied by similar mortality rates as acute myocardial infarction8,9. The underlying pathophysiological mechanisms of TTS have not been elucidated yet, but there is convincing evidence from brain imaging studies that altered physiological function within the brain heart axis might play a pivotal role10,11. A cross-sectional study based on national inpatient sample data has shown that 0.06% of hospitalization for primary acute neurological disorders are complicated by TTS and that incidence rates of TTS diagnosis among patients with acute neurological disorders have gradually increased during the entire study period12. Furthermore, the overall prevalence of acute neurological disorders is reported to be nearly 10 times higher in patients with TTS than in age- and sex-matched controls with acute myocardial infarction8.
Over the last years, uncontrolled case reports and single-center studies have added numerous acute neurological disorders as potential triggering factors of TTS13,14,15. However, a systematic characterization of neurological disorders triggering TTS is lacking. Therefore, we have designed the present study to extensively investigate frequency and spectrum of neurological disorders, clinical characteristics, and outcomes of patients with TTS and primary neurological disorders from the International Takotsubo Registry.
Methods
Study population
Patients were enrolled from the International Takotsubo Registry (InterTAK Registry, www.takotsubo-registry.com) which is a multicenter observational study including data from 45 sites in 14 countries (Australia, Austria, Czech Republic, Finland, France, Germany, Italy, New Zealand, Poland, Portugal, Russia, Switzerland, United Kingdom and United States). The rationale and methodological structure of the InterTAK Registry have been detailed previously8,16. Patients were included in the registry if International Diagnostic Takotsubo Criteria were met7. For the present study, the InterTAK Registry was screened for patients who had complete documentation on acute neurological disorders preceding the TTS event. Cases with acute neurological disorders were reviewed independently by two experienced board-certified neurologists and patients were categorized as Neuro-TTS if the following criteria were fulfilled: (i) evidence of a clearly definable disease entity; (ii) first symptoms of the acute neurological event had to be documented before the first signs of TTS, and (iii) time between first neurological symptoms and TTS diagnosis had to be < 10 days. Patients without acute neurological conditions prior to their TTS event were termed TTS and were used as controls for the Neuro-TTS group. Furthermore, patients were stratified by neurological disorders to study clinical characteristics and outcomes.
The study protocol was approved by the cantonal ethics committee Zurich (KEK-ZH Nr.: 2013-0075 and BASEC-ID 2019–02,402) and conformed to the principles of the Declaration of Helsinki. Written informed consent was waived for retrospectively included patients by the cantonal ethics committee Zurich (KEK-ZH Nr.: 2013-0075). Written informed consent was obtained from all participants included after January 1 2017 (cantonal ethics committee Zurich, BASEC ID 2019-02402).
Data acquisition
Data were collected through review of medical charts which included information on demographics, takotsubo type (i.e. apical type and non-apical types including midventricular, basal, and focal type), clinical presentation, laboratory profile, ECG, cardiovascular risk factors, comorbidities, imaging findings from echocardiography, coronary angiography, cardiac magnetic resonance imaging, clinical management and adverse events. Furthermore, detailed data on the neurological event were collected. Symptom severity was evaluated using validated measures such as the Glasgow coma scale for intracranial hemorrhage (GCS 3–15, lower score values indicate worse status)17, Hunt and Hess rating scale for subarachnoid hemorrhage (SAH, 0–5, higher score values indicate greater severity)18, Status Epilepticus Severity Score for status epilepticus (STESS, 0–6, higher score values indicate greater severity)19, and National Institutes of Health Stroke Scale for ischemic stroke (NIHSS, 0–42, higher score values indicate greater severity). Information about anatomic lesion site, presumed pathophysiology of the respective diseases was obtained from medical records as well as time interval from neurological disorders to TTS diagnosis. Follow-up data collection was conducted through phone interviews, review of medical charts, or outpatients’ visits.
Outcomes
The main outcome measure was survival at discharge. Factors associated with in-hospital mortality were assessed. Causes of in-hospital death were documented and classified as cardiovascular, non-cardiovascular, or death of unknown cause. Moreover, the incidence of major adverse cardiac and cerebrovascular events [MACCE: a composite of all-cause death, TTS recurrence, stroke or transient ischemic attack, or myocardial infarction] as well as the single components of MACCE at 10-year follow-up were studied.
Statistical analysis
Continuous data are reported as mean ± standard deviation or medians and interquartile ranges. Categorical variables are given as numbers and percentages. Comparisons of patients’ characteristics between the Neuro-TTS group and TTS controls were performed using an unpaired t-test or the Mann–Whitney U-test for continuous data and the Pearson chi-square test or Fisher’s exact test for categorical variables. Multiple group comparisons were conducted with one-way analysis of variance or Kruskal–Wallis test. Variables that were significantly different at baseline comparison were included in a logistic regression model to identify factors associated with in-hospital mortality. Multiple imputations were conducted prior to logistic regression analysis.
Kaplan–Meier method was used to study outcome estimates, which were assessed by log-rank test. A 10-year landmark analysis was performed for patients who survived the first 30 days after the TTS event. Odds ratios (OR) are given with the corresponding 95% confidence interval (C.I.). All tests were 2-sided and statistical significance was defined as P < 0.05. SPSS Version 25.0 (IBM Corp., Armon, NY, USA) was used for statistical analysis and GraphPad Prism 8 (GraphPad, La Jolla, CA, USA) for figure preparation.
Results
Patients’ characteristics
A total of 2722 patients from the InterTAK Registry with data collected between January 2011 and May 2019 were assessed for eligibility. Of these, 2402 patients had complete information on acute neurological disorders and were included in the present study. In 161 (6.7%) patients acute neurological disorders preceded the TTS event (Fig. 1). TTS was diagnosed within 2 days after neurological symptoms in 87.3% of patients (Fig. 2).
Study population. *7 patients had overlap of 2 acute neurological conditions and were classified twice: 2 with focal onset seizure and ischemic stroke, 2 with generalized onset seizure and PRES, 1 with status epilepticus and PRES, 1 with status epilepticus and SAH, and 1 with generalized onset seizure and SAH. InterTAK Registry International Takotsubo Registry, PRES Posterior reversible encephalopathy syndrome, SAH Subarachnoid hemorrhage.
Time link between neurological disorders and takotsubo syndrome. Of the 150 patients included in the analysis median time from neurological disorders to TTS was 0 (IQR 0–1) days. Notably, time from neurological disorder to TTS was less than 2 days in 87.3% of cases. In 62% of cases the neurologic event and TTS were diagnosed on the same day, while 38% of patients were already hospitalized for the underlying neurologic conditions and TTS diagnosed during the clinical course. Numbers in boxes are the number of patients diagnosed with neurological disorders on the respective day. X-axis: days from neurological event to TTS. Y-axis: different types of neurological disorders triggering TTS. 7 patients with overlap of 2 acute neurological conditions and were excluded (2 with focal onset seizure and ischemic stroke, 2 with generalized onset seizure and PRES, 1 with status epilepticus and PRES, 1 with status epilepticus and SAH, and 1 with generalized onset seizure and SAH). In 4 cases the exact time of onset of neurological disorders was unknown (1 patient with subarachnoid hemorrhage, 1 patient with ischemic stroke, 1 patient with unknown onset seizure, and 1 patient with left frontal lobe tumor with progressive aphasia). IQR Interquartile range, PRES Posterior reversible encephalopathy syndrome, SAH Subarachnoid hemorrhage, TTS Takotsubo syndrome.
Patients’ characteristics are summarized in Table 1. Patients in the Neuro-TTS group were younger (63.7 ± 15.2 years vs. 67.5 ± 12.4 years, P < 0.001) and less often female (82.6% vs. 90.9%, P = 0.001) compared to TTS controls. Neuro-TTS patients had less often cardiac symptoms including chest pain or dyspnea. While there was no difference with regard to the frequency of the classical apical ballooning pattern (66.5% vs. 70.5%, P = 0.27), Neuro-TTS patients showed more often the basal TTS variant (3.7% vs. 1.0%, P = 0.011) on echocardiography or left ventriculography. Patients in the Neuro-TTS group had higher heart rate (91.8 ± 27.6 bpm vs. 86.9 ± 21.5 bpm, P = 0.022) and lower left ventricular ejection fraction (37.4 ± 11.9% vs. 41.0 ± 11.7%, P < 0.001) compared to TTS controls. Troponin at admission levels were comparable between Neuro-TTS and TTS controls, while troponin peak values were nearly twice as high in the Neuro-TTS group compared to TTS controls [33.91 (11.50–65.75) vs. 17.35 (6.47–41.00), factor increase of upper limit of normal, P < 0.001]. Furthermore, brain natriuretic peptide on admission as well as corresponding peak values were higher in the Neuro-TTS group indicating a greater degree of myocardial injury in the Neuro-TTS group.
Clinical spectrum of neurological disorders
Of the 161 patients with neurological disorders, the most common neurological disorders were seizures (N = 64, 39.8%; Fig. 1, Table 2), intracranial hemorrhage (N = 48, 29.8%, Fig. 1, Table 3) and cerebral ischemia (N = 43, 26.7%, Fig. 1, Table 4). Transient global amnesia was identified in 4 patients, posterior reversible encephalopathy syndrome (PRES) in 4 patients, migraine or headache disorders in 3 patients, intracranial tumor with progressive aphasia upon presentation in 1 patient, and Wernicke encephalopathy in 1 patient (Supplementary Table 1). The proportion of males was significantly higher in patients with intracranial hemorrhage than in patients with seizures or cerebral ischemia, and patients with intracranial hemorrhage and seizures were younger than patients with cerebral ischemia (Table 5 and Supplementary Fig. 1). Left ventricular ejection fraction was more reduced in patients with intracranial hemorrhage, while left ventricular end-diastolic pressure was substantially higher compared to patients with seizures and cerebral ischemia. Characteristics of patients stratified by neurologic disorders are summarized in Table 5. The prevalence of seizure/epilepsy and subarachnoid hemorrhage seems to be higher in the InterTAK Registry than in the general population using data Global Burden of Disease statistics20, while numbers of ischemic stroke and intracerebral hemorrhage were similar in both cohorts (Supplementary Fig. 2).
Characteristics of patients with seizures
Of the 64 patients who had TTS secondary to seizures, 18 had status epilepticus (STESS > 2 points in 44.4%), 26 had generalized onset seizures, 7 had focal onset seizures, and 13 had unknown onset seizures (Table 2). A history of seizure was identified in 22 patients and 17 patients were on antiepileptic drug treatment prior to TTS event. Structural underlying brain lesions were present in 37 patients (data available in N = 62) and involved the right hemisphere in 22 (59.5%) patients. Seizures were considered acute symptomatic in 20 patients, including patients with SAH (N = 2), ischemic stroke (N = 3), and PRES (N = 3). Younger age and lower predominance of females seemed to be more pronounced after status epilepticus and generalized onset seizures than after seizures with focal onset or unknown onset seizure (Supplementary Fig. 1). An atypical TTS variant was observed in 37.5% after status epilepticus and 43.5% after generalized onset seizures.
Characteristics of patients with intracranial hemorrhage
Of the 48 patients who had TTS secondary to intracranial hemorrhage, 33 had SAH, 9 intracerebral hemorrhage, and 6 subdural or epidural hematoma. Mean age of patients with SAH was 58.8 ± 18.0 years, 77.4% were female (Supplementary Fig. 1), and 35.5% had an atypical TTS variant. Clinical severity of SAH was severe (median GCS 7 points and median Hunt and Hess grade 4) and 81.8% (27 / 33) of SAH were caused by aneurysmatic origin. Site of aneurysm included the anterior communicating artery or anterior cerebral artery in 12 out of 27 (44.4%) cases. Intracerebral hemorrhage occurred spontaneously in 8 out of 9 cases with overall moderate clinical severity (median GCS 9 points). Subdural or epidural hematoma were located supratentorial in all cases and had traumatic origin in 66.7% (4 / 6) of cases (Table 3).
Characteristics of patients with cerebral ischemia
Of the 43 patients with cerebral ischemia, 37 had ischemic stroke, 5 transient ischemic attack and 1 retinal ischemia. Mean age of patients with ischemic stroke was 71.6 ± 14.2 years, 77.1% were female, and 25.7% had an atypical TTS variant. Clinical stroke severity of patients with stroke was moderate (median NIHSS 6 points). Anterior circulation stroke was documented in 21 (56.8%) patients of which 11 were right-sided and 10 were left-sided. Involvement of the insular cortex was documented in 8 out of 22 patients (36.4%), and evidence of an embolic stroke origin was observed in 29 out of 32 patients (90.6%) with available descriptive imaging data, respectively. Transient ischemic attack affected the anterior circulation in 4 out of 5 of cases (Table 4).
Clinical course and outcomes
Patients in the Neuro-TTS group more often required non-invasive or invasive ventilation (47.5% vs. 15.3%, P < 0.001), catecholamine administration (30.4% vs. 11.7%, P < 0.001) and cardiopulmonary resuscitation (15.9% vs. 7.1%, P < 0.001) compared to TTS controls.
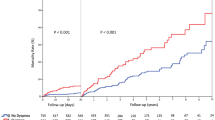
Proportions of cardiogenic shock (15.1% vs. 8.6%, P = 0.006) and in-hospital mortality (17.4% vs. 4.0%, P < 0.001) were substantially higher in Neuro-TTS patients (Table 1). When comparing causes of death, cardiovascular causes of death were substantially higher in Neuro-TTS group than in TTS controls (82.1% vs. 47.8%, P = 0.005, Supplementary Fig. 3). On multivariable logistic regression the presence of acute neurological disorders (OR 3.20, 95% C.I. 1.93–5.33, P < 0.001) was associated with increased in-hospital mortality (Fig. 3). 10-year outcome analysis demonstrated increased MACCE (32.3% vs. 15.5%, P < 0.001) and mortality (24.8% vs. 10.0%, P < 0.001) rates in TTS patients with neurological disorders, while recurrence rates were not statistically significant different in the neuro-TTS group and in TTS controls (2.5% vs. 3.1%, P = 0.66, Table 1). Moreover, we conducted a 10-year landmark analysis with a landmark set at 30-days. Landmark analysis showed substantially higher mortality within the 30 days after admission in patients with neurological disorders. After the landmark including only patients alive 30 days after TTS, patients with neurological disorders showed substantially higher long-term mortality compared to TTS controls without neurological disorders (P = 0.020, Supplementary Fig. 4).
Factors associated with in-hospital mortality. Acute neurological disorders, left ventricular ejection fraction < 45%, heart rate over 94 bpm, and WBC > 10 × 103 cells/µ were identified as factors associated with in-hospital mortality. Bpm beats per minute; C.I. confidence interval; LVEF left ventricular ejection fraction; OR odds ratio; ULN upper limit of normal; WBC white blood cell count. Errors bars indicate 95% confidence intervals. Black indicates statistically significant variables, while grey is not significant.
Discussion
The present study based on data from the InterTAK Registry demonstrates that neurological disorders are common triggering factors of TTS. TTS patients with neurological disorders exhibit a distinct clinical profile including younger age, lower female predominance, and less clinically apparent cardiac symptoms at presentation. Furthermore, patients with neurological disorders have more pronounced myocardial injury as reflected by markedly elevated cardiac biomarkers and more impaired left ventricular ejection fraction compared to patients without neurological disease. Seizures, intracerebral hemorrhage, and ischemic stroke constitute the most common neurological disorders preceding TTS.
The presence of cardiac disorders is frequently observed after neurological disorders and is linked to a high burden of morbidity and mortality1,2,3,4. Cardiac complications after acute neurological disorders comprise a wide spectrum ranging from benign ECG alterations, cardiac biomarker abnormalities, myocardial disorders, left ventricular dysfunction, malignant arrhythmias to sudden cardiac death1,2,6,21. The link between TTS and neurovascular events was underscored more than two decades ago. For instance, the first version of the Mayo Clinic Diagnostic Criteria excluded the presence of neurologic disorders for diagnosis of TTS22. Instead the term neurogenic stunned myocardium was frequently found in the literature to describe myocardial dysfunction after acute neurological injury especially after subarachnoid hemorrhage23,24. Over the last years, the interplay of the brain and heart has gained growing interest in the medical community and TTS is now acknowledged as a cardiac consequence of acute neurological events2,6,25,26,27. While the understanding of the precise pathomechanisms underlying TTS development remain incomplete, there is evidence that autonomic imbalance with excessive catecholamine release and increased cardiomyocyte response to catecholamines infer the pathophysiology28,29,30. Levels of catecholamines are reported to be 4 times higher in TTS than in patients with myocardial infarction even three to five days after hospital admission, and over 30 times higher than normal resting values29. The plasma half-lives of epinephrine and norepinephrine are approximately 1–3 min31. Thus, the maximum catecholamine levels at symptom onset will be substantially higher than any measurement taken on hospital admission considering an onset-to-door time of at least 30 min32. Prolonged elevation of circulating catecholamines for several hours has also been reported after acute neurological disorders such as seizures and stroke33,34.
Cardiovascular functions are controlled by a complex network of cortical and subcortical forebrain regions (including the amygdala, the insula, the hippocampus, as well as lateral and mesial frontal regions)1,2,35. Neuro-imaging studies on TTS patients have demonstrated altered function of brain regions which are responsible for autonomic regulation10.
In our cohort, one out of 15 TTS patients had a preceding acute neurological disorder as the underlying triggering factor. The prevalence of seizure/epilepsy and subarachnoid hemorrhage in our study population seems to be higher than in the general population using data from Global Burden of Disease statistics, while numbers of ischemic stroke and intracerebral hemorrhage were similar in both cohorts20. Elderly women after an emotional triggering factor (e.g. death of a beloved one) have historically been considered as the high-risk population for TTS. This is in contrast to patients with TTS after neurological disorders who are younger and more frequently male than expected. It has been suggested that TTS development depends on the degree of sympathetic stimulation36. This could explain the atypical clinical profile of TTS patients with neurological disorders. It may be speculated that younger individuals and males may require a more intense trigger with greater sympathetic stimulation, while only a mild trigger with low sympathetic stimulation might be sufficient to provoke TTS in elderly. The way acute neurological disorders affect the function of the central autonomic network differs among neurological disorders. In seizures and status epilepticus there is paroxysmal stimulation of the autonomic nervous system and in SAH there is an early intense sympathetic stimulation, which directly affects the hypothalamus pituitary-adrenal axis via the amygdala-insular complex. The presence of ischemic stroke lesions, on the other hand, mainly leads to loss of function or network dysfunction.
TTS secondary to seizures occurred most often after generalized onset (i.e., bilateral tonic–clonic), unknown onset motor seizures or focal to bilateral tonic–clonic seizures. In these seizure types, it has been shown that the postictal phase is dominated by sympathetic overactivation and increased heart rate with parasympathetic suppression37,38. In contrast, focal unaware seizures (complex partial seizures), which were rather uncommon in our cohort, were associated with ictal asystole or bradycardia39. Moreover, we observed that nearly 60% of structural brain lesions in patients with TTS secondary to seizures involved the right hemisphere, which may be of interest as right hemispheric lesions may promote susceptibility to autonomic dysfunction40,41,42.
Observational studies have demonstrated that TTS secondary to SAH occurs in approximately 10–15% of patients and that severity of SAH according to Hunt and Hess Scale can predict the occurrence of cardiac dysfunction24,43. This corroborates the findings of the present study as virtually all patients had severe SAH as reflected by low GCS and high Hunt and Hess Scale. Interestingly, more than half of registered SAH cases were related to ruptured anterior communicating artery aneurysms.
In our study, 36% of patients with ischemic stroke had involvement of the insular cortex. Insular cortex damage has been associated with increased sympathetic nervous system activity and cardiovascular system dysregulations including arrhythmias or myocardial injury42,44,45. Moreover, previous studies investigating TTS secondary to ischemic stroke have suggested that hemispheric lesions and especially insular cortex involvement may promote TTS occurrence46,47. Median NIHSS in these series was 16, which was higher than observed herein. Thus, our results indicate that TTS secondary to ischemic stroke may also be observed in patients with overall lower stroke severity. In our cohort, TTS has been diagnosed early after SAH, while after subdural/epidural hematoma TTS has been seen after a median of 3 days. Clinical severity was substantially higher in patients with SAH or intracerebral hemorrhage than in patients with subdural or epidural hematoma. Therefore, it can be likely that clinical disease severity of the underlying neurological disorder might impact time onset of TTS.
Patients with neurological disorders had a higher prevalence of the basal TTS phenotype. The basal TTS form constitutes a relatively rare morphological TTS variant and has been linked to neurological disorders such as SAH8,48. Furthermore, it has been suggested that the left ventricular apex is spared out after neurological disorders49. However, our findings challenge the concept of left ventricular apical-sparing as we have identified the apical TTS form in more than two-thirds of TTS patients with primary neurological disorders.
TTS secondary to neurological disorders was associated with increased rates of adverse events and in-hospital mortality. After controlling for major confounders, the presence of acute neurological disorders was associated with increased in-hospital mortality. This again highlights the importance to consider TTS patients with primary acute neurological conditions as a high-risk population despite younger age and less pre-existing cardiovascular risk factors.
Although TTS has gained heightened awareness during the last years, it likely remains an underdiagnosed and underreported disorder. Especially in patients with acute neurological diseases, TTS may remain clinically silent. Classical clinical symptoms of TTS such as chest pain or dyspnea were less often documented in the Neuro-TTS group compared with TTS controls. In addition, patients with neurological disorders had an unfavorable clinical course and therefore it might be even considered to actively screen for elevation of cardiac biomarkers and signs of heart failure. Therefore, a rise in sensitive biomarkers of myocardial disorders or dysfunction (i.e. cardiac troponin or BNP, respectively) should prompt cardiac evaluation. Additionally, prolonged clinical monitoring and non-invasive cardiac investigations should be considered and treatment with catecholamines or QT-prolonging drugs should be avoided if possible given the putative involvement in the pathogenesis.
We have shown that acute neurological disorders ranging from benign to life-threatening can provoke TTS. TTS patients with neurological disorders have substantial rates of adverse events. Outcome analysis demonstrated increased mortality in the first 30 days after the TTS event in patients with neurological disorders. The increased mortality rates within the first 30 days are likely driven by the underlying neurological state and TTS adding a secondary hit. Our findings emphasize the need of a standardized cardiac work-up in patients with acute neurological disorders to avoid underdiagnosing of TTS. The considerable rates of adverse events in patients with neurological disorders and TTS should prompt neurologist to heightened awareness of the syndrome.
Limitations
This study has inherent limitations of observational studies which do not allow to establish causal relationships. However, this study design can be particularly valuable in understudied and low-incidence diseases such as TTS. A core-team of physicians and clinical scientists reviewed all medical records and imaging data centrally to ensure correct diagnosis and data quality. However, detailed neuroimaging data beyond description of pathological findings were not available in a substantial proportion of cases. The true prevalence of TTS in patients with neurological disorders is likely to be higher as TTS might have remained clinically silent or undetected especially in elderly or in patients with milder neurological disorders.
References
Tahsili-Fahadan, P. & Geocadin, R. G. Heart-brain axis: Effects of neurologic injury on cardiovascular function. Circ. Res. 120(3), 559–572 (2017).
Scheitz, J. F., Nolte, C. H., Doehner, W., Hachinski, V. & Endres, M. Stroke-heart syndrome: Clinical presentation and underlying mechanisms. Lancet Neurol. 17(12), 1109–1120 (2018).
van der Bilt, I. A. et al. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: A meta-analysis. Neurology 72(7), 635–642 (2009).
Surges, R. The many facets of cardiac complications in epilepsy. J. Neurosci. Rural Pract. 5(1), 6–7 (2014).
Devinsky, O., Hesdorffer, D. C., Thurman, D. J., Lhatoo, S. & Richerson, G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 15(10), 1075–1088 (2016).
Sposato, L. A., Hilz, M. J., Aspberg, S., Murthy, S. B., Bahit, M. C., Hsieh, C. Y., Sheppard, M. N., Scheitz, J. F., World Stroke Organisation Brain & Heart Task Force. Post-stroke cardiovascular complications and neurogenic cardiac injury: JACC state-of-the-art review. J. Am. Coll. Cardiol. 76(23), 2768–2785 (2020).
Ghadri, J. R. et al. International expert consensus document on Takotsubo syndrome (part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 39(22), 2032–2046 (2018).
Templin, C. et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 373(10), 929–938 (2015).
Stiermaier, T. et al. Long-term excess mortality in takotsubo cardiomyopathy: Predictors, causes and clinical consequences. Eur. J. Heart Fail. 18(6), 650–656 (2016).
Templin, C. et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 40(15), 1183–1187 (2019).
Dichtl, W. et al. Functional neuroimaging in the acute phase of Takotsubo syndrome: Volumetric and functional changes of the right insular cortex. Clin. Res. Cardiol. 109(9), 1107–1113 (2020).
Morris, N. A. et al. The risk of Takotsubo cardiomyopathy in acute neurological disease. Neurocrit. Care 30, 171–176 (2018).
Finsterer, J. & Wahbi, K. CNS disease triggering Takotsubo stress cardiomyopathy. Int. J. Cardiol. 177(2), 322–329 (2014).
Nasr, D. M., Tomasini, S., Prasad, A. & Rabinstein, A. A. Acute brain diseases as triggers for stress cardiomyopathy: Clinical characteristics and outcomes. Neurocrit. Care 27(3), 356–361 (2017).
Blanc, C. et al. Takotsubo cardiomyopathy following acute cerebral events. Eur. Neurol. 74(3–4), 163–168 (2015).
Ghadri, J. R., Cammann, V. L. & Templin, C. The international Takotsubo registry: Rationale, design, objectives, and first results. Heart Fail. Clin. 12(4), 597–603 (2016).
Teasdale, G. & Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872), 81–84 (1974).
Hunt, W. E. & Hess, R. M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 28(1), 14–20 (1968).
Rossetti, A. O. et al. Status Epilepticus Severity Score (STESS): A tool to orient early treatment strategy. J. Neurol. 255(10), 1561–1566 (2008).
Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results (2020).
Chen, Z. et al. Brain-heart interaction: Cardiac complications after stroke. Circ. Res. 121(4), 451–468 (2017).
Bybee, K. A. et al. Systematic review: transient left ventricular apical ballooning: A syndrome that mimics ST-segment elevation myocardial infarction. Ann. Intern. Med. 141(11), 858–865 (2004).
Guglin, M. & Novotorova, I. Neurogenic stunned myocardium and takotsubo cardiomyopathy are the same syndrome: A pooled analysis. Congest. Heart Fail. 17(3), 127–132 (2011).
Murthy, S. B., Shah, S., Rao, C. P., Bershad, E. M. & Suarez, J. I. Neurogenic stunned myocardium following acute subarachnoid hemorrhage: Pathophysiology and practical considerations. J. Intensive Care Med. 30(6), 318–325 (2015).
Samuels, M. A. The brain-heart connection. Circulation 116(1), 77–84 (2007).
Ando, G., Trio, O. & de Gregorio, C. Transient left ventricular dysfunction in patients with neurovascular events. Acute Card. Care 12(2), 70–74 (2010).
Cammann, V. L., Wurdinger, M., Ghadri, J. R. & Templin, C. Takotsubo syndrome: Uncovering myths and misconceptions. Curr. Atheroscler. Rep. 23(9), 53 (2021).
Borchert, T. et al. Catecholamine-dependent beta-adrenergic signaling in a pluripotent stem cell model of Takotsubo cardiomyopathy. J. Am. Coll. Cardiol. 70(8), 975–991 (2017).
Wittstein, I. S. et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 352(6), 539–548 (2005).
Pelliccia, F., Kaski, J. C., Crea, F. & Camici, P. G. Pathophysiology of Takotsubo syndrome. Circulation 135(24), 2426–2441 (2017).
Feher, J. The adrenal medulla and integration of metabolic control. In Quantitative Human Physiology 2nd edn, 916–923 (2012).
Lyon, A. R., Rees, P. S., Prasad, S., Poole-Wilson, P. A. & Harding, S. E. Stress (Takotsubo) cardiomyopathy—A novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat. Clin. Pract. Cardiovasc. Med. 5(1), 22–29 (2008).
Nass, R. D. et al. Blood markers of cardiac stress after generalized convulsive seizures. Epilepsia 60(2), 201–210 (2019).
Barber, M. et al. Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc. Dis. 23(4), 260–266 (2007).
Beissner, F., Meissner, K., Bar, K. J. & Napadow, V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33(25), 10503–10511 (2013).
Wittstein, I. S. Why age matters in Takotsubo syndrome. J. Am. Coll. Cardiol. 75(16), 1878–1881 (2020).
Poh, M. Z. et al. Autonomic changes with seizures correlate with postictal EEG suppression. Neurology 78(23), 1868–1876 (2012).
Surges, R., Scott, C. A. & Walker, M. C. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology 74(5), 421–426 (2010).
van der Lende, M., Surges, R., Sander, J. W. & Thijs, R. D. Cardiac arrhythmias during or after epileptic seizures. J. Neurol. Neurosurg. Psychiatry 87(1), 69–74 (2016).
Mayer, H. et al. EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology 63(2), 324–328 (2004).
Meyer, S., Strittmatter, M., Fischer, C., Georg, T. & Schmitz, B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. NeuroReport 15(2), 357–361 (2004).
Krause, T. et al. Stroke in right dorsal anterior insular cortex Is related to myocardial injury. Ann. Neurol. 81(4), 502–511 (2017).
Naidech, A. M. et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation 112(18), 2851–2856 (2005).
Nagai, M., Hoshide, S. & Kario, K. The insular cortex and cardiovascular system: A new insight into the brain-heart axis. J. Am. Soc. Hypertens. 4(4), 174–182 (2010).
Nagai, M. et al. The insular cortex and Takotsubo cardiomyopathy. Curr. Pharm. Des. 23(6), 879–888 (2017).
Jung, J. M. et al. Takotsubo-like myocardial dysfunction in ischemic stroke: A hospital-based registry and systematic literature review. Stroke 47(11), 2729–2736 (2016).
Yoshimura, S. et al. Takotsubo cardiomyopathy in acute ischemic stroke. Ann. Neurol. 64(5), 547–554 (2008).
Shoukat, S. et al. Cardiomyopathy with inverted Tako-Tsubo pattern in the setting of subarachnoid hemorrhage: A series of four cases. Neurocrit. Care 18(2), 257–260 (2013).
Nguyen, H. & Zaroff, J. G. Neurogenic stunned myocardium. Curr. Neurol. Neurosci. Rep. 9(6), 486–491 (2009).
Acknowledgements
Dr. Helena Stengl is participant in the BIH-Charité Junior Clinician Scientist Program funded by the Charité –Universitätsmedizin Berlin and the Berlin Institute of Health. Prof. Dr. Scheitz is participant in the BIH-Charité Advanced Clinician Scientist Program funded by the Charité –Universitätsmedizin Berlin and the Berlin Institute of Health.
Funding
The International Takotsubo Registry was supported by the Biss Davies Charitable Trust. Dr. Scheitz has been supported by the Corona Foundation. Dr. Templin has been supported by the H.H. Sheikh Khalifa bin Hamad Al-Thani Research Programme and the Swiss Heart Foundation.
Author information
Authors and Affiliations
Consortia
Contributions
V.L.C. and J.S. wrote the main manuscript. V.L.C. and J.S. prepared figures. V.L.C., J.S., R.v.R., L.J., C.H.N., K.A.S., H.S., M.W., M.E., C.T., J.R.G. reviewed the manuscript. The InterTAK Consortium provided patients’ data and supported in data collection. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Nolte received research grants from German Ministry of Research and Education (BMBF), German Center for Neurodegenerative Diseases (DZNE), German Center for cardiovascular Research (DZHK) and speaker and/or consultation fees from Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer Pharma, Alexion, Abbott and W.L. Gore and Associates, all outside the submitted work. Dr. Endres reports grants from Bayer and fees paid to the Charité from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis, Pfizer, all outside the submitted work. Dr. Templin reports receiving fees outside the submitted work for serving on advisory boards from Abbott Vascular, Astra Zeneca, Boston Scientific, Fresenius Medical Care, The Medicines Company fees for serving on steering boards from Sanofi-Aventis, serving as a consultant for Schnell Medical, travel support from Abbott Vascular, Biosensors, Boston Scientific, Edwards Lifesciences, and Medtronic, and research grant support from Abbott Vascular and Biosensors, all outside the submitted work. All other authors have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cammann, V.L., Scheitz, J.F., von Rennenberg, R. et al. Clinical correlates and prognostic impact of neurologic disorders in Takotsubo syndrome. Sci Rep 11, 23555 (2021). https://doi.org/10.1038/s41598-021-01496-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01496-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.