Abstract
Several lines of evidence have suggested that patients following venous thromboembolism (VTE) are at higher risk of arterial thromboembolism (ATE). Prothrombotic fibrin clot characteristics were reported in individuals with cardiovascular risk factors. We investigated whether specific fibrin clot properties measured after 3–4 months of anticoagulation characterize VTE patients with subsequent ATE. We enrolled 320 patients following VTE aged below 70 years (median age, 46). Ten patients were lost to follow-up. ATE occurred in 21 individuals after a median 54 (31–68) months during a follow-up of 87.5 months (incidence 0.94%; 95% confidence interval [CI], 0.59–1.4 per patient-year). Patients with ATE had faster fibrin clot degradation, reflected by maximum rate of D-dimer increase during plasma clot lysis induced by tissue-type plasminogen activator (D-Drate) at baseline. Clot permeability, turbidimetric variables, clot lysis time, and thrombin generation were unrelated to ATE. Univariable Cox proportional hazards analysis showed that age, diabetes, and D–Drate were risk factors for subsequent ATE. Increased D–Drate (by 0.001 mg/L/min; hazard ratio, 1.08; 95% CI 1.02–1.14) was an independent predictor of ATE after adjustment for potential confounders. Faster fibrin clot degradation at 3 months since VTE may increase the risk of ATE among VTE patients during follow-up.
Similar content being viewed by others
Introduction
With an overall incidence of 1 to 2% of individuals per year, venous thromboembolism (VTE) involving deep-vein thrombosis (DVT) and pulmonary embolism is a common disease and its prevalence increases with age1. A two-way relationship between VTE and arterial thromboembolism (ATE), largely manifests as myocardial infarction (MI) and ischemic cerebrovascular events, has been reported, however the available data are inconclusive2,3,4,5,6.
Population-based studies have demonstrated that patients with prior unprovoked VTE had a 1.3-fold higher risk of ATE compared to controls during 20 years of follow-up6, and patients aged 20–39 years after unprovoked VTE had almost fourfold increased risk of MI in the 10-year follow-up study7. Individuals with unprovoked VTE have been found to be at a higher risk of ATE as compared to those with provoked VTE (incidence rate ratio, 1.86; 95% confidence interval [CI], 1.19–2.89) and controls (incidence rate ratio, 1.87; 95%CI, 1.32–2.65)4. It has been also reported that patients with unprovoked DVT are more likely to have carotid plaques compared with control subjects (odds ratio, 1.8; 95% CI, 1.1–2.9)8. Moreover, a higher mortality rate associated with ATE (standardized incidence ratio 1.28; 95% CI 1.00–1.56) was observed in VTE patients9.
The mechanisms underlying association between VTE and ATE are not fully understood3. VTE and ATE share some risk factors, including older age, male sex and obesity2,10,11. Trauma, immobility and thrombophilia represent well-established VTE risk factors, however thrombophilia is also reported to be associated with ATE10,12,13,14,15, while cigarette smoking, hyperlipidemia, diabetes, and hypertension are potent risk factors for ATE12. Statins and aspirin used in the prevention of ATE have been reported to reduce the risk of VTE, while anticoagulant agents in particular rivaroxaban, decrease the risk of ATE, which provides additional evidence for the role of blood coagulation in ATE16,17.
Looking for prothrombotic mechanisms involved in the pathophysiology of both ATE and VTE, it has been demonstrated that fibrin clots generated from plasma of patients with VTE are characterized by the so-called prothrombotic fibrin clot phenotype, defined as formation of denser fibrin clot networks evidenced by low permeability and reduced susceptibility to lysis, which can predict the risk of recurrent VTE18,19,20,21. Prothrombotic fibrin clot properties were also observed in patients with acute and prior ischemic stroke or MI22,23. Of note, cardiovascular risk factors including smoking, diabetes, higher blood pressure, and positive family history have been shown to be associated with prothrombotic fibrin clot characteristics18,22,24,25,26.
Given the available data suggesting a higher risk of ATE in VTE patients6,7, we put forward a hypothesis that prothrombotic fibrin clot properties might identify a subset of VTE patients at increased risk of developing ATE during long-term follow-up. We aimed to evaluate the predictive value of a comprehensive set of plasma fibrin clot parameters in terms of the risk of ATE among VTE patients.
Results
At baseline
We studied 320 DVT patients (155 men, 48.4%) at a median age of 46 (36–54) years, including 159 (49.7%) with unprovoked VTE. A total of 112 (35%) subjects smoked cigarettes, 96 (30%) individuals had hypertension, 13 (4.1%) suffered from diabetes, 256 (80%) from hypercholesterolemia and 63 (19.7%) from obesity. Heart failure was diagnosed in 10 (3.1%) patients.
ATE during follow-up
Ten patients (3.1%) were lost to follow-up. The median follow-up period was 87.5 (77–95) months. ATE was diagnosed in 21 (6.8%, 0.9% per year) patients (9 men, 12 women) after a median follow-up of 54 (31–68) months. Fourteen patients (4.5% of the whole group) had MI (including 7 ST-elevated MI and 7 non-ST elevated MI), 6 (1.9%) had ischemic stroke and 1 patient was diagnosed with peripheral arterial disease complicated with acute popliteal artery occlusion. The incidence rate of ATE was 0.94% (95% CI, 0.59–1.4%) per patient-year. The corresponding incidence rates of MI and ischemic stroke were 0.62% (95% CI, 0.36–1.02%) per patient-year and 0.27% (95% CI, 0.11–0.56%) per patient-year (Table 1). Five subjects of the 21 ATE patients (23.8%) were also diagnosed with recurrent VTE. In all of them ATE was observed after recurrent VTE when patients were on anticoagulation.
Among 310 studied patients 154 had unprovoked VTE. Those patients had lower fibrinogen compared to patients with provoked VTE (2.9 [2.5–3.6] g/L versus. 3.2 [2.6–4] g/L, p = 0.02). There were no differences in other cardiovascular risk factors between patients with unprovoked and provoked VTE (data not shown). Fibrinogen correlated negatively with permeability coefficient (Ks) (r = - 0.37), and positively with both absorbance (ΔAbs) (r = 0.61) and clot lysis time (CLT) (r = 0.24) (Supplemental Table 1).
Patients who experienced ATE during follow-up were older by about 6 years and more frequently suffered from diabetes. There were no other intergroup differences in demographics or comorbidities (Table 1, Supplemental Table 2). Regarding routine laboratory investigations patients with ATE during follow-up had 23% lower high-density lipoprotein cholesterol compared to the remainder (1.14 [1.01–1.55] mmol/L versus 1.48 [1.21–1.73] mmol/L; p = 0.009). There were no differences in fibrinogen, D-dimer, and thrombin generation between patients with ATE and the remainder.
When we divided patients into three groups, i.e. patients with recurrent VTE, patients with ATE, and those free of both ATE and recurrent VTE (Table 1), we observed that patients with ATE were older than those from the latter group. Patients with ATE had 26.7% higher low-density lipoprotein cholesterol (3.8 [3.1–4.2] mmol/L versus 3 [2.5–3.7] mmol/L; p = 0.03) along with 15.2% lower peak thrombin compared to patients with recurrent VTE (Supplemental Table 2).
Fibrin clots and ATE
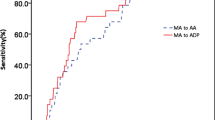
Patients with diagnosed ATE compared with the remainder had baseline lower Ks which reflects a smaller average pore size in fibrin networks, as well as a higher rate of increase in D-dimer levels (D-Drate) (Table 2, Fig. 1). The two groups did not differ in lag phase, ΔAbs, maximum D-dimer concentrations (D–Dmax), and CLT (Table 2). When 5 subjects with recurrent VTE were excluded from ATE group, then patients with subsequent ATE (n = 16) had higher D-Drate (0.077 [0.071–0.083] mg/L/min versus 0.072 [0.068–0.079] mg/L/min; p = 0.02) and D–Dmax (4.32 [4.15–4.46] mg/L versus 4.07 [3.68–4.34] mg/L; p = 0.03) (Fig. 1) compared to the remainder (n = 289), without any differences in other fibrin variables, including Ks.
The maximum rate of increase in D-dimer levels (D–Drate, panel (A,C) and maximum D-dimer concentrations (D–Dmax, panel B,D) measured during plasma clot lysis in the permeation assay in patients without arterial thromboembolism (ATE) (n = 289) and patients with subsequent ATE including (n = 21, panel A,B) and excluding (n = 16, panel C,D) patients with recurrent VTE (n = 5).
We compared patients free of both recurrent VTE and ATE during follow-up with those with recurrent VTE and those with ATE. We found that the latter group had 11.6% higher D-Drate and 25% shorter CLT than those with recurrent VTE (Table 2, Fig. 2).
There were no differences in fibrin clot properties between patients with MI and ischemic stroke (data not shown).
Risk factors of ATE
Univariable Cox proportional hazards analysis revealed that older age (hazard ratio [HR], 1.05; 95% CI, 1.01–1.1), diabetes (HR, 3.96; 95% CI, 1.17–13.45) and D–Drate (HR, 1.07; 95% CI, 1.014–1.13) predicted ATE during follow-up. Higher D–Drate was a risk factor for ATE after adjustment for age, sex, diabetes, fibrinogen and recurrent VTE (HR, 1.08; 95% CI, 1.02–1.14, Table 3). D–Drate predicted ATE also after adjustment for potential confounders including acetylsalicylic acid (ASA) (HR, 1.08; 95% CI, 1.02–1.14, Supplemental Table 3).
Fibrin clot and recurrent VTE
Individuals with recurrent VTE (n = 83) had 12% lower Ks, 9.1% lower lag phase and 23.5% longer CLT compared to subjects without both recurrent VTE and ATE during follow-up (Table 2, Fig. 2). Patients with recurrent VTE compared with those free of recurrent VTE, regardless ATE differed significantly in fibrin clot properties (Supplemental Table 4). The results correspond to the findings obtained after a median 44 months of follow-up20. Previously, seventy-seven (25%) subjects diagnosed previously with recurrent VTE were characterized by 12% lower Ks, 9% shorter lag phase in the fibrin formation assay and 25% longer CLT20.
Discussion
The present study is the first to assess a comprehensive set of plasma fibrin clot properties in a cohort of VTE patients as potential risk factors for ATE in order to address the hypothesis of the fibrin-related mechanisms linking VTE and ATE. Contrary to our expectations, we demonstrated that in a largely middle-aged cohort of VTE patients an increased rate of plasma fibrin clot degradation expressed as higher D-Drate characterized patients with subsequent ATE27. The study showed that other fibrin clot properties, including clot permeability and CLT extensively explored in VTE and cardiovascular disease, did not identify young and middle-aged VTE patients at an increased risk of ATE in the future. Our findings suggest that faster enzymatic fibrin clot degradation could reflect the instability of fibrin networks prone to fragmentation in vivo and could increase the risk of ATE. This study appears to indicate that the prothrombotic clot phenotype observed in young and middle-aged subjects after 3 months following VTE might be associated with reduced risk of ATE in the following years. The current observations suggest the complex role of fibrin clot structure and function in the pathophysiology of thromboembolism.
The reported incidence rate of ATE in VTE patients varied between studies from 0.3% per patient year for acute MI in individuals with unprovoked DVT or pulmonary embolism (mean age 46 years) to 3.8% per patient-year for ATE (MI, stroke) in patients with unprovoked pulmonary embolism at a mean age of 55 ± 17 years4. The systematic review showed that the weighted mean incidence rate of ATE was 0.65% (95% CI, 0.36–1.01; I2 = 25%) per patient-year in randomized controlled trials and 0.76% (95% CI, 0.6–0.94, I2 = 96%) per patient-year in cohort studies4. We observed a similar incidence rate of ATE, i.e. 0.94% (95% CI, 0.59–1.4%) per patient-year. In the study of Spencer et al.7 who studied 6065 young and middle-aged patients (range 20–64 years) after unprovoked VTE, the incidence rate of acute MI was 0.33% per patient-year, which is in line with our findings (0.62% [95% CI, 0.36–1.02%] per patient-year).
Of paramount importance is the plasma-based assay in which we were able to show faster clot lysis in patients who developed ATE during follow-up. In the assay introduced by Collet et al.28 in 1999 to analyze fibrin properties in nephrotic patients and healthy controls, the previously formed plasma clot was perfused with a buffer containing high tissue-type plasminogen activator (tPA) concentration, similar to that observed in patients treated with tPA-based thrombolysis29. Our modification of the original approach was used for the first time to assess the impact of statins and other drugs on fibrin clot properties in 200630. This assay was also applied by our group to evaluate efficiency of fibrinolysis in patients with ATE. We found higher D–Drate and D–Dmax in patients with cryptogenic ischemic stroke as compared to the control group23. In contrast, lower D-Drate was observed in MI patients who survived in-stent thrombosis31 and patients with a history of limb ischemia of unknown cause as compared to the control group32. In line with the present study, this measure of clot lysis did not predict recurrent VTE20. Our present observation suggests that various lysis assays should be used to highlight specific fibrin clot abnormalities in a particular disease with lysis abnormalities, without any single test appropriate to all conditions in which fibrinolysis is disturbed as shown in previous studies32,33,34. In our study the modified assay by Collet et al.28 enabled to show subtle changes in fibrin clot lysability that could be of importance in ATE prediction, while other commonly used assays did not differentiate subjects with VTE at risk of ATE. Importantly we did not observe any differences in CLT between patients who developed the subsequent ATE and the remainder.
Mechanisms behind the observed link between ATE and faster clot degradation remain unclear. Atherogenic effects of fibrin degradation products have been demonstrated in several studies. Corban et al.35 have reported that fibrin degradation products were associated with larger atherosclerotic plaques and necrotic core areas. They suggested that higher fibrin degradation products might be a marker of subclinical rupture or erosion of the plaque35. Moreover, fibrin was found to stimulate the production of proinflammatory molecules, interleukin-1, interleukin-8 and intracellular adhesion molecule-136,37,38 Fibrin degradation products stimulate the migration of monocytes and local fibrinolysis39.
On the other hand, the role of fibrin in atherothrombosis is complex. It has been suggested that fibrinogen and fibrin are involved in the development of early atherosclerotic lesions and their progression39,40,41. In hyperlipidemic and hypercoagulable mouse models (mice carrying the factor V Leiden and mice being thrombomodulin mutants) Seehaus et al.27 observed that hypercoagulabity leaded to larger atherosclerotic plaques and plaque stability with less necrotic cores, and that anticoagulant treatment reduced plaque stability27. Borissoff et al.42 reported enhanced procoagulant state and higher endogenous thrombin potential in the early atherosclerotic lesions as compared to stable advanced atherosclerotic lesions. They concluded that blood coagulation and the resultant increased formation of fibrin contribute to a more stable atherosclerotic plaques42. In animal studies it has been shown that in mice less prothrombotic phenotype was associated with reduced atherosclerosis or less early-stage atherosclerotic lesions43. Based on the current findings, it might be speculated that faster lysis with enhanced fragmentation of fibrin meshworks can predispose to embolic events. Moreover, faster clot degradation could be associated with more vulnerable plaques with more fragile fibrin deposits on the surface of atherosclerotic lesions, and therefore it could be a risk factor of ATE. It remains to be established whether similar observation can be made in all subjects at risk of ATE beyond those with a history of VTE episode.
In our cohort there were no differences in the risk of ATE between patients with unprovoked VTE versus provoked VTE. This finding, though based on a relatively small number of patients, was contrary to our expectations, because several studies, including its meta-analysis, have shown that patients after unprovoked VTE were at higher risk of ATE compared to those with provoked VTE, however some reports failed to observe such differences related to the type of VTE4,6,44. Of note, we observed an increased fibrinogen concentration at 3 months since the event in patients with provoked VTE. Elevated fibrinogen levels are observed commonly in patients with cardiovascular disease and show associated with cardiovascular risk factors45,46. This parameter is also the key determinant of fibrin clot properties18.
Several study limitations should be acknowledged. The study population was limited, however this cohort was well described with a large set of hemostatic parameters and followed for, on average, more than 7 years. All laboratory measurements were done in a single point time, at 3 months and this time point is of key importance in clinical decision making following VTE. Changes over time in all the variables measured cannot be ruled out, however in our opinion the impact of the results obtained specifically after 3 months of anticoagulation has been shown suggesting persistent abnormalities affecting clinical outcomes during follow-up19,23. The influence of drugs including ASA on fibrin clot properties is possible18. We did not perform microscopic assessment of plasma clots, but in our previous studies D-Drate showed no association with fiber diameter or pore size in clots obtained using scanning electron microscopy. The current findings cannot be easily extrapolated to elderly VTE patients in whom ATE and VTE occur more commonly, since we excluded such patients from this study. The same holds true for anticoagulated VTE patients because the majority of the current cohort stopped anticoagulation after a few months of treatment except those who developed recurrent VTE episodes.
In conclusion, we demonstrated that patients with ATE which is experienced a few years since VTE are not characterized by the prothrombotic fibrin clot phenotype in a cohort of patients aged 70 years or less, which is in contrast to the association between such phenotype and recurrent VTE. The finding suggesting that VTE patients with subsequent ATE during follow-up had a higher rate of clot degradation at high concentrations of rtPA, measured in vitro after 3 months of anticoagulation supports growing evidence for fibrin involvement in a wide spectrum of thromboembolic episodes. It needs further investigations whether D-Drate determined at 3 months since the index VTE may help identify VTE patients who need close surveillance for ATE and assessment of cardiovascular risk. Further studies should be performed to corroborate our results in larger cohorts and elucidate pathophysiological mechanisms behind the contribution of fibrin degradation and the clinical outcomes.
Patients and methods
A total of 368 patients with a history of VTE were screened for meeting the eligibility criteria for the study between October 2008 and June 2010. The cohort was described in detail in our previous paper20. Briefly, the eligible patients following the first-ever isolated DVT or combined with pulmonary embolism were recruited among those referred to our center for diagnostic work-up. The diagnosis of DVT and pulmonary embolism was established as reported20. We excluded patients with known cancer, severe thrombophilia, recent acute ATE, acute infection or severe kidney or liver failure. Patients with VTE received standard anticoagulant treatment with vitamin K antagonists for 3 to 12 months according to the guidelines47.
At enrolment data on cardiovascular risk factors were collected. Hypertension was regarded as increased blood pressure (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) or antihypertensive treatment. Hypercholesterolemia was defined as a total cholesterol level above 5 mmol/l, low-density lipoprotein cholesterol above 3 mmol/l or statin treatment; diabetes as fasting glucose of ≥ 7.0 mmol/l, non-fasting glucose ≥ 11.1 mmol/l, antidiabetic treatment or previously diagnosed diabetes. Obesity was defined as body mass index equal to or greater than 30 kg/m2. Heart failure was recognized based on typical symptoms and reduced left ventricular ejection fraction (< 40%).
All experimental protocols were approved by the Bioethics Committee of the Jagiellonian University. From all study participants the informed written consent in accordance with the Declaration of Helsinki was obtained. All methods were carried out in accordance with relevant guidelines and regulations.
Follow-up
Patients were followed-up on a 6-month basis since enrolment. Clinical data was collected every six months via a visit in the outpatient clinic or phone calls.
The primary composite endpoint was ATE defined as ischemic stroke, MI or peripheral arterial thromboembolic event. Stroke was diagnosed based on persistence of typical symptoms for more than 24 h confirmed on magnetic resonance imaging or computed tomography48. The diagnosis of MI was established based on typical symptoms, changes on the electrocardiogram and increased myocardial necrosis biomarkers49. Peripheral arterial thromboembolic event was defined as acute peripheral artery occlusion50. ATE episodes related to invasive procedures were excluded. The secondary endpoint of the study was symptomatic VTE diagnosed based on positive findings of color duplex sonography. Recurrent DVT in the same leg as the index event was diagnosed when new no-compressibility of venous segment was observed or when there was an increase of at least 4 mm in the residual diameter. Anticoagulation treatment was prescribed again in patients with recurrent VTE.
Laboratory investigations
After 3 months (12 to 15 weeks) of anticoagulant treatment since DVT, antecubital blood samples for laboratory investigation were taken from fasting patients in the morning hours (8 to 10 AM). Patients who were treated with vitamin K antagonists were first temporarily switched to a low-molecular-weight heparin for 10–14 days. Blood samples were taken after 16–24 h since the last injection. We assessed blood cell count, lipid profiles, glucose, creatinine, and international normalized ratio using routine laboratory techniques. Firstly, blood samples (vol/vol, 9:1 of 3.2% trisodium citrate) were centrifuged at 2000 × g for 10 min within 30 min of the draw, then we removed supernatant, aliquoted and stored it at − 80 °C until analysis. We determined fibrinogen using the Clauss method. High-sensitivity C-reactive protein was assessed by nephelometry (Siemens). We measured plasma concentrations of D-dimer, tPA and plasminogen activator inhibitor-1 antigens using immunoenzymatic assays (American Diagnostica). Thrombophilia screening was conducted as described51.
Fibrin clot permeability
Fibrin clot permeation was assessed as described30. Briefly, we mixed 60 µl of plasma with 60 µl of the coagulation trigger containing 1 IU/ml human thrombin and 20 mM CaCl2. Immediately, 100 µl of prepared assay was transferred to a plastic cylinder made from a serological pipette (Sarstedt, Nümbrecht, Germany). Subsequently, after 2 h of incubation at a room temperature, we connected tubes containing the clots via plastic tubing to a reservoir of a buffer (0.05 M tris–HCl, 0.15 NaCl, pH 7.5). The volume of a buffer flowing through the gels was measured within 60 min. Bromophenol blue was used after experiments to find potential leaks, thanks to this procedure we were able to detect and discard the defective clots. We calculated the permeability coefficient, an indirect measure of the average pore size in the fiber network, using the equation: Ks (× 10–9 cm2) = Q × L × η/t × A × ∆P, where Q (cm3) is the flow rate at time t (s), L (cm) is the length of the fibrin gel, η (dyne × s/cm2) is the viscosity of the liquid, A (cm2) is the cross-sectional area and ∆p (dyne/cm2) is differential pressure. The intraassay coefficient of variation was 6.8%.
Turbidity measurements
To initiate polymerization plasma citrated samples from each patient were mixed 2:1 with a Tris buffer containing 0.6 IU/mL human thrombin (Sigma) and 50 mM CaCl2. We used spectrophotometer to read absorbance (ΔAbs) at 405 nm. A lag phase as time to the start of fibrin polymerization, slope of the polymerization curve, along with maximum absorbance at plateau were assessed19.
Plasma clot lysis assays
Fibrinolysis efficiency was examined using two assays at 2 various concentrations of recombinant tPA (rtPA)30,52. In the first assay, CLT was assessed. As previously described20, 100 µl citrated plasma was mixed with 15 mmol/L CaCl2, 0.6 pM human tissue factor (Innovin, Siemens), 12 µmol/L phospholipid vesicles and 60 ng mL-1 tPA (rtPA, Boehringer Ingelheim, Ingelheim, Germany). The mixture was transferred to a microtiter plate. Absorbance was measured at 405 nm at 37 °C. CLT was determined as the time from the midpoint of the clear-to-maximum-turbid transition (clot formation), to the midpoint of the maximum-turbid-to-clear transition (the lysis of the clot).
In the second assay, fibrin clot lysis was assessed using a dynamic lysis assay according to Collet et al.28 with some modifications30. Fibrin clots obtained in the same manner as for fibrin clot permeation were washed with Tris buffer and perfused with the same buffer containing 0.2 µmol/L rtPA (Boehringer Ingelheim). The lysis rate was determined by fibrin degradation reflected D-dimer levels in the effluent using ELISA (American Diagnostica). The D-dimer level was assessing at 20 min intervals until when the fibrin gel collapsed under the pressure, usually after 80–120 min. We measured the maximum rate of increase in D-dimer levels (D–Drate) and maximum D-dimer concentrations (D–Dmax) as previously described20.
Calibrated automated thrombogram (CAT)
The commercial reagents (Thrombinoscope, BV, Maastricht, Netherlands) were used to conduct the CAT assay19. Shortly, 20 µl of a starting reagent containing 5 pM recombinant relipidated TF, 4 mM phospholipids, 100 mM CaCl2 and 2.5 mM fluorogenic substrate was added to 80 µl of plasma sample. We determined the 3 following variables: the peak thrombin, the endogenous thrombin potential, and the time to thrombin using the Fluoroskan Ascent microplate fluorometer (Thermo Fisher Scientific, Vantaa, Finland).
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (interquartile range). The Shapiro–Wilk test was used to verify the assumption of the normal distribution of continuous variables. The Student’s or the Welch’s t-test based on the equality of variances for normally distributed variables were used to compare two groups. The Mann–Whitney U-test was performed to compare two groups for non-normally distributed continuous variables. The categorical (qualitative) variables were presented as the number (percentages) and the Chi-squared test (or Fisher exact test) was used to compare them between groups and the post-hoc test was applied where it was necessary. To compare three groups the Kruskal–Wallis test was used and the post-hoc comparisons for the Kruskal–Wallis test were also performed.
The risk factors of ATE were revealed using univariable and multivariable Cox proportional hazards regression models. The variables for multivariable Cox regression model were chosen based on p-value less than 0.1 of univariable models (with the exception of Ks) and potential confounders were considered. We included in the final multivariable model: age, gender, diabetes, fibrinogen D–Drate and recurrent VTE. The proportional hazard assumption was verified by the Schoenfeld Residuals test. The predictive accuracy of presented model was estimated by C-index (c-statistics), also referred to as area under Receiver Operating Curve. The goodness of fit for the presented model was assessed by the Grønnesby and Borgan test and also with application of deviance residuals. The results of all Cox regression models are showed as HRs with 95% CIs. The level of significance for the two-sided tests was set below 0.05. The study was powered to have 81% chance of detecting deference in D–Drate, given the type I error 5%. Hence, type II error was 19%. The package R53, G*Power v. 3.1.9.454 and Statistica 12.5 software (StatSoft Inc., Tulsa, Oklahoma, United States) were used to conduct the analyses.
References
Heit, J. A., Spencer, F. A. & White, R. H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis. 41, 3–14 (2016).
Lowe, G. D. O. Common risk factors for both arterial and venous thrombosis. Br. J. Haematol. 140, 488–495 (2008).
Prandoni, P. Venous and arterial thrombosis: two aspects of the same disease?. Eur. J. Intern. Med. 20, 660–661 (2009).
Becattini, C., Vedovati, M. C., Ageno, W., Dentali, F. & Agnelli, G. Incidence of arterial cardiovascular events after venous thromboembolism: a systematic review and a meta-analysis. J. Thromb. Haemost. 8, 891–897 (2010).
Prandoni, P. et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J. Thromb. Haemost. 4, 1891–1896 (2006).
Sørensen, H. T., Horvath-Puho, E., Pedersen, L., Baron, J. A. & Prandoni, P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet 370, 1773–1779 (2007).
Spencer, F. A., Ginsberg, J. S., Chong, A. & Alter, D. A. The relationship between unprovoked venous thromboembolism, age, and acute myocardial infarction. J. Thromb. Haemost. 6, 1507–1513 (2008).
Prandoni, P. et al. An association between atherosclerosis and venous thrombosis. N. Engl. J. Med. 348, 1435–1441 (2003).
Schulman, S. et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J. Thromb. Haemost. 4, 734–742 (2006).
Anderson, F. A. Jr. & Spencer, F. A. Risk factors for venous thromboembolism. Circulation 107, I9–I16 (2003).
Greenland, P. et al. American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 56, e50–e103 (2010).
Previtali, E., Bucciarelli, P., Passamonti, S. M. & Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 9, 120–138 (2011).
Lóczi, L., Kappelmayer, J., Tarr, T. & Bagoly, Z. Antiphospholipid syndrome and the risk of myocardial infarction: Current evidence and uncertainties. Kardiol. Pol. 78, 6–14 (2020).
Mahmoodi, B. K., Brouwer, J. L., Veeger, N. J. & van der Meer, J. Hereditary deficiency of protein C or protein S confers increased risk of arterial thromboembolic events at a young age: Results from a large family cohort study. Circulation 118, 1659–1667 (2008).
Reynaud, Q. et al. Risk of venous and arterial thrombosis according to type of antiphospholipid antibodies in adults without systemic lupus erythematosus: a systematic review and meta-analysis. Autoimmun. Rev. 13, 595–608 (2014).
Lowe, G. D. O. Arterial disease and venous thrombosis: Are they related, and if so, what should we do about it?. J. Thromb. Haemost. 4, 1882–1885 (2006).
Gradolí, J., Vidal, V., Brady, A. J. & Facila, L. Anticoagulation in patients with ischaemic heart disease and peripheral arterial disease: clinical implications of COMPASS study. Eur. Cardiol. 13, 115–118 (2018).
Undas, A. Fibrin clot properties and their modulation in thrombotic disorders. Thromb. Haemost. 112, 32–42 (2014).
Zabczyk, M., Plens, K., Wojtowicz, W. & Undas, A. Prothrombotic fibrin clot phenotype is associated with recurrent pulmonary embolism after discontinuation of anticoagulant therapy. Arterioscler. Thromb. Vasc. Biol. 37, 365–373 (2017).
Cieslik, J., Mrozinska, S., Broniatowska, E. & Undas, A. Altered plasma clot properties increase the risk of recurrent deep vein thrombosis: a cohort study. Blood 131, 797–807 (2018).
Undas, A. & Casini, A. Congenital structural and functional fibrinogen disorders: a primer for internists. Pol. Arch. Intern. Med. 129, 913–920 (2019).
Undas, A. & Ariëns, R. A. S. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 31, e88–e99 (2011).
Undas, A. et al. Altered fibrin clot structure/function in patients with cryptogenic ischemic stroke. Stroke 40, 1499–1501 (2009).
Mills, J. D., Ariëns, R. A. S., Mansfield, M. W. & Grant, P. J. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation 106, 1938–1942 (2002).
Barua, R. S. et al. Effects of cigarette smoke exposure on clot dynamics and fibrin structure: an ex vivo investigation. Arterioscler. Thromb. Vasc. Biol. 30, 75–79 (2010).
Vilar, R., Fish, R. J., Casini, A. & Neerman-Arbez, M. Fibrin(ogen) in human disease: both friend and foe. Haematologica 105, 284–296 (2020).
Seehaus, S. et al. Hypercoagulability inhibits monocyte transendothelial migration through protease-activated receptor-1-, phospholipase-Cbeta-, phosphoinositide 3-kinase-, and nitric oxide-dependent signaling in monocytes and promotes plaque stability. Circulation 120, 774–784 (2009).
Collet, J. P. et al. Abnormal fibrin clot architecture in nephrotic patients is related to hypofibrinolysis: influence of plasma biochemical modifications: a possible mechanism for the high thrombotic tendency?. Thromb. Haemost. 82, 1482–1489 (1999).
Sane, D. C. et al. The Thrombolysis and Angioplasty in Myocardial Infarction Study Group Racial differences in responses to thrombolytic therapy with recombinant tissue-type plasminogen activator Increased fibrin(ogen)olysis in blacks. Circulation 83, 170–175 (1991).
Undas, A., Celinska-Löwenhoff, M., Löwenhoff, T. & Szczeklik, A. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. J. Thromb. Haemost. 4, 1029–1036 (2006).
Undas, A. et al. Altered plasma fibrin clot properties are associated with in-stent thrombosis. Arterioscler. Thromb. Vasc. Biol. 30, 276–282 (2010).
Karpińska, I. A., Nowakowski, T., Wypasek, E., Plens, K. & Undas, A. A prothrombotic state and denser clot formation in patients following acute limb ischemia of unknown cause. Thromb. Res. 187, 32–38 (2020).
Siudut, J., Natorska, J., Son, M., Plens, K. & Undas, A. Increased levels of histidine-rich glycoprotein are associated with the development of post-thrombotic syndrome. Sci. Rep. 10, 14419 (2020).
Siudut, J., Iwaniec, T., Plens, K., Pieters, M. & Undas, A. Determinants of plasma fibrin clot lysis measured using three different assays in healthy subjects. Thromb. Res. 197, 1–7 (2021).
Corban, M. T. et al. Elevated levels of serum fibrin and fibrinogen degradation products are independent predictors of larger coronary plaques and greater plaque necrotic core. Circ. J. 80, 931–937 (2016).
Luyendyk, J. P., Schoenecker, J. G. & Flick, M. J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 133, 511–520 (2019).
Qi, J. & Kreutzer, D. L. Fibrin activation of vascular endothelial cells. Induction of IL-8 expression. J. Immunol. 155, 867–876 (1995).
Harley, S. L., Sturge, J. & Powell, J. T. Regulation by fibrinogen and its products of intercellular adhesion molecule-1 expression in human saphenous vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20, 652–658 (2000).
Spronk, H. M. H., van der Voort, D. & Ten Cate, H. Blood coagulation and the risk of atherothrombosis: a complex relationship. Thromb. J. 2, 12 (2004).
Borissoff, J. I., Spronk, H. M. H., Heeneman, S. & ten Cate, H. Is thrombin a key player in the “coagulation-atherogenesis” maze?. Cardiovasc. Res. 82, 392–403 (2009).
Borissoff, J. I., Spronk, H. M. H. & ten Cate, H. The hemostatic system as a modulator of atherosclerosis. N. Engl. J. Med. 364, 1746–1760 (2011).
Borissoff, J. I. et al. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation 122, 821–830 (2010).
Kalz, J., ten Cate, H. & Spronk, H. M. Thrombin generation and atherosclerosis. J. Thromb. Thrombolysis. 37, 45–55 (2014).
Golemi, I. et al. Registro Informatizado de Enfermedad Tromboembólica Investigators. Incidence of major adverse cardiovascular events among patients with provoked and unprovoked venous thromboembolism: Findings from the Registro Informatizado de Enfermedad Tromboembólica Registry. J. Vasc. Surg. Venous Lymphat. Disord. 8, 353–359 (2020).
Stec, J. J. et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation 102, 1634–1638 (2000).
Emerging Risk Factors Collaboration, Kaptoge, S., et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 367, 1310–1320 (2012).
Kearon, C. et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141, e419S-e496S (2012).
Sacco, R.L. et al., American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 44, 2064–2089 (2013).
Ibánez, B. et al., ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 39, 119–177 (2018).
Hirsch, A.T. et al., American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 113, e463-e654 (2006).
Undas, A. et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 114, 4272–4278 (2009).
Kupis, R. W., Goldman-Mazur, S., Polak, M., Ząbczyk, M. & Undas, A. Faster fibrin clot degradation characterizes patients with central pulmonary embolism at a low risk of recurrent peripheral embolism. Sci. Rep. 9, 72 (2019).
R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/.
Erdfelder, E., Faul, F. & Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 28, 1–11 (1996).
Funding
This work was supported by the Jagiellonian University School of Medicine (K/ZDS/005802) (A.U.) and the Polish National Science Centre (UMO-2013/09/B/NZ5/00254) (A.U.).
Author information
Authors and Affiliations
Contributions
S.M. and E.W. interpreted data and wrote the article, E.B. performed statistical analysis. A.U. designed the study, recruited patients, collected data and approved the article for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mrozinska, S., Wypasek, E., Broniatowska, E. et al. Accelerated fibrin clot degradation is associated with arterial thromboembolism in patients following venous thrombosis: a cohort study. Sci Rep 11, 21003 (2021). https://doi.org/10.1038/s41598-021-00411-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00411-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.