Abstract
Surgical cutting guides are increasingly used for maxillofacial reconstruction. They are usually provided by laboratories. In recent years, surgical teams have published studies on the possibility of manufacturing their own cutting guides thanks to 3D printers. The object of this study is to analyze the impact of the sterilization on the surface of those personalized models and to assess the effectiveness of sterilization. Using the data from high-resolution CT scan of patient, 3D models were generated through computerized assisted design and fabricated with a 3D printer using Acrylonitrile Butadiene Styrene (ABS). For the sterilization, a Sterrad method was used. In order to evaluate the effectiveness of sterilization, 3D models were artificially contaminated with several bacterial reference strains, sterilized and finally cultured. The surfaces and mechanical modifications were analyzed before and after sterilization with infrared spectrometry, surface contact angle, extensometer, scanning electron microscopy and atomic force microscopy. Ten models of different shapes and 24 samples were fabricated, sterilized and analyzed. The 3D models were designed in 48 h, printed in an average of 122 min and underwent a 47 min cycle of sterilization. All experimentally contaminated 3D models were negative in culture, with at least, a six log reduction of the initial inoculum. The hydrophobicity and roughness of the surface suffered few changes. The reproducibility of this procedure was proved by identical results in the three sterilization rounds. Using Sterrad process for the sterilization of ABS printed material doesn’t represent a bacterial risk for the patient. It is a feasible and safe innovative reconstructive method that can save time particularly for oncological cases.
Similar content being viewed by others
Introduction
Three-dimensional printing technology, also called additive manufacturing is having an increasing role in the medical field particularly for surgical planning and production of anatomical models, surgical instruments, implants and prostheses1,2,3.
In maxillofacial and reconstructive surgery, the challenge of is to restore the anatomical structure with the best aesthetic and functional aspect. Personalized surgery is essential in this territory4. 3D guides are the best tools to achieve an accurate, symmetrical and functional reconstruction.
The commercialization of low-cost 3D printers allowed us to internalize the 3D printing process and integrate it into our therapeutic plan avoiding a long and expensive external providing process5. Working with 3D printers implies the employment of several technologies: fused deposition modeling, stereolithography, selective laser sintering, and digital light processing. A diverse range of materials that are consistently evolving can be use in this printing process. In order to be positioned in open surgical wounds directly applied at the surface of the bone structure that will be cut or reshaped, these materials must have undergone an effective sterilization process. It is also essential to have a good assessment of the specific bacteriological risk associated with the use of these printed surgical guides. The ANSM (Agence Nationale de Sécurité du Médicament), which is the French equivalent of the FDA (Food and Drug Administration) has established a classification for medical devices with specific guidelines for sterilization procedures corresponding to their proximity to sterile tissue. The 3D printed guides fall into the class IIa (Class I for the FDA) of medical devices and therefore require fulfilling a specific set of security criteria in terms of sterilization6.
In the present study, the surgical guides were printed by the fused deposition technique with pure Acrylonitrile Butadiene Styrene (ABS). ABS has a glass transition temperature close to 105 °C (121°F) that is incompatible with classic steam autoclave sterilization (121 °C (250°F) for 18 min). The alternative could be a chemical sterilization process at low temperature with hydrogen peroxide: the Sterrad7. A study by Peniston et al.8 showed that hydrogen peroxide plasma and ethylene acid can be used to sterilize polylactic acid (glass transition temperature: 55 °C, 131°F) without causing any significant change to their biomechanical properties. Diab-Elschahawi et al., emphasized the importance of cleaning medical devices before being exposed to a subsequent hydrogen peroxide sterilization process9. Although hydrogen peroxide plasma can induce some changing on polymer surfaces, no published studies assessing its impact on biomechanics and its bactericidal efficiency are available for ABS10,11.
The aim of this study was to assess if 3D printed material used as medical device temporarily applied in surgical wounds can be effectively sterilized with the Sterrad method without alteration of the final shape, surface and functionality.
Material and methods
Conception and printing
Figure 1 summarizes the experimental protocol carried out by a trained plastic surgeon. For the conception of the digital model of the guides, we have used, for each case, anonymized high-resolution CT data in DICOM format (Fig. 1a) that was transformed into Standard Tesselation Language (STL) format and transferred to a computer 3D modeling software (MeshMixer 2: Autodesk, Inc) for the image segmentation and the generation of the digital 3D models12,13,14 (Fig. 1b).
Manufacturing process of the surgical cutting guides with the ABS 3D printer. (a) DICOM format images from a CT-scan of the inferior limbs used for the volume rendering and 3D reconstruction, (b) Personalized 3D printed guides designed to anatomically fit the fibula and guide the osteotomies in order to have exactly the osseous segments needed for the mandibular reconstruction, and avoiding all important structures as vessels and nerves, (c) 3D printer to obtain ABS cutting guides from STL format digital files, (d) Cutting guides before sterilization, (e) The Sterrad 100NX is a system that allows dry, low temperature sterilization of heat-sensitive instruments using hydrogen peroxide vapor gas plasma technology without leaving any toxic residues. (f) Bacteriological tests after sterilization, (g) Surface analysis of the 3D printed samples.
The 3D models were sliced with the printer software Z-Suite with the following settings: ABS material, 0.14 mm of layer thickness, normal speed, full infill, support lite and autoprint cooling (Fig. 1c).
The 3D printer used was the Zortrax M200 that converted the digital model into a physical object by layer-to-layer deposition method using ABS (Fig. 1d). This 3D printing technique consists in liquefying the ABS by heating it beyond its melting point, and then pushing it through an extruder mounted on a movable gantry that moves according to the instructions generated by the slicing program. By laying down, layer after layer that rapidly cools and bond to each other, the digital model is thus converted into a physical model (Fig. 1d). The time needed for each step of the conception of the cut guides was also recorded.
Sterilization
After the fabrication, and once the support was taken out manually, the guides were treated with a chemical sterilization process at low temperature with hydrogen peroxide: the Sterrad 100NX (ASP c/o Ethicon SAS, Issy les Moulineaux, Fr) (Fig. 1e). All guides underwent before going into the Sterrad a classic 15 min manual wash with a detergent made of didecyldimethylammonium chlorure, digluconate chlorhexidine and non-ionic surfactants (Anios’clean EXCEL D at 5 mL/1 L of water, Anios, France), a 5 min sonication, drying at room temperature with clean medical air and packaging into a double specific Sterrad bag. The Sterrad 100NX is based on 58–59.5% aqueous hydrogen peroxide, which is concentrated to about 95% through removal of water from the peroxide solution before evaporation. The standard cycle sterilizer-setting intended for the sterilization of most surgical instruments was chosen for our experiments. We use the standard Sterrad cycle of 47 min that is the cycle used for most heat-sensitive surgical instruments.
In order to validate each batch and according to ISO recommendations, a microbiological control: Attest (3 M, Cergy-Pontoise); containing spores of Geobacillus stearothermophilus undergoes the sterilization cycle with the Sterrad method (Fig. 1f)15.
Surface analysis (Fig. 1 g)
Textured samples have been printed for surface analysis. Samples were analyzed before and after sterilization.
Contact angle
Contact angle measurements were carried out using a Digidrop apparatus from GBX (Bourg de Péage, France) by doing static contact angle measurements with a water drop.
Fourier-transform infrared spectroscopy (FT-IR)
The spectrometer apparatus was a Perkin-Elmer (Courtaboeuf, France) Spectrum 2000. It was used in transmission mode directly on the extruded film (disposed perpendicularly to the beam). The wavelength range was set from 4000 to 400 cm−1 with a resolution of 4 cm−1 during 16 scans. The styrene (1490 cm−1)/carbonyl (1733 cm−1) and the one between carbonyl and polybutadiene at 966 cm−1 were studied.
Scanning electron microscope
Surface observations was carried out using a Hitachi Flex Sem 100 operating at 15 kV equipped with secondary electron detector (SE) with a magnification factor × 55, × 250 and × 1000.
Atomic force microscopy
Surface roughness of the implants was analyzed with atomic force microscopy (AFM). Measurements were performed in ambient air with an Innova AFM (Bruker, France) using the tapping mode. The cantilever resonant frequency was 320 kHz using a silicon probe tip with a nominal spring constant of 42 N/m (NCHV-A, Bruker) and a radius of 10 nm. The scan rate was 0.5 Hz. The root mean squared roughness (Rq), as well as the profile were calculated using the Gwyddion 2.35 software.
Mechanical properties
The mechanical properties of ABS before and after sterilization were measured with an AGS-X extensometer (Shimadzu). The elongation kinetics was 50 mm/min. The force needs to reach the breaking point and the Young’s modulus were recorded with Trapezium X software. Two batch of ABS were tested. 5 samples before non-sterile and 5 samples sterile were used. All samples were considered as the worst cases material (rectangular band of 2 mm thickness, 15 mm width and 60 mm length).
Sterilization effectiveness (Fig. 2 )
In order to measure the effectiveness of the Sterrad method for sterilizing 3D models, specific models have been designed and artificially contaminated with several bacterial species. Afterwards, the bacterial load on the surface of these models and the effectiveness of the sterilization protocol were assessed in parallel. A preliminary study was carried out to determine the sensitivity of the Schadler broths used to assess the sterilization effectiveness.
Contamination of the 3D models
Contamination protocol
In order to assess the efficiency of sterilization, 3D specific models were artificially contaminated with four different reference bacterial strains: E. coli (ATCC 25922), S. aureus (ATCC 29213), E. faecalis (ATCC 29212) and P. aeruginosa (ATCC 27853). The models were immersed in a suspension calibrated at 1 McFarland (≈ 3.108 bacteria/ml) during 48 h at 35 ± 2 °C. After incubation, the models were removed from the 1 McFarland solutions and dried at room temperature to undergo immediately the protocols 5.a.ii or 5.b.ii.
Estimation of the bacterial load on 3D models surface after contamination protocol
Twelve 3D models (tree for each strain) were artificially contaminated following the contamination protocol (5.a.i). After incubation, the models were removed from the 1 McFarland solutions, dried at room temperature and transferred into a sterile water tube. Then, the tubes were placed in an ultrasonic tank (Bandelin, Berlin, Germany) for 15 min (frequency 35 kHz, power 160 W) in order to remove the bacteria from the 3D models16. After centrifugation (2500 g, 5 min) and removal of the supernatant, the centrifugation base was dissolved in 1 ml and 10 µl were isolated on blood agar (Biorad, Marne-la-coquette, France). After 48 h incubation at 35 ± 2 °C, the colonies were counted.
Assessment of Sterrad sterilization protocol
Preliminary evaluation of the sensitivity of Schaedler broths (supplementary data)
For each strain, 100 µl of bacterial suspensions, approximately calibrated to 103 102 and 10 CFU/ml were introduced in triplicate in a Schaedler broth. The actual concentrations were simultaneously verified on blood agar culture in triplicate (Supplementary data). The broths were incubated at 35 ± 2 °C and checked at, 24 h, 48 h, 5 and 7 days. After 48 h of culture, sub-cultures with 100 µl of each broth were performed on blood agar plates and incubated at 48 h at 35 ± 2 °C. A second subculture, performed under the same conditions, was carried out after 7 days of broth incubation, when the first one was negative.
Assessment of sterilization effectiveness
Twenty-four 3D models (6 for each strain) were artificially contaminated following the contamination protocol (5.a.i). In each case, one of the six models was used as growth control and was directly incubated in Schaedler broth (Biomerieux, Marcy-l'Étoile, France) at 35 °C + /− 2 °C for 48 h, whereas the five other models underwent the different sterilization steps (see above). After sterilization, each model was incubated in a Schaedler broth at 35 °C + /− 2 °C until clouding or for a total of seven days. Afterwards, 100 µl of each model broth (growth control and sterilized models) were sub-cultured on blood agar plates and have been read after 48 h incubation at 35 + /− 2 °C. This in-house protocol was carried out in triplicate.
Statistic analysis
Categorical variables are presented as counts and percentages and were compared by means of the Fisher’s exact test. Continuous variables following a normal distribution are presented as mean ± Standard Deviation (SD) and were compared with a Student t test with 8 degrees of freedom (dof). A two-way variance analysis (ANOVA) test was applied to compare the roughness differences between all three samples before and after sterilization. All p-values are two-sided and a value of p < 0.05 was considered significant. All analyses were performed with the use of PRISM, version 7 (Graph Pad, USA). All the authors had full access to all of the data and take full responsibility for the integrity of the data and the accuracy of the data analysis. All methods were carried out in accordance with relevant guidelines and regulations without experiment on humans or animals. No member of our research team named in the author list of the paper had access to identifying patient’s information when analyzing the data.
Results
The same surgeon from the Plastic Surgery department completed the generation and design, then the 3D printing of the ten models. The 3D printed models used were 10 cutting guides for fibular and mandibular osteotomies used for oral cancer and mandibular reconstruction with a free fibular flap. These guides had different complex shapes including holes, narrow corners, and thin arches. The size of both holes and slots were adapted to the size of the screws (3.5 × 12 mm) and the thickness of the oscillating saws (0.3 mm) used in usual clinical practice.
The 3D models were printed in an average time of 122 min. We did not encounter any technical problem through the fabrication process. The sterilization program lasted for each round 47 min.
Surface contact angle
Before the sterilization the surface contact angle measure with water at room temperature was about 70.2 °C. After sterilization, the SCA increase to 82.3 °C revealing highest surface tension as before treatment by Sterrad. This difference was statistically significant (t test of student, α = 0.05; p < 0.001).
The Fig. 3 shows the mean profiles of the mechanical properties of the models tested. The Young’s modulus was equivalent in all samples from the batch production with or without sterilization. A non-significant difference of the strength needed to reach the breaking point was calculated. Breaking strengths were 1304 N for non-sterile bands of ABS and 1388 N for sterilized bands (t test of student 8 dof, α 5%, p 0.12). Also, a non-significant difference was calculated for the elongation before. Elongations were 3.08 mm for non-sterile ABS band and 3.23 mm for sterilized bands (t test of student 8 dof, α 5%, p 0.53).
The sterilization of the ABS did not induce strong changes of the surface. For the FT-IR spectrum, the ratios of each signal were the same. The styrene/carbonyl ratio was not significantly different between samples before and after sterilization (t test of student, α = 0.05; p = 0.127). The butadiene/carbonyl ratio did not significantly change after sterilization (t test of student, α = 0.05; p = 0.764).
Profile modifications were analyzed by SEM from × 55 magnification to × 1000.
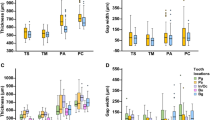
The sterilization did not change the appearance of the profiles of the surfaces made of regular ABS-polymer lines. Nevertheless, some gaps in the lines of impression were observed at × 200 magnification. This was identified as the consequences of artifacts during the molding process. These artifacts were about 100 to 300 µm. At a highest magnification (× 1000), the roughness of the impression appeared. Although these textures were under 50 µm, their number decreased after sterilization.
AFM
All samples had homogeneous surface properties as for the mean square roughness (Rq) or the surface profiles. Texture was made of regular peaks of approximately 2 µm high and 20–25 µm width.
There was no roughness difference between all three samples before and after sterilization (A two-way ANOVA, α = 0.05) nevertheless profiles were less regular as before the sterilization. These observations confirmed those made with the SEM.
Sterilization effectiveness
Preliminary evaluation of the sensitivity of Schaedler broths
For the lowest concentration of E. coli (ATCC 25922), S. aureus (ATCC 29213), E. faecalis (ATCC 29212) and P. aeruginosa (ATCC 27853) suspension, the actual concentrations were respectively 6.6, 3.33, 20 and 13 CFU/ml. The theoretical number of bacteria present in 100 µl inoculated into the broths was respectively 0.66, 0.33, 2 and 1.3. After 7 days of incubation, 2/3, 1/3, 3/3 and 2/3 of the subcultures corresponding respectively to the 4 tested strains were positive (Supplementary data).
Estimation of the bacterial load on 3D models surface
The bacterial load on the surface of the models after 48 h of incubation was estimated at 6.6*104 for E. coli models, 3.3*106 for S. aureus models and 107 for E. faecalis and P. aeruginosa models (Table 1).
Assessment of sterilization effectiveness
All internal controls of the Sterrad sterilization cycle, including Attest control, were successfully completed. All subcultures of the growth control broths were positive. None of the Schaedler broths containing the sterilized 3D models were cloudy after 7 days of incubation. Moreover, all the corresponding subcultures incubated for 48 h were also negative (Table 2).
Furthermore, after sterilization the 3D guides didn’t show any macroscopic alteration in terms of shape, structure or hardness.
Discussion
This preliminary study shows that the Sterrad process enables to obtain complete sterilization of the cutting guides manufactured in ABS without altering their morphology, with acceptable surface modifications.
Personalized surgery is essential to practice, more so in the maxillofacial field where reconstruction has to be as patient specific as possible to gain in symmetry and functionality, allowing a better quality of life.
Authors believe that virtual planning technologies are an emerging criterion standard in mandible reconstruction13,14,17. The use of computer assisted designed osteotomy guides for helping mandible and maxilla reconstruction has been demonstrated since more than 20 years17,18. A study on “in house” manufacturing of bone cut guides has been already published with an evaluation of clinical outcomes12. The results showed that the rates of complications and post-operative infections were not different than before the use of 3D printing. Over the past ten years, many authors have published mandibular reconstruction cases with the help of a model based on rapid prototyping technology, which assists accurate contouring of plates and/or planning of bone graft harvest geometry before surgery19.
The 3D personalized guided surgery is not performed in many hospitals because of the hefty fee of the 3D guides that commercial companies providing those services set up and the delay of average three weeks to have the guides via those companies. The software programs that we used are all free of charge and downloadable via the internet. Surgeons of our maxillofacial department made the conception and production of the guides without specialized training in either computer 3D modeling or engineering. The downside of the in-doors guide production in that it is time consuming5 so we could imagine that it should be ideally be place into the hands of a specific hospital technician.
Numerous materials are available for fused deposition printing. We tested several materials: with glass and PLA (polylactid acid) we encountered issues as obstruction of the extruder, and fragility of the guides, with ABS we didn’t had any technical problem in the printing process. Moreover, post-processing tasks after printing are very simple and can be performed directly in the sterilization room without complex equipment.
The guides are used directly into the body of the patient, applying them to the bone, therefore they are considered as a “critical item” meaning they are associated with a high risk of infection if they are contaminated with any microorganism. It is recommended for critical items8 that are heat sensitive to be sterilized by chemical process as hydrogen peroxide gas plasma.
In our hospital two types of sterilization process are used: the steam autoclave and the Sterrad. There is no standard procedure for the sterilization of this ABS medical device.
We chose the Sterrad system because it combines the use of hydrogen peroxide vapor and plasma safely to rapidly sterilize most medical heat-labile instruments and it is known to be less prone to affect fragile material as endoscopic tools.
This study shows that our process of printing, hand washing, and hydrogen peroxide gas plasma sterilization is effective in terms of bacteriological risk without macroscopic shape changes and few surface structure modifications20. The surface analyses show a regular high roughness and hydrophobicity that could both explain the initial bacterial colonization before sterilization. The sterilization procedure has increased the hydrophobicity but has decreased the global roughness in the same time. The first observation is reliable to the oxidation of the superficial layers of the ABS material that could have changed the surface energy on the material and, thereby its interaction with water drop21. A higher hydrophobicity is a risk of bacterial colonization. The roughness changes could be attributable to the friction of the handwashing on polymer surface. Nevertheless, smoother surfaces have less bacterial colonization than rough surfaces. Moreover, in term on biocompatibility, smoother surface present less risk than rough ones22,23.
The sterilization by plasma gas has not a significant impact on mechanical properties tested of ABS polymer. We estime that the non-significant modifications of both elongation and breaking point (Fig. 3a,b) are tolerable because they could increase the strength of the device for the surgery. Moreover, the difference observed seems to be more dependent of the batch of production than the sterilization of the polymer.
To study the effectiveness of the Sterrad method on bacterial colonisation of our models, we created an in-house experimental protocol with four reference strains from clinically relevant species24. According to Standard 11737-2:201925, the recommended method to control the sterilization process is the immersion of the sterilized product in a liquid culture medium. However, this standard does not specify which type of medium should be used. Schaedler broth have been chosen for its ability to grow fastidious bacteria, including anaerobes, and its use in the diagnosis of prosthetic infections26,27. Moreover, the Schaedler broth also allows the culture of E. coli, P. aeruginosa, E. faecalis and S. aureus species, according to previous publications28,29,30. The preliminary study aiming to assess the sensitivity of Schaedler broths shows a good match between the theoretical number of bacteria inoculated and the proportion of positive schaedler broth with the lowest bacterial incolum. Whichever the tested strain, the detection threshold for Schaedler broth was close to 1 bacteria, attesting the good sensitivity of our protocol. It also demonstrated its capacity to grow the strains used in this study.
The differences observed between the models contaminated with E. coli and those contaminated with the other strains may reflect discrepancies between the contamination abilities of the tested strains. Indeed, we can hypothesize that the E. coli strain was less able to colonize the 3D models and thus be present in lesser concentration.
Quantification of the bacterial load on the surface of the 3D models, in parallel with the negativity of the cultures and the determination of the threshold of the Schaedler broth makes it possible to estimate that sterilization by the Sterrad method gives, at least, a 6 log reduction of the initial inoculum for the S. aureus, E. faecalis and P. aeruginosa contamination protocol. Concerning the E. coli contamination protocol, an approximate 5 log reduction of the bacterial load was observed, due to a lower initial inoculum. Altogether, the Sterrad sterilization method appears to provide good conditions for material implantation, even in case of very high bacterial inoculum.
This study has several limitations. First, the mechanical tests used to investigate the properties of the 3D models before and after the sterilization step were focused on the research of modifications of both elasticity and breaking points of the 3D models printed in ABS. But there are many others mechanical properties which could be tested, and which could give information about the hardness of the final product. Since the guides are used to guide the saw during osteotomies, we expect them to have high strength and that they retain it after sterilization. But the clinical studies already published with this material did not show any fragility of the guides after sterilization, when they were used in the operating room. This is one of the important issues for clinical transfer that needs to be assessed in future studies.
Second, only one shape of model was used to assess the sterilization efficiency. This model included a slit and two holes to simulate the complexity of the 3D models used in practice, while being small enough to be immersed in a tube of Schaedler broth. However, other shapes, with a higher level of reality should be tested to confirm our results.
Third, the statistical power to determine the detection threshold of the Schaedler broth is low; a test on a larger sample size would allow a more accurate estimate of this parameter.
Fourth, the ability of sonication to detach all bacteria from the 3D models is uncertain, leading to a possible underestimation of the initial inoculum.
Fifth, as defined in the ISO 11138-715 standard, the sterilization assurance level (SAL) corresponds to the probability that a single unit will not be sterile at the end of a sterilization cycle. The commonly SAL accepted is 10–6. Given the complete reduction of the starting inoculum, the calculation of this indicator was impossible with this protocol. A concentration gradient greater than 1McFarland should be tested to calculate the SAL. However, the reduction of inoculum was comparable to that of the internal control ATTEST, which contained at least 106 spores of G. stearothermophilus.
Sixth, surface analysis was performed on 10 different cutting guides. Since the guides are personalized, they are different for each patient and for each surgical procedure. So, they have different shapes and masses. In order to obtain an experimental protocol that should be closer to the actual conditions of use of this material, we considered that it was wiser to carry out three series of tests on each guide rather than repeating the tests on a single guide printed in ten copies. However, such variations are also likely to occur in the clinical practice.
In contrast, manufacturing conditions and print parameters of the guides were strictly the same: layer thickness, ABS density and print speed. 3D printing was always performed in the operating room in a dedicated room with controlled airflow.
Seventh, we performed this study with cutting guides designed by a single surgeon. It is likely that the design and the shapes of 3D models could vary with other operators due to differences in cutting guides usage. This is another important issue for clinical transfer that needs to be assessed in future studies.
Conclusion
In this study, we have shown that it is feasible to fabricate with the hospital’s resources an anatomically accurate patient specific guide by using a low-cost 3D printer and a specific Sterrad sterilization program. Even if in-house three-dimensional printing with ABS is feasible, affordable and may represent a gain of time with an acceptable bacteriological added risk, we cannot extend this process to other kind of materials for 3D printers and we think that all new material should be specifically tested.
References
Schott, D. H., Collins, R. N. & Bretscher, A. Secretory vesicle transport velocity in living cells depends on the myosin V lever arm length. J. Cell Biol. 156, 35–39 (2002).
Chae, M. P. et al. Emerging applications of bedside 3D printing in plastic surgery. Front. Surg. 2, 25 (2015).
Malik, H. H. et al. Three-dimensional printing in surgery: a review of current surgical applications. J. Surg. Res. 199, 512–522 (2015).
Arnedos, M., Soria, J.-C., Andre, F. & Tursz, T. Personalized treatments of cancer patients: a reality in daily practice, a costly dream or a shared vision of the future from the oncology community?. Cancer Treat. Rev. 40, 1192–1198 (2014).
Huang, S., Peng, L., Abhiram, M. & Liang, H. Additive manufacturing and its societal impact: a literature review. Int. J. Adv. Manuf. Technol. 67, 1191–1203 (2013).
Morrison, R. J. et al. Regulatory considerations in the design and manufacturing of implantable. Clin. Transl. Sci. 8, 594–600 (2015).
Jacobs, P. & Kowatsch, R. Sterrad sterilization system: a new technology for instrument sterilization. Endosc. Surg. Allied Technol. 1, 57–58 (1993).
Peniston, S. J. & Choi, S. J. Effect of sterilization on the physicochemical properties of molded poly(L-lactic acid). J. Biomed. Mater. Res. B Appl. Biomater. 80, 67–77 (2007).
Diab-Elschahawi, M., Blacky, A., Bachhofner, N. & Koller, W. Challenging the Sterrad 100NX sterilizer with different carrier materials and wrappings under experimental ‘“clean”’ and ‘“dirty”’ conditions. Am. J. Infect. Control 38, 806–810 (2010).
Lerouge, S., Tabrizian, M., Wertheimer, M. R., Marchand, R. & Yahia, L. Safety of plasma-based sterilization: surface modifications of polymeric medical devices induced by Sterrad and Plazlyte processes. Biomed. Mater. Eng. 12, 3–13 (2002).
Lerouge, S. et al. Plasma-based sterilization: effect on surface and bulk properties and hydrolytic stability of reprocessed polyurethane electrophysiology catheters. J. Biomed. Mater. Res. 52, 774–782 (2000).
Bosc, R. et al. Mandibular reconstruction after cancer: an in-house approach to manufacturing cutting guides. Int. J. Oral Maxillofac. Surg. 46, 24–31 (2017).
Seruya, M., Fisher, M. & Rodriguez, E. D. Computer-assisted versus conventional free fibula flap technique for craniofacial reconstruction: an outcomes comparison. Plast. Reconstr. Surg. 132, 1219–1228 (2013).
Fischer, M. et al. Preclinical usability study of multiple augmented reality concepts for K-wire placement. Int. J. Comput. Assist. Radiol. Surg. 11, 1007–1014 (2016).
ISO/AWI 11138-7 : Sterilization of health care products - Biological indicators - Part 7: Guidance for the selection, use and interpretation of results. 2019.
Zips, A., Schaule, G. & Flemming, H. C. Ultrasound as a means of detaching. Biofouling 2, 323–333 (1990).
Saad, A., Winters, R., Wise, M. W., Dupin, C. L. & St Hilaire, H. Virtual surgical planning in complex composite maxillofacial reconstruction. Plast. Reconstr. Surg. 132, 626–633 (2013).
Leiggener, C., Messo, E., Thor, A., Zeilhofer, H.-F. & Hirsch, J.-M. A selective laser sintering guide for transferring a virtual plan to real time surgery in composite mandibular reconstruction with free fibula osseous flaps. Int. J. Oral Maxillofac. Surg. 38, 187–192 (2009).
Hou, J.-S. et al. Application of CAD/CAM-assisted technique with surgical treatment in reconstruction of the mandible. J. Cranio-Maxillo-fac Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-fac Surg. 40, 32–37 (2012).
Cohen, A., Laviv, A., Berman, P., Nashef, R. & Abu-Tair, J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg. 108, 661–666 (2009).
Kyi, M. S., Holton, J. & Ridgway, G. L. Assessment of the efficacy of a low temperature hydrogen peroxide gas plasma sterilization system. J. Hosp. Infect. 31, 275–284 (1995).
Boldizar, A. & Möller, K. Degradation of ABS during repeated processing and accelerated ageing. Polym. Degrad. Stab. 81, 359–366 (2003).
Anderson, J. M., Rodriguez, A. & Chang, D. T. Foreign body reaction to biomaterials. Innate Adapt Immune Responses Tissue Eng. 20, 86–100 (2008).
Tande, A. J. & Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 27, 302–345 (2014).
ISO/AWI ISO 11737-2: Sterilization of health care products—Microbiological methods—Part 2: tests of sterility performed in the definition, validation and maintenance of a sterilization process (2019).
Technical documentation : Bouillon Schaedler gélosé 0,02% + vit. K3 (SCHAEDK3 0.02%-T), Biomerieux, (2013).
Bémer, P. et al. How many samples and how many culture media to diagnose a prosthetic joint infection: a clinical and microbiological prospective multicenter study. J. Clin. Microbiol. 54, 385–391 (2016).
Scythes, K. D., Louie, M. & Simor, A. E. Evaluation of nutritive capacities of 10 broth media. J. Clin. Microbiol. 34, 1804–1807 (1996).
Bartlett, R. C., Mazens, M. & Greenfield, B. Acceleration of tetrazolium reduction by bacteria. J. Clin. Microbiol. 3, 327–329 (1976).
Jones, R. N., Packer, R. R., Fuchs, P. C., Barry, A. L. & Borchardt, K. Stability of antimicrobials in schaedler’s anaerobic and brain heart infusion broths stored at -20 Degrees °C. J. Antibiot (Tokyo). 31, 226–228 (1978).
Acknowledgements
The authors would like to thank the central pharmacy and the bacteriologic department of the Centre Hospitalier Universitaire Henri Mondor for their contribution.
Author information
Authors and Affiliations
Contributions
Each author has materially participated in the research and the article preparation. The conception and design of the study: R.B., P.A., L.T. and R.L. Figures, acquisition and analysis of data: R.B., B.H., L.T. and, P.L.W. Interpretation of data: L.T., C.L., M.O., P.L.W. Draft: P.A., R.B., B.H. and R.L. Revising: J.-P.M., R.B. and P.l.W. Responses to the reviewers: L.T., R.LeG., R.B. and P.L.W. Final Version: R.B. and L.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bosc, R., Tortolano, L., Hersant, B. et al. Bacteriological and mechanical impact of the Sterrad sterilization method on personalized 3D printed guides for mandibular reconstruction. Sci Rep 11, 581 (2021). https://doi.org/10.1038/s41598-020-79752-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79752-7
This article is cited by
-
A survey regarding the organizational aspects and quality systems of in-house 3D printing in oral and maxillofacial surgery in Germany
Oral and Maxillofacial Surgery (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.