Abstract
The safety of endovascular revascularization in patients with carotid artery near occlusion (CANO) is unknown. We aimed to evaluate the peri-procedural risk in CANO patients receiving carotid artery stenting (CAS). A prospective data base with retrospective review was performed to identify patients who underwent CAS with CANO from July 2006 to July 2020, and had at least 1-month clinical follow-up data. The primary endpoints were stroke, hyperperfusion syndrome, and death within 30 days after CAS. A total of 198 patients with carotid artery stenosis were enrolled including 92 patients with CANO and 106 age and sex-matched patients with 70–99% conventional carotid stenosis. Full distal carotid collapse was found in 45 CANO patients (45/92, 49%). The technical success rate was 100%. The CANO patients had significantly longer lesion lengths compared with those of the non-CANO group. The incidence of hyperperfusion syndrome was comparable (CANO: 2.2%, non-CANO: 0.9%, P = 0.598). The risks of ischemic stroke and death within 30 days were 1.1% and 0% in the CANO group; and 1.9% and 0.9%, in the non-CANO group, respectively, without statistical difference. In conclusion, CAS is safe for patients with CANO, with a similar low 30-day peri-procedural event rate comparable to those of non-CANO.
Similar content being viewed by others
Introduction
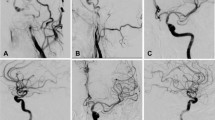
Carotid artery near occlusion (CANO) is defined as a reduced internal carotid artery (ICA) lumen diameter distal to a prominent bulb stenosis1. The actual stroke risk in CANO remains elusive. There are 2 subtypes of CANO, severe form with full collapse (thread like distal ICA lumen) versus partial form without full collapse (a normal-appearing but narrowed distal ICA lumen) (Fig. 1), and their prognosis could be different2. Current guideline suggests medical treatment for CANO3, but the recommendations are based on experts’ consensus on an assumed low stroke risk and high revascularization complication rates. Recent studies, however, suggested high stroke risk and poor clinical outcome in CANO with full collapse, compared with those of severe carotid stenosis without CANO features4,5. In addition, recent meta-analysis showed that medical treatment alone may not be enough6. Evidences are urgently needed for the optimal management recommendation in CANO patients4,7.
Illustration of different variants of carotid stenosis. (A) Carotid artery near occlusion with full collapse (B) Carotid artery near occlusion without full collapse (C) Severe conventional carotid stenosis without near occlusion. Black star: external carotid artery. White arrow: internal carotid artery.
None of the modern prospective carotid revascularization trials, such as the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST)8,9 and International Carotid Stenting Study (ICSS)10,11, included CANO patients. Several recent observational studies reported improvement in cerebral hemodynamics and cognitive functions, and reduced risk of stroke after carotid artery stenting (CAS) in CANO patients12,13,14,15. However, the technical difficulty and risk of periprocedural complications, especially hyperperfusion syndrome, may be underestimated and should be investigated12,16. We hereafter reported our results of CAS in CANO patients.
Methods
Patients
Patients underwent CAS for carotid stenosis at the National Taiwan University Hospital from July 2006 to July 2020, with complete 1-month clinical follow-up, were reviewed from a prospective CAS database. Of these patients, 92 CANO patients were identified, and another 106 age- and sex-matched patients with conventional internal carotid stenosis (70–99% diameter narrowing without CANO features) were enrolled as controls. The definition of CANO was based on the previously reported angiographic criteria as evidences of narrowing of the post-stenotic ICA. The ipsilateral ICA/ contralateral ICA ratio, ipsilateral ICA/ipsilateral external carotid artery (ECA) ratio, the evidence of reduced flow in the distal ICA including delayed arrival of contrast into the distal ICA or evidence of intracranial collateral flow toward the affected cerebral hemisphere from other arterial territories were used to help correctly define post-stenotic distal ICA narrowing1,17. Patients with post-stenotic distal ICA narrowing due to anatomic variants such as ICA asymmetry associated with circle of Willis variations were excluded from this study18. CANO with full collapse was defined as a distal ICA lumen diameter ≤ 2 mm and/or ipsilateral ICA/contralateral ICA diameter ratio ≤ 0.422. The angiograms were retrospectively reviewed by 2 independent investigators experience in cerebral angiography, who were blinded to the procedural and clinical information. In case of disagreement, consensus was reached after joint review and discussion.
The patients’ medical information, including demographics, medications, and neurological conditions were reviewed. A “symptomatic” lesion was defined as causing hemispheric transient ischemic attack (TIA), transient monocular blindness, or a stroke within 6 months, associated with ipsilateral carotid artery stenosis according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) definitions19. All patients received baseline biochemistry studies, non-invasive image studies including carotid duplex, computed tomography (CT) angiography or magnetic resonance (MR) angiography, before conventional angiography and ICA stenting. All patients were followed at the outpatient clinic after 7 days and 30 days post discharge, with full neurological evaluation. This study was approved by the Institutional Review Board of National Taiwan University Hospital and was performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all patients before enrollment.
Intervention protocol
All patients received 7 days of standard dual antiplatelet therapy before the procedure. All interventions were performed via an 8Fr femoral sheath, with heparin administered to maintain an activated clotting time within 200–250 s. The severity of ICA stenosis was measured according to NASCET method19. Intracranial collaterals from ipsilateral and contralateral origins were carefully examined. The target common carotid artery (CCA) was engaged with an 8Fr JR 4 guiding catheter. A distal embolic protection device (EPD) was deployed if technically feasible. After careful wiring and balloon pre-dilatation if necessary, properly sized self-expanding stents were deployed to scaffold the stenosis. Balloon post-dilation was done to achieve adequate stent expansion. Technical success was defined as residual diameter stenosis ≤ 20% and establishing grade 3 Thrombolysis in Cerebral Infarction (TICI) antegrade flow. After the procedure, all patients were sent to the intensive care unit for continuous blood pressure and neurological symptoms monitoring for 24 h. During the procedure and the intensive care unit stay, the systolic blood pressure was strictly controlled within 100 to 140 mmHg. All patients received 100 mg aspirin and 75 mg clopidogrel daily for at least 1 month after the intervention.
Outcome definitions
Hyperperfusion syndrome, ischemic stroke, and mortality within 30 days after CAS were defined as the primary endpoints. Hyperperfusion syndrome was defined as ipsilateral headache, seizures, focal neurological deficits, encephalopathy, intracranial hemorrhage with evidence of hyperperfusion (on transcranial Doppler, single photon emission tomography, CT or MR perfusion imaging) within 30 days after CAS, without evidence of new cerebral ischemia, carotid stent occlusion, or metabolic causes20. Ischemic stroke was defined as an acute neurologic event with focal symptoms and signs, lasting for ≥ 24 h, with focal cerebral ischemia8. Ischemic stroke was categorized as being a major stroke if the National Institute of Health Stroke Scale (NIHSS) score was ≥ 9, or it was fetal or disabling8. TIA was defined as an episode of focal neurologic dysfunction attributed to focal cerebral ischemia, with resolution within 24 h.
Statistical analysis
Statistical analysis was performed using SPSS version 25 for Windows (SPSS Inc., IL, USA). The data were first tested for normality using Kolmogorov–Smirnov test. Normal distribution data were expressed as mean ± standard deviation. Categorical data were expressed as number and percentage (%). Differences between proportions were calculated using the chi-square test or Fisher’s exact test. Comparisons of data between two groups were performed using the independent T test (normally distributed data). A two-sided P value less than 0.05 was defined as being statistically significant.
Results
Patients demographics and angiographic characteristics
A total of 92 patients with CANO and 106 patients with conventional carotid stenosis (NASCET diameter stenosis 70–99%) were enrolled in this study. Of the patients with CANO, 45 patients (49%) had full collapse. The demographic and angiographic characteristics are listed in Table 1. Less patients in the CANO group had history of head/neck radiation, but more co-morbid coronary artery disease. Within the CANO group, more patients with full collapse were symptomatic than those without. The delay from the last presenting events of neurological symptoms to CAS in the symptomatic patients were 67.4 ± 44.9 days in the CANO group and 75.9 ± 45.0 days in the non-CANO group (P = 0.469). The lesion lengths were significantly longer in the CANO group, especially in those with full collapse group than in the without full collapse group.
Procedural data
The procedural data are shown in Table 2. The technical success rate was 100%, and no device failure or procedural strokes were observed. An EPD was used in all except for two CANO patients. Of note is that some of the CANO lesions, especially those with full collapse, even required pre-dilatation before EPD deployment. CANO lesions were also more likely to receive pre-dilatation to facilitate stent delivery due to tight stenosis. But direct stenting without pre-dilatation was the majority in the current cohort. Three (3.3%) patient in CANO group and two (1.9%) patients in the conventional stenosis group experienced TIA, but all recovered within 12 h after CAS without neurological deficit.
Endpoints
The clinical outcomes are listed in Table 3. Hyperperfusion syndrome occurred in two patients (2.2%) in the CANO group: one patient presented with headache, focal weakness, and aphasia 1 day after CAS. Perfusion CT showed reginal brain swelling and hyperperfusion. Another patient experienced headache and transient right upper limb weakness, resolved 12 h after the CAS. The CT showed mild left brain swelling and the hyperperfusion syndrome was suspected. Hyperperfusion syndrome occurred in one patient (0.9%) in the non-CANO group who presented with acute onset headache and focal seizure 1 week after CAS. Magnetic resonance imaging documented regional cerebral hyperperfusion. All these patients recovered completely after conservative treatment, and new infarction was ruled out by follow-up imaging studies.
Three ipsilateral strokes occurred within 30 days post CAS, including one (1.1%) major stroke in the CANO group, one major stoke (0.9%) and one minor stroke (0.9%) in the non-CANO group. The composite outcome of all stroke and mortality within 30 days after CAS was 1.1% in the CANO group and 1.9% in the non-CANO group.
Discussion
The prevalence of CANO was variable in the past literatures, due to different diagnostic methods, criteria, and the study designs. Recent systemic review suggested that the prevalence of CANO in symptomatic carotid stenosis may be as high as 34%21. CANO with full collapse can be easily misdiagnosed as complete occlusion, and CANO without full collapse may simply be classified as conventional stenosis. Therefore, the prevalence of CANO may be under-reported in clinical routine practice22, and careful examination of diagnostic images using a uniform definition is important.
The severity of carotid stenosis is known to be generally associated with the risk of ipsilateral ischemic stroke in both symptomatic and asymptomatic patients19,23,24,25,26. As the stenosis progresses to the near-occlusion, however, the reported risk becomes controversial. Rothwell et al. reported a relatively low risk of ipsilateral stroke in medically treated patients with severe carotid stenosis with post-stenotic narrowing of the ICA27. In contrast, Paciaroni et al. reported that 30% of medically treated severe carotid stenosis would progress to total occlusion, and that this was associated with impaired brain perfusion and ipsilateral stroke in the long-term follow-up28. In a recent study, Gu et al. reported that symptomatic CANO was associated with a high short-term risk of recurrent ipsilateral ischemic stroke, especially those with distal vessel full collapse29. The natural history of CANO, therefore, remains elusive.
Current guideline recommends conservative treatment for patients with CANO, unless it is associated with recurrent ipsilateral symptoms despite optimal medical therapy (OMT)3. Recent prospective carotid revascularization trials such as CREST and ICSS, unfortunately, have excluded these patients8,9,10,11. Therefore, the current guideline recommendations are based on previous surgical endarterectomy trials data and do not consider contemporary advance in surgical techniques, OMT, or carotid stenting. A recent meta-analysis suggested the risk of stroke was higher in CANO patients receiving OMT alone, compared with those receiving revascularization (OMT 8.4%, endarterectomy 1.5%, CAS 1.8%)30. Modification of management guidelines for CANO is therefore necessary, mandating studies to collect more data.
Perioperative stroke rate was 6.3% in CANO patients receiving endarterectomy in the NASCET trial, similar to that in patients with 70–94% stenosis23. Kim et al. reported similar periprocedural complication rates in symptomatic CANO patients receiving revascularization, 5.9% in CAS and 3.5% in endarterectomy13. In our study, the 30-day ischemic stroke and death rate in the CANO patients was only 1.1%, which is significantly lower than in prior reports. In addition, the 30-day complication rate was similar in the CANO and non-CANO patients. Overall improvement of CAS devices and techniques, high percentage of embolic protection device usage, and direct stent delivery without balloon pre-dilatation were the possible explanations.
Hyperperfusion syndrome is a potentially life-threatening complication following carotid revascularization, especially in those with critical carotid stenosis. However, its risk has been under-reported and underestimated. In a recent meta-analysis, hyperperfusion was seen in up to 4.6% CAS procedures, and nearly half of these progressed to stroke16. Its incidence has rarely been described in previous studies in CANO31,32, which may be theoretically high due to the impaired hemodynamics and autoregulation. The incidence of hyperperfusion in this study, however, was low and comparable in patients with or without CANO. Possible explanations included the modest procedural anticoagulation, strict blood pressure control protocol, and high incidence of established intracranial collaterals. Recent study has identified insufficient collateral and unregulated hypertension were predictors of hyperperfusion after endarterectomy33. Admittedly, we might have missed hyperperfusion with only subtle non-specific symptoms. It should be emphasized that awareness and proper management of hyperperfusion in CANO revascularization is very important, and modalities such as transcranial Doppler may be mandatory to improve detection and monitoring in future research.
Limitation
There were several limitations to this study. First, this was a retrospective study with a limited number of cases. Second, we only provided 30-day event rates to examine the safety of CAS in CANO and non-CANO patients. Longer-term effect of CAS in CANO, therefore, cannot be demonstrated. Third, the delay from the last presenting events of neurological symptoms to CAS in the symptomatic patients were relatively long. Therefore, the findings of this study might not be applicable in urgent CAS. Finally, we only investigated hyperperfusion with clinical suspicion, so its incidence might be underestimated.
Future considerations and clinical implications
Endovascular revascularization is an alternative for carotid artery stenosis in patients with high surgical risk. Current guideline suggests OMT for CANO patients, and the benefit and safety of CAS in CANO remain unclear despite encouraging recent data. The present study compared results of CAS in severe carotid stenosis with and without CANO and demonstrated low 30-day peri-procedural event rates in CANO, comparable with those in non-CANO. Future large prospective trial including patients receiving OMT, endarterectomy, and CAS are mandatory to establish new management guidelines for CANO patients.
Conclusion
The present study demonstrated low peri-procedural event rates of CAS in CANO patients, comparable to those in non-CANO patients.
Abbreviations
- CANO:
-
Carotid artery near occlusion
- CCA:
-
Common carotid artery
- CT:
-
Computed tomography
- ECA:
-
External carotid artery
- EPD:
-
Embolic protection device
- ICA:
-
Internal carotid artery
- MR:
-
Magnetic resonance
- NASCET:
-
North American Symptomatic Carotid Endarterectomy Trial
- NIHSS:
-
National Institute of Health Stroke Scale
- OMT:
-
Optimal medical therapy
- TIA:
-
Transient ischemic attack
- TICI:
-
Thrombolysis In Cerebral Infarction
References
Fox, A. J. et al. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am. J. Neuroradiol. 26, 2086–2094 (2005).
Johansson, E., Gu, T. & Fox, A. J. Defining carotid near-occlusion with full collapse: A pooled analysis. Neuroradiology https://doi.org/10.1007/s00234-021-02728-5 (2021).
Aboyans, V. et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 39, 763–816. https://doi.org/10.1093/eurheartj/ehx095 (2018).
Johansson, E., Ohman, K. & Wester, P. Symptomatic carotid near-occlusion with full collapse might cause a very high risk of stroke. J. Intern. Med. 277, 615–623. https://doi.org/10.1111/joim.12318 (2015).
Ogata, T. et al. Outcomes associated with carotid pseudo-occlusion. Cerebrovasc. Dis. 31, 494–498. https://doi.org/10.1159/000324385 (2011).
Meershoek, A. J. A. et al. Meta-analysis of the outcomes of treatment of internal carotid artery near occlusion. Br. J. Surg. 106, 665–671. https://doi.org/10.1002/bjs.11159 (2019).
de Borst, G. J., Antonopoulos, C. N., Meershoek, A. J. A. & Liapis, C. D. Carotid artery near occlusion: Time to rethink the management?. Eur. J. Vasc. Endovasc. Surg. 60, 169–170. https://doi.org/10.1016/j.ejvs.2020.04.010 (2020).
Brott, T. G. et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N. Engl. J. Med. 363, 11–23. https://doi.org/10.1056/NEJMoa0912321 (2010).
Brott, T. G. et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N. Engl. J. Med. 374, 1021–1031. https://doi.org/10.1056/NEJMoa1505215 (2016).
International Carotid Stenting Study et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 375, 985–997. https://doi.org/10.1016/S0140-6736(10)60239-5 (2010).
Bonati, L. H. et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet 385, 529–538. https://doi.org/10.1016/S0140-6736(14)61184-3 (2015).
Cay, F. et al. Relevance of distal arterial collapse in stenting of atherosclerotic near-occlusion of the carotid artery. AJNR Am. J. Neuroradiol. 41, 1054–1060. https://doi.org/10.3174/ajnr.A6570 (2020).
Kim, J., Male, S., Damania, D., Jahromi, B. S. & Tummala, R. P. Comparison of carotid endarterectomy and stenting for symptomatic internal carotid artery near-occlusion. AJNR Am. J. Neuroradiol. 40, 1207–1212. https://doi.org/10.3174/ajnr.A6085 (2019).
Song, L. P., Zhang, W. W., Gu, Y. Q., Ji, X. M. & Zhang, J. Cognitive improvement after carotid artery stenting in patients with symptomatic internal carotid artery near-occlusion. J. Neurol. Sci. 404, 86–90. https://doi.org/10.1016/j.jns.2019.07.023 (2019).
Oka, F. et al. Cerebral hemodynamic benefits after carotid artery stenting in patients with near occlusion. J. Vasc. Surg. 58, 1512–1517. https://doi.org/10.1016/j.jvs.2013.05.103 (2013).
Huibers, A. E. et al. Editor’s Choice—Cerebral hyperperfusion syndrome after carotid artery stenting: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 56, 322–333. https://doi.org/10.1016/j.ejvs.2018.05.012 (2018).
Rothwell, P. M., Gutnikov, S. A., Warlow, C. P. & European Carotid Surgery Trialist’s C. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke 34, 514–523 (2003).
Johansson, E., Aviv, R. I. & Fox, A. J. Atherosclerotic ICA stenosis coinciding with ICA asymmetry associated with Circle of Willis variations can mimic near-occlusion. Neuroradiology 62, 101–104. https://doi.org/10.1007/s00234-019-02309-7 (2020).
North American Symptomatic Carotid Endarterectomy Trial C et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 325, 445–453. https://doi.org/10.1056/NEJM199108153250701 (1991).
Bouri, S. et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur. J. Vasc. Endovasc. Surg. 41, 229–237. https://doi.org/10.1016/j.ejvs.2010.10.016 (2011).
Johansson, E. & Fox, A. J. Near-occlusion is a common variant of carotid stenosis: Study and systematic review. Can. J. Neurol. Sci. https://doi.org/10.1017/cjn.2021.50 (2021).
Johansson, E., Gu, T., Aviv, R. I. & Fox, A. J. Carotid near-occlusion is often overlooked when CT angiography is assessed in routine practice. Eur. Radiol. 30, 2543–2551. https://doi.org/10.1007/s00330-019-06636-4 (2020).
Morgenstern, L. B. et al. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Neurology 48, 911–915 (1997).
Barnett, H. J. et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 339, 1415–1425. https://doi.org/10.1056/NEJM199811123392002 (1998).
Halliday, A. et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 363, 1491–1502. https://doi.org/10.1016/S0140-6736(04)16146-1 (2004).
Inzitari, D. et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 342, 1693–1700. https://doi.org/10.1056/NEJM200006083422302 (2000).
Rothwell, P. M. & Warlow, C. P. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 31, 622–630 (2000).
Paciaroni, M. et al. Long-term clinical and angiographic outcomes in symptomatic patients with 70% to 99% carotid artery stenosis. Stroke 31, 2037–2042 (2000).
Gu, T., Aviv, R. I., Fox, A. J. & Johansson, E. Symptomatic carotid near-occlusion causes a high risk of recurrent ipsilateral ischemic stroke. J. Neurol. 267, 522–530. https://doi.org/10.1007/s00415-019-09605-5 (2020).
Antonopoulos, C. N., Giosdekos, A., Mylonas, S. N. & Liapis, C. D. Management of internal carotid artery near-occlusion: The need for updated evidence. Ann. Transl. Med. 8, 1263. https://doi.org/10.21037/atm.2020.03.148 (2020).
Medel, R., Crowley, R. W. & Dumont, A. S. Hyperperfusion syndrome following endovascular cerebral revascularization. Neurosurg. Focus 26, E4. https://doi.org/10.3171/2009.1.FOCUS08276 (2009).
Zhang, L. et al. Risk factors for hyperperfusion-induced intracranial hemorrhage after carotid artery stenting in patients with symptomatic severe carotid stenosis evaluation. J. Neurointerv. Surg. https://doi.org/10.1136/neurintsurg-2018-013998 (2018).
Manojlovic, V. et al. Cerebrovacular reserve predicts the cerebral hyperperfusion syndrome after carotid endarterectomy. J. Stroke Cerebrovasc. Dis. 29, 105318. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105318 (2020).
Acknowledgements
We thank the staff of the Eighth Core Laboratory, National Taiwan University Hospital for technical support during the study. We also acknowledge Taiwan health Foundation and Dr. TY Lin’s Medical Research Foundation for their support.
Author information
Authors and Affiliations
Contributions
H.L.K., Y.H.C. and C.H.T. conceived and designed the study. H.L.K. and Y.H.C. executed the study. CCH and CFY reviewed the angiography. C.H.T., S.F.L., S.C.T., C.C.C. collected and analyzed the data. C.H.T., Y.H.C. and H.L.K. wrote the paper. M.S.L., C.C.H., C.S.H. and C.F.Y. made scientific comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsai, CH., Chen, YH., Lin, MS. et al. The periprocedural and 30-day outcomes of carotid stenting in patients with carotid artery near-occlusion. Sci Rep 11, 21876 (2021). https://doi.org/10.1038/s41598-021-01286-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01286-3
This article is cited by
-
Diagnosing carotid near-occlusion is a difficult task—but it might get easier
Neuroradiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.