Abstract
Fluid flux through Earth’s surface and its interior causes geochemical cycling of elements in the Earth. Quantification of such process needs accurate knowledge about the composition and properties of the fluids. Knowledge about the fluids in Earth’s interior is scarce due to limitations in both experimental methods and thermodynamic modeling in high/ultrahigh pressure–temperature conditions. In this study, we present halogen (Cl, F) measurements in apatite grains from the mafic (metagabbro), and felsic (two-pyroxene granulite, charnockite, hornblende-biotite gneiss) rocks preserved in the Nilgiri Block, southern India. Previous experiments show that it is difficult to incorporate Cl in apatite compared to F at high pressure and temperature conditions. Based on regional trends in Cl and F content in apatite (with highest Cl content 2.95 wt%), we suggest the presence of acidic C–O–H fluids in the lower crust (~20–40 km deep) during the high-grade metamorphism of these rocks. These fluids are capable of causing extreme chemical alterations of minerals, especially refractory ones. They also have significant potential for mass transfer, causing extensive geochemical variations on a regional scale and altering the chemical and isotope records of rocks formed in the early Earth. Our findings have important relevance in understanding speciation triggered by acidic fluids in the lower crust, as well as the role of fluids in deep Earth processes.
Similar content being viewed by others
Introduction
Earth as a system witnesses constant exchange of elements through its different reservoirs, such as atmosphere–hydrosphere–solid Earth1,2,3. Understanding geochemical cycles is crucial in solving the uncertainties in evolution models of the solid Earth and its environment2,3,4 with implications on the habitability of the planet. Fluids play a crucial role in the geochemical cycle of the Earth’s interior1. However, our knowledge on the nature and composition of these fluids including redox potential and acid–base mechanisms at high/ultrahigh pressure (P)-temperature (T) conditions in the lithosphere is limited5,6. The only fluid remnants observed so far in deep crustal rock inclusions are either pure CO2 of variable density or high salinity brines, either miscible or immiscible7. Recent thermodynamic5 and experimental6 models propose a mildly alkaline nature of deep fluids, derived from subduction. It needs to be evaluated whether these fluids are unique or represent the possibility of unknown extreme conditions8,9,10,11.
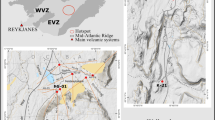
Here we report fluid-assisted recrystallization (during metamorphism) processes that occurred in deep crustal rocks as old as 2.7–2.5 Ga12,13,14 from the Nilgiri Block, southern India (Fig. 1), a major high-grade crustal block located to the south of the Archean Dharwar Craton in Peninsula India15,16 (Fig. 1a). Extensive mineralogical, textural, geochemical, geochronological and thermodynamic P–T–H2O activity (aH2O) calculations of equilibrium mineral assemblages show that these mafic and felsic rocks were formed due to differentiation of underplated mafic magma in the magma chamber during arc magmatic processes (ca. 2.7 Ga), and have then undergone anhydrous granulite facies metamorphism (ca. 2.5 Ga) at depths of ~20 to 40 km14,17,18,19,20. Fluid inclusions in the granulites from this terrane, and related mineral textural and thermodynamic data suggest that fluids played an important role during the transformation of mineral assemblages in the magmatic protolith to the recrystallized high-grade metamorphic rock20,21,22,23,24. In addition, fluid inclusions and textural studies in granulites worldwide suggest that CO2 and highly saline fluids/brines might represent the major fluid compositions25,26,27,28,29. These data, along with textural and experimental results suggest the possibility of fluid-assisted metamorphism in the presence of H2O–CO2-saline fluids/brines30,31,32,33,34,35,36.
Regional geology and sample locations. (a) Regional geology and tectonic framework of southern India (after Ref.37); Blue box outlines the Nilgiri Block. An isotopic boundary divides the Madurai Block into Archean and Mesoproterozoic based on Sm–Nd whole rock and U–Pb ages38. (b) Geological map of the Nilgiri massif. Sample locations annotated by sample numbers are plotted on the map. The numbers are the same as those used in Ref.20, and are also listed in the Extended Data Table 1. Brown-dashed line separates areas of relatively high (> 800 °C) and low (< 800 °C) peak-metamorphic temperatures. CHAR charnockite samples, TPG two-pyroxene granulite samples and MG metagabbro samples. (a) Is reproduced by permission from Elsevier (see acknowledgements). (b) Is created using QGIS 2.6.1 (https://www.qgis.org/en/site/); (a) and (b) are modified using Inkscape version 0.92.2 (https://inkscape.org).
Apatite is a common accessory mineral in the lower crustal rocks, and is widely used to trace fluid compositions8,32,39,40,41,42,43,44. Apatite undergoes halogen-(F,Cl)-exchange reactions with fluids such that its final composition reflects the chemical nature of the associated fluids8,41,42,43,44. In previous natural examples of high Cl bearing apatite (3–6 wt% Cl) reported in metamorphosed rocks, their high Cl contents were incorporated during contact metamorphism on a local scale in the presence of fluids enriched in Cl (F is minor/absent) released from the shallower sources, such as sediment deposits or country rocks40,42. Further, it is difficult to incorporate Cl and easier to incorporate F in apatite at high P–T conditions from a fluid containing both F and Cl8,44. However, high P–T experimental investigations suggest that acidic H2O–HCI or H2O–CO2 fluids, even with modest Cl and F concentrations, results in high Cl composition in apatite at high P–T conditions8. In this contribution, we provide first evidence for the presence of acidic fluids in the lower crust (~20–40 km) based on the trends in Cl and F content in naturally-occurred apatite grains (with a high Cl value of 2.95 wt%) under high-grade granulite facies metamorphic rocks (viz., metagabbro, two-pyroxene granulite and charnockite) preserved in the Nilgiri Block, southern India.
Geological setting and apatite composition
The Nilgiri Block mainly preserves granulite-facies metagabbro exposures in the north, two-pyroxene granulite at the central domain, charnockite in the south, and amphibolite facies hornblende (Hb)-biotite (Bt) gneiss in the west20 (Fig. 1a,b). Detailed field geological settings, metamorphic history, and major mineral compositions are provided in a previous study20, and are summarized here in Extended Data Table 1. Metagabbro samples were equilibrated at P–T conditions of ~ 1 GPa—~ 850°C, and the two-pyroxene granulite at ~ 0.8 GPa— ~ 800 °C, whereas the charnockite samples show a regional variation in pressure and temperature of ~800–750 °C and ~0.8–0.7 GPa, respectively20. Samples of this study (Fig. 1b; Extended Data Table 1) were selected based on the extensive regional scale petrological study20. The apatite grains in these rocks are euhedral to subhedral, and show no obvious preferred mineral or textural association. They occur (1) as inclusions in major minerals, (2) along their boundaries, (3) within the quartz-feldspar matrix, (4) near K-feldspar micro-veins, and (5) in association with oxide-sulphide minerals and their veins (Fig. 2). Textural features suggest that apatite coexists in equilibrium with the other minerals that were formed during peak metamorphism (Fig. 2). The apatite grains show no compositional zoning. Similar compositional homogeneity is also observed for major minerals in these samples20. Grain boundaries of garnet, pyroxene and plagioclase are intact (Fig. 2). Previous geochronological studies indicate that these rocks were not subject to younger thermal disturbance or alteration following peak metamorphism at ~ 2.5 Ga13,17,19.
High contrast back-scattered secondary electron (BSE) imaging of phosphate-silicate-oxide-sulfide mineral textures using an Electron Probe Micro Analyzer (EPMA). In metagabbro and two-pyroxene granulite, apatite occurs in equilibrium with (a) clinopyroxene-magnetite-quartz, (b) orthopyroxene-hemo-ilmentite-Mt-feldspars, where the apatite grain is surrounded by K-feldspar veins, (c) orthopyroxene-hemo-ilmentite-Mt-feldspars, (d) hemo-ilmenite-plagioclase-K-feldspar-quartz; (e) garnet-plagioclase-quartz, (f) orthopyroxene-plagioclase-magnetite, and (g) in charnockite, apatite exists in equilibrium with orthopyroxene-rutile-ilmenite-biotite-feldspar-quartz; (h) in Hb-Bt gneiss, apatite is associated with hemo-ilmenite-magnetite-biotite-hornblende-plagioclase-quartz. Qtz quartz, Plg plagioclase, Kfs K-feldspar, Opx orthopyroxene, Cpx clinopyroxene, Gt garnet, Amph amphibole, Bt biotite, Hm-Ilm hematite–ilmenite exsolutions. Mt magnetite, Ilm-Rt ilmenite-rutile assemblage, Po pyrrhotite, Ap apatite, Aln alanite, and Mnz monazite. This figure is created using Inkscape version 0.92.2 (https://inkscape.org).
An electron probe micro analyzer (EPMA) was used to measure the elemental oxides and halogen composition of the apatite in the rock samples. Analytical conditions for the EPMA measurements are provided in “Methods”. Compositions of 462 apatite grains were measured from the 34 samples selected for this study (Extended Data Table 2–6). Apatite in all four rock types studied here mainly consisted of Ca, P, F, Cl, OH with slight enrichment in Fe (0.1–0.8 wt%). Highest Cl content of 2.95 wt% (sample no. 105 on Fig. 1b, Extended Data Table 3), 0.52 wt%, (sample no. 84 on Fig. 1b, Extended Data Table 4) 1.27 wt% (sample no. 42 on Fig. 1b, Extended Data Table 5) and 0.11 wt% (sample NIL27-14, Extended Data Table 6), were obtained from the metagabbro, two-pyroxene granulite, charnockite, and Hb-Bt gneiss samples, respectively. In the highest Cl bearing metagabbro sample (no. 105 on Fig. 1b), out of total 23 grains, ten grains have mean Cl content of 2.75 wt% and thirteen grains have mean Cl content of 1.41 wt%. Another high Cl bearing metagabbro sample (no. 106 on Fig. 1b) has total seven grains with mean Cl content of 1.03 wt%. The mean Cl content variation in apatite (5–30 apatite grains from each sample) was found to be 0.1 (n = 6, no. 102 on Fig. 1b)–2.75 (n = 10, no. 105 on Fig. 1b) wt% in metagabbro, 0.1 (n = 16, no. 85 on Fig. 1b)–0.2 (n = 2, no. 84 on Fig. 1b) wt% in two-pyroxene granulite, 0–0.49 (n = 13, no. 42 on Fig. 1b) wt% in charnockite, and 0–0.08 (n = 4, NIL27-14) wt% in Hb-Bt gneiss (Extended Data Table 2). There is no preferred textural association with respect to these variations (Fig. 2).
Characteristics of acidic metamorphic conditions
Observed Cl content in the apatite shows a decreasing trend with respect to the metamorphic grade (regional variation trend; Fig. 3a). Among these, three charnockite samples, which formed at a higher P–T conditions20 (sample no. 6, 8, 42 on Fig. 1b) and have high Cl content, are shown separately from those bearing low Cl content (sample no. 12–61 except 42 on Fig. 1b). F content in the apatite, on the other hand, was found to be low in metagabbro, although it displays a constant value of ~ 3 wt% in two-pyroxene granulite, charnockite, and Hb-Bt gneiss (Fig. 3a).
Regional distribution of Cl, F in apatite, and comparison of apatite composition with previous experimental results. Plots representing (a) regional distribution of log10 Cl wt % and log10 F wt% in apatite. Each data point represents the average Cl and F wt% in apatite grains in each sample (vertical bar-lowest and highest Cl or F content in apatite grains in each sample); (b) Mean XCl/XOH values with vertical bar representing lowest and highest values in each sample. This data is compared with XCl/XOH apatite data from experiments (the experimental data is normalized to 1 wt% Cl in the fluid) involving apatite-aqueous HCl and apatite-aqueous NaCl from Ref.8; (c) Mean XF/XOH values with vertical bar representing lowest and highest values in each sample. The data are compared with XF/XOH apatite data from experiments (the experimental data is normalized to 0.1 wt% F in the fluid) involving apatite-aqueous HCl and apatite-aqueous NaCl from Ref.8. CHAR charnockite samples, TPG two-pyroxene granulite samples, MG metagabbro samples, HBG hornblende-biotite gneiss samples. This figure is created using MS Excel version 16.16.27 (https://www.microsoft.com/en-us/microsoft-365/excel) and modified using Inkscape version 0.92.2 (https://inkscape.org).
Partitioning of F and Cl from the co-existing fluids into apatite has been demonstrated to be highly sensitive to the fluid composition, and the prevailing P–T conditions8,41,42,43,44. In the Nilgiri samples, the metagabbro, two-pyroxene granulite and three high-grade high-Cl-bearing charnockites show XCl/XOH in the range of 0.01–2.5 and XF/XOH in the range of 1–20. Based on an earlier experimental investigation8, in a piston-cylinder apparatus that equilibrated natural fluorapatite with aqueous HCI, NaCl, NaOH, Na2CO3 and CO2–H2O mixtures at 1–2.0 GPa and 950–1050 °C, such high XCl/XOH values, in Cl- and F-bearing apatite, indicate apatite equilibrating in acidic fluids (e.g., HCl with ~ 15 wt% Cl, or ~ 8 wt% Cl in CO2–H2O fluids—considering the linear relationship observed in experiments8). At these P–T conditions, neutral pH is ~ 4 because of the increase in dissociation constant9 to ~ 10–8, and therefore the acidic nature of the fluid co-existing with apatite is relative to this neutral pH. Abundance of hemo-ilmenite-magnetite-pyrite in the metagabbro and two-pyroxene granulite samples show that these rocks are relatively oxidized20, and also could be considered as supportive evidence for the acidic environment during peak metamorphism. Apatite equilibration in aqueous NaCl solutions with ~ 50 wt% Cl at 1GPa (water activity: 0.4 at 1 GPa35,36) can only result in XCl/XOH in the range of 0.5–0.6 (considering the linear relationship observed in experiments with aqueous NaCl fluids at 1050 °C and 2 GPa8). Additionally, rock-buffered experimental results44 of subduction zone lithologies also confirm the experimental results8 that suggest the acidic nature of evolving fluids (containing both Cl and F) in that incorporating high Cl content in apatite cannot be excluded. Such studies strongly suggest that it is difficult to incorporate high Cl content in apatite equilibrating with a fluid containing both F and Cl under normal or high pH conditions at high pressure and high temperature. Therefore, it is reasonable to infer the presence of an acidic environment, due to interaction of fluids containing high-Cl with rocks, during the metamorphism of these rock samples. Thus, comparing our results with observations from an experimental study8 (using both aqueous HCl and aqueous NaCl) shows that XCl or F/XOH values in most of our samples formed in an acidic environment (Fig. 3b,c).
According to high P–T experiments (950–1050 °C and 1–2 GPa)8, if we assume constant Cl, F content in the acidic fluid, the XCl/XOH of the apatite will rise with temperature at constant pressure, but drop with pressure at constant temperature. In our samples, XCl/XOH of apatite increases with pressure and temperature (Fig. 3b). In acidic fluids containing F, an increase in temperature has been demonstrated to significantly decrease XF/XOH content in apatite8 (Fig. 3c). In our samples XF/XOH decrease with pressure and temperature from two-pyroxene granulite to metagabbro. In contrast, XF/XOH in charnockite samples show a constant trend, and amphibolite facies Hb-Bt gneiss samples show a decreasing trend.
An apparent increase in the equilibrium constant (K), [considering ideal conditions39, (KCl/OH = (fHCI × aOH-ap)/(fH2O × aCl-ap)) and (KF/OH = (fHF × aOH-ap)/(fH2O × aF-ap)), for the exchange reactions Ca5(PO4)3Cl + H2O = Ca5(PO4)3OH + HCl and Ca5(PO4)3F + H2O = Ca5(PO4)3OH + HF respectively] with respect to increasing pressure and temperature can be expected at the granulite facies conditions based on data available from previous low P–T studies30,31 (extrapolating the values of K at low P–T conditions (0.1–0.4 GPa, 400–700 °C)41,42 to granulite facies conditions). We also need to bear in mind the low fH2O nature of these fluids in producing anhydrous minerals in these rocks. Assuming these conditions, we estimated the variation of fugacity of the fluid components (fHCl/fH2O and fHF/fH2O). Assuming a constant fHCl or HF/fH2O, as the P–T conditions increase, we expect a decreasing trend in XCl/F/XOH. Contrary to expectations, XCl/XOH increase in our samples, and XF/XOH remains constant from charnockite to two-pyroxene granulite samples and decrease from two-pyroxene granulite to metagabbro samples. This might be due to extremely high fHCl/fH2O in metagabbro, two-pyroxene granulite and the three high-Cl-bearing charnockite samples formed at high-grade conditions. However, in the remaining charnockite samples and amphibolite facies Hb-Bt gneiss samples, fHCl/fH2O shows a gradual decrease, with respect to decrease in P–T. The value of fHF/fH2O seems to be extremely low in metagabbro, and gradually increase from two-pyroxene granulite to charnockite samples. In summary, the comparison of our observations with both high and low P–T experimental data suggest that a change in P, T or the equilibrium constant (based on available data from previous studies) are not the only cause for the observed regional trend.
Similar trends with Cl (relatively low) and F content in apatite have been observed along the Krishnagiri to Salem traverse in the Shevaroy Hills, adjoining the Nilgiri Block32. In this case, the two-pyroxene granulite (named as clinopyroxene-orthopyroxene zone) has the highest Cl content (~ 0.5 wt%) in apatite. Relatively lesser Cl content has been detected in charnockite and amphibolite facies regions (Hb-Bt gneiss).
High P–T acidic conditions (observed in metagabbro, two-pyroxene granulite and the three high-grade charnockite samples) favor partitioning of more F into fluids relative to Cl during fluid-mineral interactions32. Relationship between XCl/XOH in apatite and wt% Cl in fluids observed in experiments also suggests that a change in fluid composition has a significant effect on the Cl content in apatite8. A gradual lowering of Cl in apatite below 800 °C and 0.8 GPa can be seen in our data (Fig. 3). Thus, possibly below 800 °C and 0.8 GPa, the acidic fluid is enriched in F compared to Cl and its composition may gradually shift from high Cl to high F. Another possibility is that Cl is shielded by metal complexes formed during speciation below 800 °C and 0.8 GPa.
Nature of fluids and their implications
Abundant CO2 fluid inclusions in minerals are reported from the major rock types in the Nilgiri and Shevaroy high-grade terranes20,21,22,23. No salt composition is detected in these inclusions. Comparing these results with our observations and previous experimental results8,43 suggests that Cl and F bearing C–O–H fluids could have been more prevalent agents of metamorphism in the lower crust than aqueous NaCl (saline fluids/brine). Moreover, textures like apatite surrounded by K-feldspar veins as well as oxidation textures indicate a convergence of mineralogical and textural features with an acidic environment created by fluids. However, the capacity of C–O–H fluids to cause metamorphic recrystallization was considered low due to the non-polar nature of CO2, and relatively less solubility of minerals in them (compared to saline fluids at low salt concentration)35,36. Recent thermodynamic calculations at 900 °C and 5.0 GPa show a pH drop during irreversible C–O–H fluid-rock reactions, suggesting that at lower pH, C–O–H fluids could be a good agent of metamorphic recrystallization45. Therefore, we propose that fluid–rock reactions in the granulite facies conditions might have reduced the pH of C–O–H-Cl–F fluids, causing high Cl content in apatite, and formation of extensive regional anhydrous granulites.
The source of these high—Cl, F—bearing fluids at a depth of ~20–40 km is currently unknown, although it is possible to speculate different possible scenarios. Cathodoluminescence (CL) images and U–Pb data of zircon grains from both mafic and felsic rock types, together with field and textural evidence of mineral assemblages in this terrane clearly show that they were extensively recrystallized during metamorphism after their magmatic crystallization12,13,14,17,18,19,20. Since these rocks were not thermally perturbed after their peak granulite facies metamorphism, any possibility of contact metamorphism in the presence of fluids released from shallower sources40,42, such as sediment deposits or country rocks, can be excluded. Also, no shallow sedimentary contacts are reported in the vicinity of these rocks. Another possible source is the residual Cl and F enriched fluids left after the partial melting of protoliths at high-grade conditions35. However, there is no evidence for formation of crustal-scale migmatite during a partial melting of these outcrops20. Further, the charnockite or two-pyroxene granulite might not represent a residual phase after partial melting because they are enriched in incompatible elements and LREE14. In addition, they cannot be considered as a melt phase from partial melting of the mafic granulite/metagabbro, because of their concurrent magmatic and metamorphic ages14,18,19. If the two-pyroxene granulite and charnockite were partial melts of metagabbro, then the metamorphic age of metagabbro should have been similar to the magmatic (core) age of charnockite and two pyroxene granulite. Previous geochemical studies using compatible elements shows that, these rocks (both felsic and mafic) could have formed by differentiation of underplated mafic magma produced in an arc magmatic setting14,20. Solid state recrystallization of these rocks during metamorphism could have been assisted by fluids infiltrating from deep or within. Three likely scenarios can be considered for the origin of fluids during metamorphism. The first possibility is that the fluids were auto-generated in the rocks during heating and dehydration reactions within the rock. Second possibility is that they were mantle derived46,47. A third scenario is that the fluids might have originated from crystallizing mafic magma stalled beneath the lower crust (e.g. basaltic underplating) that assisted the metamorphic process during collision with the Dharwar Craton at ca. 2.45–2.5 Ga. The solubility of Cl and F in mafic magma is markedly high48. Therefore, during the initial stages of underplated magma crystallization, relatively higher Cl and F may be expected to preferentially partition into the fluids relative to the solids48. Such acidic fluids (C–O–H–Cl–F or H–Cl–F) infiltrating into the crustal column above could participate in metamorphism. The Cl and F content in such underplated mafic magma could be mantle-derived or due to mixing of Cl-rich fluids released from subducting lithologies such as oceanic crust or subducted sediments in the mantle wedge during formation of mafic magma49,50,51. Irrespective of the uncertainty in their sources, fluid-rock interactions at granulite facies conditions could result in an acidic environment (drop in pH) as is evident from the results reported in this study.
Future directions
Acidic fluids have high potential in causing mass transfer of important elements used for geochemical modeling of petrogenesis of rocks, and also of economically important elements regionally8,9,10,11. Further experimental and theoretical estimations are needed to establish the link between the fugacity of HCl/HF in minerals with that of evolving fluid pH in a rock-buffered system. If such data could be generated then it would be possible to deduce a solution model for OH–F–Cl apatite or to estimate fHF and fHCl to compute the speciation of C–O–H solvent at granulite facies conditions. Improved understanding about the speciation properties and role of acidic fluids in lower crustal processes, such as subduction, arc magmatism, formation of economic mineral deposits, etc., can be sought through further field, analytical, experimental, and thermodynamic studies. This would allow us to improve the uncertainties in modelling the long-term geochemical evolution of Earth’s lithosphere and its environment.
Methods
Electron microprobe (EPMA) analyses of apatite grains given in Extended Data Tables 2–6 were conducted on a 5 channel—JEOL JXA-8100 Superprobe, Electron Probe Micro Analyzer (EPMA), housed at the Department of Earth System Sciences, Yonsei University, Seoul, South Korea. Analytical conditions are accelerating voltage of 20 kV; beam current of 15 nA; counting time of 10 s for F, Cl, Ca, Na, Al, Si, P, Mn and Fe and 50 s for trace elements; an electron beam spot size of 15 μm52,53. F and Cl were kept first in the analysis list due to their volatile nature52,53. F X-ray excitation tends to increase with time during EMPA, therefore elongated apatite grains were chosen for analysis52,53 (Fig. 2). This has been done based on the assumption that grains are oriented parallel to [0001] and would minimize the increase in F X-ray excitation with time. Standards (F-apatite, Cl-apatite, and REE-Y-PO4) supplied by Prof. Daniel E. Harlov, GFZ Potsdam, Germany were used for calibration of F, Cl, Ca, P, and REE’s. During standardization and analysis, TAP crystal (L value ~ 199.2 mm) is used to diffract F X-rays and PET crystal is used for Cl (L value ~ 151.8 mm). Standardization and analysis (including interference correction) for all other elements were done based on Ref.52. Natural and synthetic silicates and oxides supplied by JEOL and ASTIMEX Standards Ltd., Canada, were used for calibration of Na, Al, Si, Mn and Fe. Relative errors in EMPA are estimated to be < 1% at the > 10 wt% level, 5–10% at the 1–10 wt% level, 10–20% at the 0.2 to 1 wt % level, and 20–40% at the < 0.1 wt% level26. Detection limits were ~ 500 ppm for (Y + REE) and ~ 100 ppm for the remaining elements. The data were reduced using the ZAF correction procedures supplied by JEOL. For most samples, we have chosen an average of between 5 and 35 grains. Mineral recalculation (Extended Data Table 2–6) applied the method used in Ref.32. Mole fractions of OH in apatite are calculated assuming the halogen site is filled with F, Cl, and OH (XOHAp = 1 − XFAp − XClAp).
References
Fyfe, W. S., Price, N. & Thompson, A. B. Fluids in the Earth’s Crust (Elsevier, 1978).
Lyons, T., Reinhard, C. & Planavsky, N. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014).
Korenaga, J., Planavsky, N. J. & Evans, D. A. D. Global water cycle and the coevolution of Earth’s interior and surface environment. Philos. Trans. R. Soc. A 375, 20150393 (2017).
Korenaga, J. Crustal evolution and mantle dynamics through Earth history. Philos. Trans. R. Soc. A 376, 20170408 (2018).
Galvez, M. E., Connolly, J. A. & Manning, C. E. Implications for metal and volatile cycles from the pH of subduction zone fluids. Nature 539, 420–424 (2016).
Hwang, G. C. et al. A role for subducted albite in the water cycle and alkalinity of subduction fluids. Nat. Commun. 12, 1155 (2021).
Newton, R. C. & Manning, C. E. Role of saline fluids in deep-crustal and upper-mantle metasomatism: Insights from experimental studies. Geofluids 10, 58–72 (2010).
Brenan, J. M. Partitioning of fluorine and chlorine between apatite and aqueous fluids at high pressure and temperature: Implications for the F and Cl content of high P-T fluids. Earth Planet Sci. Lett. 117, 251–263 (1993).
Eugster, H. P. Minerals in hot water. Am. Mineral. 71, 655–673 (1986).
Korzhinskii, D. S. The theory of metasomatic zoning. Mineral. Depos. 3, 222–231 (1968).
Azimov, P. Y. & Bushmin, S. A. Solubility of minerals of metamorphic and metasomatic rocks in hydrothermal solutions of varying acidity: Thermodynamic modeling at 400–800°C and 1–5 kbar. Geochem. Int. 45, 1210–1234 (2007).
Jayananda, M. & Peucat, J. J. Geochronological Framework of Southern India (Field Science Publishers Osaka, 1996).
Raith, M., Srikantappa, C., Köhler, H. & Buhl, D. The Nilgiri enderbites: Nature and age constraints on protolith formation, high-grade metamorphism and cooling history. Precambr. Res. 98, 129–150 (1999).
Samuel, V. O., Santosh, M., Liu, S., Wang, W. & Sajeev, K. Neoarchean continental growth through arc magmatism in the Nilgiri Block, southern India. Precambr. Res. 245, 146–173 (2014).
Radhakrishna, B. P. Suspect tectono-stratigraphic terrain elements in the Indian sub-continent. J. Geol. Soc. India 34, 1–24 (1989).
Jayananda, M., Santosh, M. & Aadhiseshan, K. R. Formation of Archean (3600–2500 Ma) continental crust in the Dharwar Craton, southern India. Earth Sci. Rev. 181, 12–42 (2018).
Raith, M., Srikantappa, C., Ashamanjari, K. G. & Spiering, B. The granulite terrane of the Nilgiri hills (southern India): characterization of high-grade metamorphism. In Granulites and Crustal Evolution (eds. Vielzeuf, D. & Vidal, P.) 339–365 (Kluwer Academic Publishers, 1990).
Samuel, V. O., Sajeev, K., Hokada, T., Horie, K. & Itaya, T. Neoarchean arc magmatism followed by high-temperature, high-pressure metamorphism in the Nilgiri Block, southern India. Tectonophy. 662, 109–124 (2015).
Samuel, V. O., Santosh, M., Yang, Q. Y. & Sajeev, K. Geochemistry and zircon geochronology of the Neoarchean volcano-sedimentary sequence along the northern margin of the Nilgiri Block, southern India. Lithos 263, 257–273 (2016).
Samuel, V. O., Harlov, D. E., Kwon, S. & Sajeev, K. Mineralogy, petrology, and geochemistry of high-grade, neo-Archean rocks from the Nilgiri Block, southern India: Oxide and sulphide trends. J. Petrol. 60, 1027–1062 (2019).
Newton, R. C., Smith, J. V. & Windley, B. F. Carbonic metamorphism, granulites and crustal growth. Nature 288, 45–50 (1980).
Santosh, M. Carbonic metamorphism of charnockites in the south-western Indian Shield: A fluid inclusion study. Lithos 19, 1–86 (1986).
Touret, J. L. R. & Hansteen, T. H. Geothermobarometry and fluid inclusions in a rock from the doddabetta charnockite complex, Southern India. Soc. Ital. Mineral. Petrol. 43, 65–82 (1988).
Srikantappa, C., Raith, M. & Touret, J. L. R. Synmetamorphic high-density carbonic fluids in the lower crust: Evidence from the Nilgiri granulites, southern India. J. Petrol. 4, 733–760 (1992).
Touret, J. L. R. & Huizenga, J. M. Fluids in granulites. Geol. Soc. Am. Mem. 207, 25–37 (2011).
Newton, R. C., Touret, J. L. R. & Aranovich, L. Y. Fluids and H2O activity at the onset of granulite facies metamorphism. Precambrian Res. 253, 17–25 (2014).
Xiao, Y. L., Hoefs, J., Van-Den-Kerkhof, A. M. & Li, S. G. Geochemical constraints of the eclogite and granulite facies metamorphism as recognized in the Raobazhai complex from North Dabie Shan, China. J. Metamorph. Geol. 19, 3–19 (2001).
Van den Berg, R. & Huizenga, J. Fluids in granulites of the southern marginal zone of the Limpopo belt, South Africa. Contrib. Mineral. Petrol. 141, 529–545 (2001).
Nehring, F., Foley, S. F., Holtta, P. & Van Den Kerkhof, A. M. Internal differentiation of the Archean continental crust: Fluid-controlled partial melting of granulites and TTG–amphibolite associations in Central Finland. J. Petrol. 50, 3–35 (2009).
Putnis, A. Mineral replacement reactions. Rev. Mineral. Geochem. 70, 87–124 (2009).
Putnis, A. & Austrheim, H. Fluid induced processes: Metasomatism and metamorphism. Geofluids 10, 254–269 (2010).
Hansen, E. C. & Harlov, D. E. Whole-rock, phosphate, and silicate compositional trends across an amphibolite- to granulite-facies transition, Tamil Nadu, India. J. Petrol. 86, 1641–1680 (2007).
Safonov, O. G., Kosova, S. A. & Van Reenen, D. D. Interaction of biotite–amphibole gneiss with H2O–CO2–(K, Na)Cl fluids at 550 MPa and 750 and 800 °C: Experimental study and applications to dehydration and partial melting in the middle crust. J. Petrol. 12, 2419–2456 (2014).
Aranovich, L. Y., Zakirov, I. V., Sretenskaya, N. G. & Gerya, T. V. Ternary system H2O–CO2–NaCl at high P–T parameters: An empirical mixing model. Geochem. Int. 48, 446–455 (2010).
Manning, C. E. & Aranovich, L. Y. Brines at high pressure and temperature: Thermodynamic, petrologic and geochemical effects. Precambrian Res. 253, 6–16 (2014).
Manning, C. E. Fluids of the lower crust: Deep is different. Annu. Rev. Earth Planet. Sci. 46, 67–97 (2018).
Ishwar-Kumar, C. et al. A Rodinian suture in western India: New insights on India-Madagascar correlations. Precambrian Res. 236, 227–251 (2013).
Plavsa, D. et al. Delineating crustal domains in Peninsular India: Age and chemistry of orthopyroxene-bearing felsic gneisses in the Madurai Block. Precambrian Res. 198–199, 77–93 (2012).
Tacker, R. C. & Stormer, J. C. Jr. A thermodynamic model for apatite solid solutions, applicable to high-temperature geologic problems. Am. Mineral. 74, 877–888 (1989).
Nijland, T. G., Jansen, J. H. & Maijer, C. Halogen geochemistry of fluid during amphibolite–granulite metamorphism as indicated by apatite and hydrous silicates in basic rocks from the Bamble Sector, South Norway. Lithos 30, 167–189 (1993).
Yardley, B. W. D. Apatite composition and the fugacities of HF and HCl in metamorphic fluids. Mineral. Mag. 49, 77–79 (1985).
Sisson, V. B. Halogen chemistry as an indicator of metamorphic fluid interaction with the Ponder pluton, Coast Plutonic Complex, British Columbia, Canada. Contrib. Mineral. Petrol. 95, 123–131 (1987).
Zhu, C. & Sverjensky, D. A. Partitioning of F–Cl–OH between minerals and hydrothermal fluids. Geochem. Cosmochim. Acta 55, 1837–1858 (1991).
Li, H. & Hermann, J. Apatite as an indicator of fluid salinity: An experimental study of chlorine and fluorine partitioning in subducted sediments. Geochem. Cosmochim. Acta. 166, 267–297 (2015).
Sverjensky, D. A. & Huang, F. Diamond formations due to a pH drop during fluid–rock interactions. Nat. Commun. 6, 8702 (2015).
Dunai, T. J. & Touret, J. L. R. A noble gas study of a granulite sample from the Nilgiri Hills, southern India: Implications for granulite formation. Earth Planet. Sci. Lett. 119, 271–281 (1993).
Klemme, S. & Stalder, R. Halogens in the Earth’s mantle: what we know and what we don’t. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle (eds. Harlov, D. E. & Aranovich, L.) 847–869 (Springer, 2018).
Guo, H. & Audétat, A. Transfer of volatiles and metals from mafic to felsic magmas in composite magma chambers: An experimental study. Geochem. Cosmochim. Acta 198, 360–378 (2017).
Barnes, J. D., Manning, C. E., Scambelluri, M. & Selverstone, J. The Behavior of Halogens During Subduction-Zone Processes In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle (eds. Harlov, D. E. & Aranovich, L.) 545–590 (Springer, 2018).
Wallace, P. J. Volatiles in subduction zone magmas: Concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geotherm. Res. 140, 217–240 (2005).
Harlov, D. E. & Aranovich, L. Y. The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle (Springer, 2018).
Pyle, J. M., Spear, F. S. & Wark, D. A. Electron microprobe analysis of REE in apatite, monazite and xenotime: Protocols and pitfalls. In Phosphates—Geochemical, Geobiological, and Materials Importance (eds. Kohn, M. J. Rakovan, J. & Hughes, J. M.) 337–362. (Mineralogical Society of America, 2002).
Goldoff, B., Webster, J. D. & Harlov, D. E. Characterization of fluor-chlorapatites by electron probe microanalysis with a focus on time-dependent intensity variation of halogens. Am. Mineral. 97, 1103–1115 (2012).
Acknowledgements
This research was supported by 2017R1A6A1A07015374 (Multidisciplinary study for assessment of large earthquake potentials in the Korean Peninsula) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, Korea to V.O.S, NRF-2019R1A2C1002211 to S.K., and NRF-2021R1C1C101057011 to Y.J. Funding from the Ministry of Earth Sciences, Government of India projects MoES/ATMOS/PP-IX/09 and MoES/P.O. (Geosci)/26/2014 for field work and sample preparation while V.O.S. was a PhD student at the Centre for Earth Sciences, Indian Institute of Science, Bangalore, India is also acknowledged. Figure 1a is republished with permission of Elsevier Science & Technology Journals, from “A Rodinian suture in western India: New insights on India-Madagascar correlations”, Ishwar-Kumar, C. et al., 236, 2013; permission conveyed through Copyright Clearance Center, Inc.
Author information
Authors and Affiliations
Contributions
V.O.S., S.K., and Y.J. conceived the project. V.O.S. performed petrographic and EPMA analyses, and data evaluation. V.O.S., S.K., and Y.J. contributed to interpretation and the writing of an earlier draft. All authors equally contributed to interpretation and final manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samuel, V.O., Santosh, M., Jang, Y. et al. Acidic fluids in the Earth’s lower crust. Sci Rep 11, 21146 (2021). https://doi.org/10.1038/s41598-021-00719-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00719-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.