Abstract
Bats are potential natural reservoirs for emerging viruses, causing deadly human diseases, such as COVID-19, MERS, SARS, Nipah, Hendra, and Ebola infections. The fundamental mechanisms by which bats are considered “living bioreactors” for emerging viruses are not fully understood. Some studies suggest that tolerance to viruses is linked to suppressing antiviral immune and inflammatory responses due to DNA damage by energy generated to fly. Our study reveals that bats' gut bacteria could also be involved in the host and its microbiota's DNA damage. We performed screening of lactic acid bacteria and bacilli isolated from bats' feces for mutagenic and oxidative activity by lux-biosensors. The pro-mutagenic activity was determined when expression of recA increased with the appearance of double-strand breaks in the cell DNA, while an increase of katG expression in the presence of hydroxyl radicals indicated antioxidant activity. We identified that most of the isolated bacteria have pro-mutagenic and antioxidant properties at the same time. This study reveals new insights into bat gut microbiota's potential involvement in antiviral response and opens new frontiers in preventing emerging diseases originating from bats.
Similar content being viewed by others
Introduction
Over the past years, bats have been widely studied as primary reservoirs for emerging deadly human viruses. There is direct evidence that Nipah and Hendra viruses, which lead to outbreaks in Southeast Asia and Australia, originated from bats1,2,3. The evidence of bat origin of the Ebola virus is debatable as it is based only on serological studies and detection of Ebola-related viruses in these animals4,5,6. A similar situation is with SARS-CoV, MERS-CoV, and especially SARS-CoV-2, as there is no unequivocal worldwide consensus on the origin of these viruses7,8. However, the involvement of bats in the interspecies transmission of emerging viruses is undeniable, as evidenced by the discovery of probable virus precursors of the abovementioned coronaviruses in them9,10,11.
Future epidemic and pandemic outbreaks of emerging viral diseases are inevitable. Even before the COVID-19, several papers were published where the possibility of occurrence of emerging zoonic bat virus has been discussed12,13. Therefore, one of the main priorities today is to find ways to prevent or delay the spillover of highly virulent viruses from animals to humans. Bats should be the primary target in such studies as the range of zoonotic pathogens in them is the highest among other known mammalian species14. Also, these animals pose an additional thread as reservoirs of emerging zoonotic infections as they are the only mammalians with the ability to fly and a relatively long lifespan15. This results in a high rate of contact with humans, and most importantly, other animals16.
The secret of viral biodiversity in bats lies in limited immune and inflammatory responses to infections. The central concept behind this unique feature is the relationship between the ability to fly and resistance to viruses mediated by high levels of free radicals produced during flight and subsequent DNA damage17. In addition, some suggest that the gut microbiota of bats also could be involved in tolerance to viruses. However, the exact mechanisms have not yet been revealed18.
In this study, we screened lactic acid bacteria and bacilli isolated from Nyctalus noctula, Pipistrellus kuhlii, and Eptesicus serotinus bats' feces for oxidative and mutagenic activity in vitro using lux-biosensors.
Results
Mass-spectrometry identification
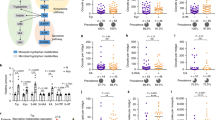
Sixty-five isolates of lactic acid bacteria and bacilli were isolated from the litter according to the following parameters: growth on MRS agar medium with sorbic acid (pH 6.4) in a microaerophilic chamber, tinctorial properties (Gram-positive bacilli and coccobacilli), and mass spectrometric biotyping (Figs. 1, 2). Out of 89 bats, lactic acid bacteria and bacilli were isolated only from 59 animals. For the lux-biosensors study, we used microorganisms identified by mass spectrometry to a genus level. Detailed results of mass spectrometric biotyping are shown in supplementary Table 1S.
Mutagenic and antioxidant properties of bat gut commensals
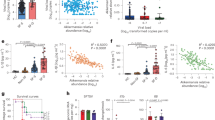
According to the lux-biosensors study, most isolated lactic acid bacteria and bacilli have pro-mutagenic (Fig. 1) and antioxidant (Fig. 2) properties. Pro-mutagenic and prooxidant activities were considered when induction changes based on calculations of KatG/RecA expressions reached negative values, while DNA-protective and antioxidant properties were considered at positive values. Only 9 out of 65 isolates showed a beneficial DNA-protective effect, while most isolates except for seven showed beneficial antioxidant properties. The mean of pro-mutagenic effect evaluated and calculated relative to dioxidine is -4.796%. The mean of antioxidant effect evaluated and calculated relative to peroxide is 24.926 %. Thus, we did not observe a significant association between the mutagenic and oxidative properties (Table 1). Also, there were no statistical differences in mutagenic and oxidative activities in lactic acid bacteria and bacilli isolated from different bat species, which indicates that all isolated bacteria from bat species included in the study have similar properties: both pro-mutagenic (Fig. 3) and antioxidant (Fig. 4). According to the mosaic plot, most of the isolated bacteria have pro-mutagenic and antioxidant properties. At the same time, there is an almost complete absence of isolates with DNA-protective (anti-mutagenic) and prooxidant activity with little presence of such bacteria in P. kuhlii and E. serotinus (Fig. 5).
Discussion
Bats are one of the most dangerous reservoirs of viral infections among mammals due to their ability to fly and spread viruses capable of interspecies spillover. The proper mechanisms making these animals perfect "living bioreactors" for emerging viruses due to viral immune tolerance and subsequent unpredictable replication and recombination of viruses are still not fully understood16. Luo et al. suggest that the gut microbiota of bats can be involved in the unique antiviral response of these animals, even naming it a "missing link" between the ability of flight and tolerance for viruses in bats18. Our study opens new frontiers in this concept, revealing expressed pro-mutagenic and antioxidant activities of bat gut bacteria at the same time.
Unlike other animals, bats' gut microbiota composition strongly depends on their environment rather than on an evolutionary predisposition to host-specific bacteria19. This is related to the following features of the bat gut. Intestines in bats are comparatively shorter from one-third to one-fifth than in animals of the same size due to the absence of the ascending and transverse colon20,21. Some bat species do not even have a whole colon22. Additionally, most bat species lack cecum and appendix, which are essential parts of the gastrointestinal tract for the gut microbial community in other animals21,23. The shorter length of the gastrointestinal tract and absence of some of its parts results in reduced transit time of food digest compared to mammalians of the same size24. It should be noted that along with the rapid chyme movement through the intestine, a relatively high metabolic rate has been described in bats due to energy expenditure on flights17. All of these features contribute to a different environment from other animals for bats gut bacteria. Most important is lower anaerobic volumes, which reduce resident anaerobic gut microbiota and increase the proportion of transient environmental microbes25. Our study supports this statement, as we did not isolate anaerobic or microaerophilic bacteria from the examined bats. The reason for this could be the absence or low numbers of lactic acid bacteria and bacilli in bats' feces. For studying microbial biodiversity in bats, other screening methods should be used, for example, PCR24. Our study is aimed at investigating mutagenic and oxidative properties of bat gut lactobacilli and spore-forming bacilli.
We used lux-biosensors to determine the properties of the microorganisms isolated from the bats' feces. Lux-biosensors are E. coli MG1655, containing plasmids with the operon luxCDABE of Photorhabdus luminescens put under the control of the E. coli recA and katG promoters. The expression of recA increases with the appearance of double-strand breaks in the cell DNA, which leads to activation of the SOS response system26,27, while the expression of katG increases in the presence of hydroxyl radicals28.
As shown in Fig. 1, the supernatants of most of the studied bacteria increased recA induction when supplemented simultaneously with the DNA damage inducer. However, it should be noted that the supernatants themselves did not cause a significant change in recA expression.
Since lux-biosensors do not show double-stranded breaks in the cell DNA, but only the cell response to them, two occasions can lead to the increase recA expression in the presence of metabolites. First, bacterial metabolites and the dioxidin used as an inducer can act synergistically as the number of double-strand breaks in the cell increases compared with the action of dioxidin alone. Therefore, the cellular response to damage also increases. On the other hand, metabolites can directly enhance the cell response by interfering with the SOS response in the early stages at the same level of DNA damage.
Initially, RecA protein expression is at a low level. The recA promoter, like other SOS response protein promoters, is repressed by the LexA protein. When double-strand breaks occur, RecA protein binds to the damaged ssDNA and forms activated RecA protein, under the influence of which LexA protein is autocatalytically cleaved, leading to a rapid increase in the expression of recA and other genes involved in the SOS response26,27,28. It can be speculated that metabolites of bat intestinal bacteria can increase the affinity of RecA for ssDNA or interact with LexA, accelerating its autocatalysis. The following can only be considered as a very cautious assumption; however, in eukaryotes, there is a system of homologous repair of double-strand breaks, and the RAD51 protein playing an essential role in its regulation29. Moreover, RAD51 protein is homologous and similar to RecA30,31. Thus, it is possible that the action of metabolites of the intestinal microbiota can enhance the SOS-response of bacteria and the processes occurring in the eukaryotic cells of the host. On the one hand, the homologous repair system works only in dividing cells in the S- and G2-stages of the cell cycle. So it does not belong to the primary cell repair systems32. On the other hand, due to the ability to fly, the cells of bats produce increased levels of prooxidants17, which leads to an increase in DNA damage, among other things. There are many genetically anchored adaptations in bats to protect various systems from prooxidants17,33, including enhanced DNA repair systems34,35. It is possible that the gut microbiota of bats also contributes to the enhanced DNA repair systems of bats.
RAD51 also plays a prominent role in the replication of viruses, including single-stranded RNA viruses such as coronaviruses and retroviruses. For instance, the human immunodeficiency virus can enhance RAD51 expression, increasing cell survival after treatment with genotoxic agents and the transcription level of viral proteins36,37. On the other hand, high levels of RAD51 limit the integration of HIV into cells38,39,40. There was a positive correlation between RAD51 expression and the proviral load of the human T-lymphotropic virus41. Compound B02, a human RAD51 inhibitor, showed activity against SARS-CoV-2 by inhibiting its replication42. The resistance of volatiles to viruses may depend, among other things, on the regulation of RAD51, in which the gut microbiota may be involved.
This study speculates on new possible mechanisms in the bat-gut bacteria relationship, particularly pro-mutagenic and antioxidant action of isolated lactic acid bacteria and bacilli from bats' feces. We suggest that antioxidant properties of bat gut microbiota could be a direct response to the high level of free radicals produced during flight, which increases their survival chances in bat intestines17. Interestingly, antioxidant properties are also described in lactic acid bacteria and bacilli studied by lux biosensors earlier, but at the same time, they showed anti-mutagenic (DNA-protective) properties, making them good candidates for probiotics43,44. However, mass screening of bat gut bacteria revealed that most of them have pro-mutagenic action with expressed antioxidant activity. One explanation may be that bat gut bacteria adapt to the intestinal environment of these animals, as they strive to occupy a niche that strongly depends on the environment because of unique features of bat intestines physiology. That is why they develop properties promoting DNA damage to take out other bacteria and antioxidant properties to counteract high levels of free radicals. Another explanation may lie in the transient environmental microbes, which are relatively easy to enter the bat gut because of lower anaerobic volumes. Thus, uncontrolled contact of entire bat populations with environmental bacteria, which may have pro-mutagenic properties, may affect the known mechanisms of interaction with DNA damage caused by the production of free radicals during flight and antiviral immune response altering viral tolerance of these animals17. This can result in unpredicted replication and recombination of emerging viruses, which could spillover to humans and cause epidemics.
Reported data on possible pro-mutagenic and antioxidant activity of bat gut bacteria places the fundament in further research of the relationship between bats, bacteria, and viruses interaction. These properties require more in-depth studies. In particular, screening gut microbiota of bats from distant regions, as the microbiota composition of Chiroptera is different in various environments19. We studied bats from regions with different climates and environments and did not find significant differences; however, more evidence should be obtained, especially in countries with unfavorable epizootic scenarios. Also, the metagenomic approach can be used for a more thorough screening of bat gut microbiota composition as it allows the identification of uncultivated microorganisms. Our study points to the necessity to reveal the genetic mechanisms of possible pro-mutagenic and antioxidant activity of bats gut commensals, which should be done on samples from animals from distant geographical areas and natural habitats. We also suggest that in vitro studies based on gastrointestinal model simulators can help reveal the relationship between the length and structure of the bats' intestine and the pro-mutagenic and antioxidant properties of their gut bacteria45, as the in vivo controlled studies in bats could be unethical, biologically-threatening, and challenging to conduct. However, the existing artificial gastrointestinal systems, such as TIM, SHIME, ESIN, DIDGI, and SIMGI, are not suitable for the mentioned studies as is, since they were not designed for bat gastrointestinal tract45. Therefore, they should be modified for these purposes, or a conceptually new bat simulating system should be designed. Bat-based in vitro gastrointestinal model can also reveal the direct relationship between lower gastrointestinal anaerobic volumes and lower anaerobic and microaerophilic gut bacteria rates, which is observed in our study by the relatively low rate of lactobacilli and spore-forming bacilli isolates. Most importantly, this study reveals the possible mechanism of bats-bacteria-viruses interaction that humankind can control by bacterial modulation of the bats' environment to prevent new emerging viruses spillover.
Methods
Bats fecal samples collection
The study involved fecal samples of N. noctula (n=43), P. kuhlii (n=22), and E. serotinus (n=24) from southern regions of Russia (Table 2) collected from April 15th to May 31st, 2021. Minimum 0.5 g of fecal samples were taken from each bat, then they were placed at sterile containers and transported to the laboratory at 7 °C.
Microorganisms isolation and mass-spectrometry identification
Samples were aseptically removed from containers for subsequent extraction by grinding in sterile phosphate-buffered saline (pH 7.4) at a 1:10 ratio. Then the extracts were inoculated into a liquid MRS medium. Inoculates were incubated at 37 °C for 24 hours, then serial tenfold dilutions in sterile PBS (pH 7.4) were made. Each dilution was plated on MRS agar medium (6.4), and the cultures were incubated in a microaerophilic chamber at 37 °C for 24 hours. The selection of colonies for further research was carried out based on colony morphology, microscopy of smears with Gram stain, and catalase activity.
Pure overnight bacterial cultures were used for mass spectrometric biotyping. Sample preparation for the mass MALDI-TOF spectrometry was carried out by the direct deposition method. The study was performed on a Microflex LT instrument (Bruker Daltonics GmbH, Leipzig, Germany) using Biotyper (version 3.0) software (Bruker Daltonics GmbH, Leipzig, Germany).
Determination of DNA-destructive and antioxidant properties using lux-biosensors
Sample preparation included inoculation of cultures into a test tube with 10 ml of liquid LB medium and cultivation for 48 hours for lactic acid bacteria and 24 hours for bacilli at a temperature of 37 °C44,46. Cell-free supernatants were obtained by centrifugation (Minispin-plus; Eppendorf, Leipzig, Germany) at 11000 g for 7 min.
Determination of mutagenic and oxidative activity was based on bacterial lux-biosensors - genetically modified strains of E. coli MG1655 (RecA-lux) and E.coli MG1655 (KatG-lux) responding to DNA damage and oxidative stress, respectively, by the expression of bioluminescence genes46,47. The strains contain plasmids with the operon luxCDABE of Photorhabdus luminescens put under the control of the corresponding E. coli promoters. This operon contains the luciferase genes and their regulators and provides the bioluminescence used as a reporter function in this test. As an inducer of DNA damage, we used dioxidine (2,3-Quinoxalinedimethanol,1,4-dioxide, Biosintez, Russia) at a 2.25·10−5 M concentration was used. For the induction of oxidative damage, we used hydrogen peroxide (Aquatest Ltd, Rostov on Don, Russia) at a concentration of 10−3 M. Bacteria were cultured in a liquid nutrient medium at 37 °C until the early- to mid-logarithmic phase. The overnight culture was diluted with fresh medium to a density of 0.01 to 0.1 McFarland unit (3·106–3·107 cells/mL). Density measurements were performed using a DEN-1B densitometer (Biosan, Riga, Latvia). The suspension was then incubated for 2 hours to the early logarithmic phase. Aliquots of this culture (90 μl each) were transferred into sterile microplate wells. Then we added 10 μl of the test cell-free supernatant preparations to each well with culture aliquot following the addition of 10 µl of each studied inducers (dioxidine and hydrogen peroxide). In control wells, we added 10 µl of deionized water and 10 µl of sterile distilled water.
After treatment, the plate with samples was placed in a luminometer and incubated at 30 °C. Bioluminescence intensity was measured every 10 min over a 110-min period.
A microplate reader FLUOstar Omega (BMG Labtech Germany) was used for luminescence measurements. All experiments were performed in three independent replicates.
To evaluate KatG and RecA expression, we calculated the induction factor (Is) according to the formula:
where Le and Lk are the luminescence intensities of samples from the experimental and control groups, respectively.
The index of mutagenic and oxidative activity (A, %) was calculated by the formula:
where Ip and Ia are the SOS-response induction factors in the presence of the cell-free supernatants preparation and the control, respectively.
The detailed calculation algorithms are provided in supplemented R statistical code (Supplementary File S1).
Statistical analysis
Statistical analysis was performed using R v4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). The sets obtained from the mass spectrometer were analyzed and processed, then bar charts characterizing the SOS response and oxidative activity of the isolates under study were constructed. Mutagenic and oxidative activities were then compared between different bat species. All data were not normally distributed according to the Shapiro–Wilk test. For the median comparison between groups, the Kruskal-Wallis test was used. Fisher exact test was used to evaluate the strength of the association between the pro- or antioxidant and pro-mutagenic or DNA-protective properties of isolated lactic acid bacteria and bacilli.
Ethical statement
The experimental protocols of the reported study were approved by the ethics committee of the Don State Technical University, Rostov-on-Don, Russia (protocol number 67-43-4). Experimental procedures for this report did not include any in vivo studies. ARRIVE guidelines: not applicable48. The collection of fecal samples was carried out according to the Sanitary and Epidemiological Regulations SP 3.2.1288-03. Studies of lactobacilli and spore-forming bacilli were carried out according to the Sanitary and Epidemiological Regulations SP 1.3.2322-08.
References
Edson, D. et al. Routes of Hendra virus excretion in naturally-infected flying-foxes: Implications for viral transmission and spillover risk. PLoS ONE 10, e0140670. https://doi.org/10.1371/journal.pone.0140670 (2015).
Islam, M. S. et al. Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011–2014. Emerg. Infect. Dis. 22, 664–670. https://doi.org/10.3201/eid2204.151747 (2016).
Gentles, A. D., Guth, S., Rozins, C. & Brook, C. E. A review of mechanistic models of viral dynamics in bat reservoirs for zoonotic disease. Pathog. Glob. Health 114, 407–425. https://doi.org/10.1080/20477724.2020.1833161 (2020).
Fairhead, J., Leach, M. & Millimouno, D. Spillover or endemic? Reconsidering the origins of Ebola virus disease outbreaks by revisiting local accounts in light of new evidence from Guinea. BMJ Glob. Health 6, e005783. https://doi.org/10.1136/bmjgh-2021-005783 (2021).
Caron, A. et al. Ebola virus maintenance: If not (only) bats, what else?. Viruses 10, 549. https://doi.org/10.3390/v10100549 (2018).
Goldstein, T. et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol. 3, 1084–1089. https://doi.org/10.1038/s41564-018-0227-2 (2018).
Al-Salihi, K. A. & Khalaf, J. M. The emerging SARS-CoV, MERS-CoV, and SARS-CoV-2: An insight into the viruses zoonotic aspects. Vet. World 14, 190–199. https://doi.org/10.14202/vetworld.2021.190-199 (2021).
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C. & Garry, R. F. The proximal origin of SARS-CoV-2. Nat. Med. 26, 450–452. https://doi.org/10.1038/s41591-020-0820-9 (2020).
Lau, S. K. et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 102, 14040–14045. https://doi.org/10.1073/pnas.0506735102 (2005).
van Boheemen, S. et al. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 3, e00473-e512. https://doi.org/10.1128/mBio.00473-12 (2012).
Shang, J. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 581, 221–224. https://doi.org/10.1038/s41586-020-2179-y (2020).
Wang, L. F. & Anderson, D. E. Viruses in bats and potential spillover to animals and humans. Curr. Opin. Virol. 34, 79–89. https://doi.org/10.1016/j.coviro.2018.12.007 (2019).
Calisher, C. H., Childs, J. E., Field, H. E., Holmes, K. V. & Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19, 531–545. https://doi.org/10.1128/CMR.00017-06 (2006).
Letko, M., Seifert, S. N., Olival, K. J., Plowright, R. K. & Munster, V. J. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 18, 461–471. https://doi.org/10.1038/s41579-020-0394-z (2020).
Gorbunova, V., Seluanov, A. & Kennedy, B. K. The world goes bats: Living longer and tolerating viruses. Cell Metab. 32, 31–43. https://doi.org/10.1016/j.cmet.2020.06.013 (2020).
Donnik, I. M. et al. Coronavirus infections of animals: Future risks to humans. Biol. Bull. Russ. Acad. Sci. 48, 26–37. https://doi.org/10.1134/S1062359021010052 (2021).
Subudhi, S., Rapin, N. & Misra, V. Immune system modulation and viral persistence in bats: Understanding viral spillover. Viruses 11, 192. https://doi.org/10.3390/v11020192 (2019).
Luo, J., Liang, S. & Jin, F. Gut microbiota in antiviral strategy from bats to humans: A missing link in COVID-19. Sci. China Life Sci. 64, 942–956. https://doi.org/10.1007/s11427-020-1847-7 (2021).
Lutz, H. L. et al. Ecology and host identity outweigh evolutionary history in shaping the bat microbiome. mSystems 4, e00511-e519. https://doi.org/10.1128/mSystems.00511-19 (2013).
Caviedes-Vidal, E. et al. The digestive adaptation of flying vertebrates: High intestinal paracellular absorption compensates for smaller guts. Proc. Natl. Acad. Sci. U. S. A. 104, 19132–19137. https://doi.org/10.1073/pnas.0703159104 (2007).
Gadelha-Alves, R., Rozensztranch, A. M. D. S. & Rocha- Barbosa, O. Comparative intestinal histomorphology of five species of phyllostomid bats (phyllostomidae, microchiroptera): Ecomorphological relations with alimentary habits. Int. J. Morph. 26, 591–602 (2008).
Makanya, A. N. & Maina, J. N. The morphology of the intestine of the insectivorous horseshoe bat (Rhinolophus-Hildebrandti, Peters)—A scanning electron and light-microscopic study. Afr. J. Ecol. 32, 158–168. https://doi.org/10.1111/j.1365-2028.1994.tb00566.x (1994).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32. https://doi.org/10.1038/nrmicro3552 (2016).
Sun, D. L., Gao, Y. Z., Ge, X. Y., Shi, Z. L. & Zhou, N. Y. Special features of bat microbiota differ from those of terrestrial mammals. Front. Microbiol. 11, 1040. https://doi.org/10.3389/fmicb.2020.01040 (2020).
Song, S. J. et al. Comparative analyses of vertebrate gut microbiomes reveal convergence between birds and bats. MBio 11, e02901-02919. https://doi.org/10.1128/mBio.02901-19 (2020).
Maslowska, K. H., Makiela-Dzbenska, K. & Fijalkowska, I. J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 60, 368–384. https://doi.org/10.1002/em.22267 (2019).
Baharoglu, Z. & Mazel, D. SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol. Rev. 38, 1126–1145. https://doi.org/10.1111/1574-6976.12077 (2014).
Bell, J. C. & Kowalczykowski, S. C. RecA: Regulation and mechanism of a molecular search engine. Trends Biochem. Sci. 41, 491–507. https://doi.org/10.1016/j.tibs.2016.04.002 (2016).
Godin, S. K., Sullivan, M. R. & Bernstein, K. A. Novel insights into RAD51 activity and regulation during homologous recombination and DNA replication. Biochem. Cell Biol. 94, 407–418. https://doi.org/10.1139/bcb-2016-0012 (2016).
Lin, Z., Kong, H., Nei, M. & Ma, H. Origins and evolution of the recA/RAD51 gene family: Evidence for ancient gene duplication and endosymbiotic gene transfer. Proc. Natl. Acad. Sci. U. S. A. 103, 10328–10333. https://doi.org/10.1073/pnas.0604232103 (2006).
Shinohara, A. et al. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat. Genet. 4, 239–243. https://doi.org/10.1038/ng0793-239 (1993).
Hustedt, N. & Durocher, D. The control of DNA repair by the cell cycle. Nat. Cell. Biol. 19, 1–9. https://doi.org/10.1038/ncb3452 (2016).
Lagunas-Rangel, F. A. Why do bats live so long?-Possible molecular mechanisms. Biogerontology 21, 1–11. https://doi.org/10.1007/s10522-019-09840-3 (2020).
Zhang, G. et al. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science 339, 456–460. https://doi.org/10.1126/science.1230835 (2013).
Croco, E. et al. DNA damage detection by 53BP1: Relationship to species longevity. J. Gerontol. A. Biol. Sci. Med. Sci. 72, 763–770. https://doi.org/10.1093/gerona/glw170 (2017).
Chipitsyna, G. et al. HIV-1 Tat increases cell survival in response to cisplatin by stimulating Rad51 gene expression. Oncogene 23, 2664–2671. https://doi.org/10.1038/sj.onc.1207417 (2004).
Chipitsyna, G., Sawaya, B. E., Khalili, K. & Amini, S. Cooperativity between Rad51 and C/EBP family transcription factors modulates basal and Tat-induced activation of the HIV-1 LTR in astrocytes. J. Cell Physiol. 207, 605–613. https://doi.org/10.1002/jcp.20612 (2006).
Thierry, S. et al. Dual and opposite effects of hRAD51 chemical modulation on HIV-1 integration. Chem. Biol. 22, 712–723. https://doi.org/10.1016/j.chembiol.2015.04.020 (2015).
Cosnefroy, O. et al. Stimulation of the human RAD51 nucleofilament restricts HIV-1 integration in vitro and in infected cells. J. Virol. 86, 513–526. https://doi.org/10.1128/JVI.05425-11 (2012).
Kaminski, R. et al. Interplay of Rad51 with NF-κB pathway stimulates expression of HIV-1. PLoS ONE 9, e98304. https://doi.org/10.1371/journal.pone.0098304 (2014).
Ramezani, S. et al. Assessment of HTLV-1 proviral load, LAT, BIM, c-FOS and RAD51 gene expression in adult T cell leukemia/lymphoma. Med. Microbiol. Immunol. 206, 327–335. https://doi.org/10.1007/s00430-017-0506-1 (2017).
Biering, S. B. et al. Screening a library of FDA-approved and bioactive compounds for antiviral activity against SARS-CoV-2. ACS Infect. Dis. https://doi.org/10.1021/acsinfecdis.1c00017 (2021).
Prazdnova, E. V. et al. DNA-protection and antioxidant properties of fermentates from Bacillus amyloliquefaciens B-1895 and Bacillus subtilis KATMIRA1933. Lett. Appl. Microbiol. 61, 549–554. https://doi.org/10.1111/lam.12491 (2015).
Chistyakov, V. A., Prazdnova, E. V., Mazanko, M. S. & Bren, A. B. The use of biosensors to explore the potential of probiotic strains to reduce the SOS response and mutagenesis in bacteria. Biosensors (Basel) 8, 25. https://doi.org/10.3390/bios8010025 (2018).
Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 4, 308–319. https://doi.org/10.1016/j.crfs.2021.04.004 (2021).
Prazdnova, E. V. et al. SOS response inhibitory properties by potential probiotic formulations of bacillus amyloliquefaciens B-1895 and bacillus subtilis KATMIRA1933 obtained by solid-state fermentation. Curr. Microbiol. 76, 312–319. https://doi.org/10.1007/s00284-018-01623-2 (2019).
Bazhenov, S. et al. Influence of the luxR regulatory gene dosage and expression level on the sensitivity of the whole-cell biosensor to acyl-homoserine lactone. Biosensors (Basel) 11, 166. https://doi.org/10.3390/bios11060166 (2021).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Acknowledgements
The reported study was funded by RFBR according to the research Project № 20-04-60263. I.V.P., M.S.M., S.N.G., M.L.C., and A.M.E. acknowledge support from the Ministry of Science and Higher Education of the Russian Federation (Project Number 075-15-2019-1880). P.E.V. was financially supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the state task in the field of scientific activity (Southern Federal University, No. 0852-2020-0029). We thank professor Manukhov I.V. (Moscow Institute of Physics and Technology, Laboratory for Molecular Genetics, Moscow, Russia) for providing E. coli MG1655, containing plasmids with the operon luxCDABE of Photorhabdus luminescens put under the control of the E. coli recA and katG promoters.
Author information
Authors and Affiliations
Contributions
I.V.P., M.L.C. and A.M.E. planned the research and contributed to the initial study design. A.V.M. conducted zoological research and obtained fecal samples. S.N.G. isolated microorganisms from feces and performed primary identification. I.S.A., A.V.A. and T.I.T. conducted mass spectrometry biotyping, M.S.M. and E.V.P. performed biosensor studies, E.D.K. and I.V.P. performed the statistical analysis, I.V.P., M.S.M., and E.D.K. wrote the draft of the manuscript. T.I.T., A.M.E., and M.L.C. revised the manuscript. All authors reviewed the final variant of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Popov, I.V., Mazanko, M.S., Kulaeva, E.D. et al. Gut microbiota of bats: pro-mutagenic properties and possible frontiers in preventing emerging disease. Sci Rep 11, 21075 (2021). https://doi.org/10.1038/s41598-021-00604-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00604-z
This article is cited by
-
Detection of coronaviruses in insectivorous bats of Fore-Caucasus, 2021
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.