Abstract
Clostridioides difficile infection (CDI) is a major healthcare-associated diarrheal disease. Consistent with trends across the United States, C. difficile RT106 was the second-most prevalent molecular type in our surveillance in Arizona from 2015 to 2018. A representative RT106 strain displayed robust virulence and 100% lethality in the hamster model of acute CDI. We identified a unique 46 KB genomic island (GI1) in all RT106 strains sequenced to date, including those in public databases. GI1 was not found in its entirety in any other C. difficile clade, or indeed, in any other microbial genome; however, smaller segments were detected in Enterococcus faecium strains. Molecular clock analyses suggested that GI1 was horizontally acquired and sequentially assembled over time. GI1 encodes homologs of VanZ and a SrtB-anchored collagen-binding adhesin, and correspondingly, all tested RT106 strains had increased teicoplanin resistance, and a majority displayed collagen-dependent biofilm formation. Two additional genomic islands (GI2 and GI3) were also present in a subset of RT106 strains. All three islands are predicted to encode mobile genetic elements as well as virulence factors. Emergent phenotypes associated with these genetic islands may have contributed to the relatively rapid expansion of RT106 in US healthcare and community settings.
Similar content being viewed by others
Introduction
The Gram-positive and spore-forming anaerobic bacterium Clostridioides difficile (formerly named Clostridium difficile) is a leading cause of antibiotic-associated diarrhea that may be self-limiting, or progress to severe and fulminant (pseudomembranous) colitis or toxic megacolon1,2,3,4. There has been an increased incidence of C. difficile infection (CDI) over the past two decades5,6,7,8 and, in the USA, this coincides with the emergence and spread of ribotype 027 strains [also called RT027 or BI or NAP1 based on the phylogenetic test9,10]. While RT027 remains the most prevalent healthcare-associated C. difficile ribotype, its frequency has been steadily declining11. Multiple surveillance studies indicate a changing trend in the C. difficile ribotype frequency distribution, particularly the emergence of RT106 (also called Group “DH” or “NAP11”) in regions where it was previously rarely found. In 2008, RT106 was second to RT027 as the most dominant ribotype in England, and was also identified in neighboring European countries including Spain and Ireland12,13,14. However, during the same period, RT106 was rarely identified elsewhere in Europe, or in the USA and Canada15, where RT027 and RT014/020 were predominant13,15. By 2012, RT106 emerged as the second most dominant C. difficile molecular type in the ten US states participating in the Centers for Disease Control and Prevention (CDC) Emerging Infections Program (EIP) surveillance16. From 2014 to 2017, RT106 replaced RT027 as the most prevalent ribotype recovered from community-associated CDIs16,17,18,19,20,21.
Currently, Arizona is not a participant in the CDC EIP program, and no molecular typing data or epidemiological trends are available for this state. As part of an ongoing surveillance to rectify this gap in knowledge, we determined the ribotype frequency of C. difficile isolates recovered from patients at a tertiary University Medical Center in Tucson, Arizona between August 2015 and July 2018. Consistent with broader trends in the country, we noted increased prevalence of RT106 strains in our patient population. Since little is known about these strains22, we focused on genomic and phenotypic characterization of all recovered RT106 isolates with the goal of identifying genetic factors contributing to the increased prevalence of this molecular type.
Results
Clostridioides difficile RT106 is the second-most prevalent molecular type in an acute-care teaching hospital in Tucson, Arizona
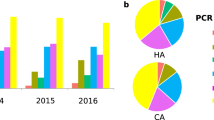
From August 2015 to July 2018, we recovered 788 C. difficile isolates from adult patients confirmed to be CDI-positive via a PCR test (employed until February 2017) or a “two-step” GDH/EIA test [Glutamate Dehydrogenase (assesses live C. difficile); Enzyme Immunoassay (detects C. difficile glycosyltransferase toxins TcdA and TcdB)] employed from March 2017. To ensure test-result consistency, we first verified the presence of tcdB, the same gene assayed in the PCR test, in all samples collected from March 2017 to July 2018. Overall, 519/788 isolates contained tcdB or expressed EIA-detectable levels of TcdA/B. Ribotype analysis revealed a diversity of strains in the patient population, with RT027 being the most frequently isolated strain (n = 144) (Fig. 1). RT106 (n = 38) was the second most frequently identified ribotype over the 3-year period.
RT106 is the second most prevalent molecular type in a Tucson-area hospital. Top chart depicts ribotype distribution of 519 tcdB PCR-positive and/or TcdA/B ELISA-positive C. difficile strains from patient stool samples collected from August 2015 to July 2018 (8.2015 to 7.2018). Ribotype frequency and percent of total sample size are shown in parenthesis. Overall, RT106 is the second most frequently isolated molecular type, while RT027 is the most prevalent ribotype. Bottom charts depict ribotype distribution in 12-month periods. RT106 ranked second to RT027 as the most frequently isolated molecular type during 8.2015 to 7.2016 and 8.2017 to 7.2018. RT106 was the third most dominant ribotype during 8.2016 to 7.2017.
RT106 isolates are virulent in an animal model of infection
Prior to detailed characterization of RT106 isolates, we verified the virulence of the representative strain GV599 in the Golden Syrian hamster model of acute C. difficile infection. All infected animals succumbed to disease within 6 days of spore inoculation (Supplemental Fig. S1a). Microscopy-based visualization of colonic tissue sections revealed classic C. difficile infection pathology including gross hemorrhage, epithelial erosion and inflammatory infiltrates (Supplemental Fig. S1b).
RT106 strains harbor one clade-specific novel genetic element
Whole genome sequencing was performed on all 38 RT106 strains recovered in our surveillance (Supplemental Table S2), and data were compared to 1425 publicly available C. difficile strain sequences. Based on single nucleotide polymorphism (SNP) analyses23,24, the strains were not clonal, and the two closest-related isolates (GV597 and GV753) were divergent by 113 SNPs. Overall, RT106 genomes were most-closely related to RT002 strains25.
Our 38 RT106 strains mapped closely to 33 previously sequenced RT106 strains from pediatric patients26,27 and 23 other strains of unknown ribotype (highlighted in red in Fig. 2b). Evolutionary analysis of the 94 strains containing the entire GI1 was performed using MEGA X (Fig. 2c). We performed in silico ribotyping on the 23 strains, and 13/23 (those with currently available closed genome sequence) generated a clear RT106 PCR fragment pattern. For an additional assessment of genome relatedness, we performed in silico Multi-Locus Sequence Typing (MLST) on all 94 strains; this method differentiates organisms into Sequence Types [STs28]. 92/94 strains were sequence type ST42, whereas 2/94 belonged to the closely-related sequence type ST2829. Taken together, all 94 strains interrogated in these analyses grouped together in a distinct RT106 clade (Fig. 2b,c)30.
The RT106 clade may harbor up to three novel genomic islands. (a) Genetic islands GI1, GI2, and GI3 associated with RT106 are at three different locations in the genome. GV364, used as a representative genome, contains GI1 and GI3. Outer ring: Insertion site (green) of GI2 is shown relative to GI1 and GI3 locations; Blue indicate CDS (protein-coding DNA sequences). Inner ring: Purple denotes lower % GC compared to the overall % GC of the genome; Green denotes higher % GC compared to the overall % GC of the genome. Artemis DNA Plotter was used to generate genome circular map. (b) A composition vector tree of 1425 publicly available C. difficile and 38 RT106 genome sequences shows that RT106 strains clade together with 56 other strains (highlighted in red). (c) The relatedness of the 94 strains within the RT106 clade is shown in the maximum likelihood tree (log likelihood = − 29,380.67) based on the 3306 core SNPs identified using Panseq. Tree scale: 0.01 represents 0.01 substitutions per nucleotide site. Our clinical isolates are designated as “GV”, whereas pediatric isolates from Chicago, Illinois27 are designated “DH or ST”. All 94 strains within the RT106 clade harbor the complete GI1. GI2 is present in 7 RT106 strains (green). Thirteen strains harboring GI3 (yellow) belong to 2 different subclades. (d) Gene arrangement, size and functions of GI1, GI2 and GI3. Functions of genes within GI1 and GI3 are named either via GO term or gene name. Genes within GI2 were previously identified and reported27.
Up to three unique genomic islands GI1, GI2 and GI3 are associated with the RT106 clade (Fig. 2a), and GI1, a novel 46 kb element reported for the first time herein, is invariantly carried by all RT106 strains. GI1 and GI3 were also predicted as genomic islands in our analysis of an RT106 strain BR81 genome using IslandViewer 4, which used SIGI-HMM and IslandPath-DIMOB as horizontal gene transfer predictors31,32,33,34. GI2 (also 46 kb) was previously identified in RT106 strains recovered from pediatric patients27, and its overall prevalence in the RT106 clade is 7.4% (7/94 strains). GI3 (a 29.4 kb element) prevalence is 13.8% (13/94 strains). GI1 has features of conjugative mobile genetic elements and contain DNA integration and transposition genes (Locus IDs FE556_11090, FE556_11095, FE556_11065, FE556_11085, FE556_11205, FE556_11240, FE556_11260, FE556_11275 in Supplemental Table S3). GI3 also contains genes associated with conjugative transfer (Locus IDs FE556_02435, FE556_02450, FE556_02470 in Supplemental Table S4). Genes predicted to encode anti-restriction modification, antibiotic-resistance and cell adhesion functions are also present in GI1 and GI3 (Fig. 2d; Supplemental Tables S3 and S4). No plasmid-like genes were found. All three islands display higher percentage GC content (38%, 45% and 37% for GI1, GI2 and GI3, respectively) than the rest of the C. difficile genome (28–29%).
Currently, the 46 kb GI1 appears to be uniquely and specifically associated with RT106 (Fig. 2b,c), and all sequenced strains belonging to this clade (38 from this study and 56 others identified in publicly available databases) harbor a complete GI1 island. GI1 has 99.91% pairwise identity among strains (100% GI1 identity in 48 strains; 44 strains with 1–2 SNPs; 2 strains with > 3 SNPs). Fragments of GI1 were, however, detected in some non-RT106 strains. GI2, previously identified in pediatric RT106 isolates27, is present in only 1/38 adult RT106 strains from our surveillance (Fig. 2c); we also identified this island in the non-RT106 strain Y358 (GCF_00451525.2). The 29.4 kb GI3 is present in 8/38 of our adult RT106 strains, as well as 5 other RT106 isolates in publicly available databases (Fig. 2c). We also identified GI3 in one non-RT106 strain (VRECD0053, GCF_900164815.1).
The 46 kb genomic island 1 is unique to RT106/ST42/ST28 strains
BLASTN analysis of the 46 kb GI1 against 1425 publicly available C. difficile genome sequences at the NCBI database resulted in the identification of 265 C. difficile strains that contain either segments (> 7.7 kb, 98% identity) of or the entire genomic island. We concomitantly performed in silico MLST analysis to determine the respective sequence types, and then generated a maximum likelihood tree based on the core genome SNPs of 265 C. difficile strains harboring segments of or the entire G1 using Mega X35. GI1-related genes found in each strain were annotated based on gene function. Only RT106/ST42/ST28 strains harbor the complete 46 kb GI1, while other ST strains included in this analysis contain only shorter segments of the genomic island (Fig. 3).
RT106 strains harbor a complete and unique 46 kb genomic island 1. The relatedness of the 265 C. difficile strains that carry GI1 segments (> 7.7 kb, 98% identity) is shown in a maximum likelihood tree (log likelihood = − 479,911.97) based on 40,879 core SNPs identified using Panseq. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. GI1 is drawn to scale on the right to illustrate regions present in different sequence types (ST). Tree scale: 0.01 represents 0.01 substitutions per nucleotide site. The complete 46 kb GI1 is present in RT106/ST28/ST42. Genes were colored based on functional categories from gene ontology (GO) analysis. A 7.1 kb region carried by all the strains (black dashed box) was used for determining progenitor STs of the element in the molecular clock analysis in Fig. 4.
A 7.1 kb gene segment (demarcated within a black dashed box; Fig. 3) is common to all MLST sequence type strains shown. SNP analysis was performed on the 7.1 kb gene segment to generate a molecular clock of GI1 via Mega-X36 using maximum likelihood (ML) approach (Fig. 4). The molecular clock revealed gradual and progressive acquisition of gene elements in different strains, finally leading to an intact GI1. CD105KSE6, which branches most distantly from RT106 based on the alignment of the 7.1 kb segment of GI1, contained the least number of GI1-associated genes as opposed to STs branching closer to RT106/ST42/28.
Molecular clock analysis reveals organization of the genomic island 1 via acquisition of distinct sub-elements. A timetree using the 7.1 KB consensus region in the 265 strains highlighted in black dashed box in Fig. 3 may offer clues towards the acquisition of sub-elements leading to the formation of GI1. Divergence times shown are relative times as no calibrations were provided. The estimated log likelihood value of the tree is − 14,227.79. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. The phylogenetic tree is rooted using CD105KSE6. CD105KSE6 branches most distantly from RT106/ST28/ST42 clade based on alignment of the 7.1 kb region in GI1.
To further interrogate whether GI1 was acquired via horizontal transfer, we compared the molecular clock of the 7.1 kb GI segment (Fig. 4) with the a molecular tree based on genes assumed to be refractory to horizontal gene transfer37,38. Thus, a minimum spanning tree was generated using the seven housekeeping genes utilized in MLST characterization to establish genetic relatedness of strains harboring the core 7.1 kb GI1 fragment (Supplemental Fig. S2). ST28, a sequence type that is included within the RT106 clade, is closely related to ST16, ST18 and ST46 based on sequence similarity of the core GI1 fragment (Fig. 4). However, only ST16, ST18 and ST28 are closely related based on the seven MLST gene loci; ST46 is distantly placed from ST16, ST18 and ST28 (Supplemental Fig. S2). A similar case is observed with the more predominant sequence type, ST48, within the RT106 clade. ST48 is closer to ST42 and ST7 based on the seven housekeeping genes (Supplemental Fig. S2), and yet these ST strains map distantly in the core GI1-based molecular clock (Fig. 4). Since the tree topologies do not exhibit the same pattern, it is likely that GI1 is acquired laterally. Further analysis of tree topologies via likelihood ratio tests showed that the ML tree based on the 7.1 kb shared region within GI1 was significantly different from the ML tree based on core genome SNPs (Pvalue = 6.83E−74 calculated using approximately unbiased test) and the ML tree based on the seven MLST housekeeping genes (Pvalue = 1.17E−71).
The entire GI1 is not found in any other bacteria. However, two regions (8.4 kb and 13.7 kb) within GI1 were detected in other enteric bacteria (Fig. 5). The 13.7 kb gene segment was found in Enterococcus faecium EnGen0312 UAA407 at 99% sequence identity, while the 8.4 kb gene segment occurs in the same gene order but with some sequence plasticity in Enterococcus faecium EnGen0312 UAA407, Anaerostipes hadrus BPB5-Raf3-2-5, Clostridium sporogenes YH-Raf3-2-5 and Roseburia intestinalis M50/1 strains (89.8%, 90.4%, 90.5% and 92.2% DNA sequence identity, respectively).
Human commensal microbiota may contribute to the acquisition of the 46 kb genomic island 1. The complete 46 kb GI1 is not present in any other microbial genome or plasmid sequence, but two gene segments (8.4 kb and 13.7 kb) within the island are found in other human enteric bacteria. The 8.4 kb gene segment is present in Enterococcus faecium EnGen0312 UAA407, Anaerostipes hadrus BPB5-Raf3-2-5, Clostridioides sporogenes YH-Raf3-2-5 and Roseburia intestinalis M50/1 strains (89.8%, 90.4%, 90.5% and 92.2% DNA sequence identity, respectively). E. faecium also harbors a 13.7 kb gene segment at 99% sequence identity. These two gene segments are found in E. faecium as part of a 36 kb genomic element. RT106 strains do not carry this 36 kb genomic element, but other C. difficile strains (VL0228 and 17-314-01071) strains have the identical 36 kb genomic element. Tree scale: 0.01 represents 0.01 substitutions per nucleotide site.
Phenotypic characterization of RT106 isolates
Clade-specific properties, including those conferred by genes within GI1 could explain the emergence and spread of RT106 strains. Therefore, we assessed various virulence-associated phenotypes including antibiotic susceptibility, motility, toxin production, biofilm production and adhesion to collagen on the first 21 of the 38 RT106 strains chronologically obtained from our clinical surveillance.
RT106 strains display variable antibiotic susceptibility, with some isolates displaying multi-drug resistance
We determined the susceptibility of RT106 isolates to the antibiotics cefotaxime, vancomycin, erythromycin, clindamycin, levofloxacin, moxifloxacin, metronidazole, and tetracycline. All isolates were resistant to cefotaxime (minimum inhibitory concentration (MIC) > 32 mg/ml), but susceptible to vancomycin, metronidazole, and tetracycline (Table 1). 18/21 strains had intermediate resistance to clindamycin (MIC = 4–6 mg/ml). Three isolates (GV371, GV423, GV432) were highly resistant to erythromycin (MIC > 256 mcg/ml). Clindamycin and erythromycin belong to the macrolide-lincosamide-streptogramin B (MLSB) group of protein synthesis inhibitors. MLSB resistance in C. difficile has been associated with the acquisition of erm genes39 or nucleotide substitution (C → T) at position 656 within the 23S rDNA40. None of the RT106 strains harbor the erm genes, while only GV415 had the 23S rDNA 656C>T substitution (Supplemental Table S1); however, GV415 has low-level resistance to clindamycin (MIC = 4 mg/ml) and is susceptible to erythromycin.
All RT106 isolates, except GV597, were susceptible to the fluoroquinolone levofloxacin. GV597, GV453, GV587, and GV642 had intermediate resistance to the fluoroquinolone moxifloxacin (MIC = 4–6 mg/ml). However, these fluoroquinolone-resistant RT106 isolates do not encode mutations in GyrA (T82I, T82V, D71V, D81N and A118T) or GyrB (D426V, D426N, R447L, R447K, S366A and S416A) associated with fluoroquinolone resistance41,42,43,44,45 (Supplemental Table S1). The levofloxacin-resistant GV597 strain harbors an A421T mutation within the primary dimer interface of the conserved topoisomerase domain of gyrase A (Supplemental Table S1), but GyrA A421T mutation is not previously known to be associated with fluoroquinolone resistance in C. difficile.
GI1 harbors a gene encoding a VanZ family protein (locus ID FE556_11215; Supplemental Table S3). VanZ family proteins were previously implicated in teicoplanin resistance46. The GI1-encoded vanZ gene, present in all RT106 strains, is not found within C. difficile strains 630, VPI and BI-1 (Supplemental Table S1). Consistent with this, all RT106 isolates exhibit modest increase in resistance to teicoplanin compared to reference strains (T7, BI-1, 630, VPI; Table 1); the teicoplanin CLSI and EUCAST breakpoint values for C. difficile have not been established. Cultivation of RT106 strains in sub-inhibitory concentration (MIC) of teicoplanin (0.0125 mg/mL) resulted in increased teicoplanin resistance in 7/21 strains (Supplemental Table S5).
RT106 strains display collagen-dependent biofilm formation
Biofilm formation could facilitate intestinal colonization and persistence, and possibly contribute to recurrence47. RT106 strains display variable biofilm densities on an abiotic plastic surface (Fig. 6a). Since GI1 encodes a putative SrtB-anchored collagen-binding adhesin (locus ID FE556_11350; Supplemental Table S3), we tested the ability of RT106 strains to form biofilms on type I and type III collagen, the major collagen types present in the extracellular matrix of normal human intestines48.
Clinical RT106 isolates display collagen-dependent biofilm formation. (a) 21 clinical RT106 strains (blue circles) and 3 non-RT106 toxigenic C. difficile strains (VPI, BI-1, and 630 designated as green, yellow and black circles, respectively) were cultured in uncoated or collagen-coated (combined types I and III) plastic wells for 72 h. RT106 strains displayed variable levels of biofilm on abiotic plastic wells. (b) Relative changes in biofilm densities (ΔA570nm) were determined by comparing A570nm of crystal violet-stained biofilms formed on human collagen (combined types I and III) vs. on uncoated plastic wells. Filled blue circles denote Pvalue < 0.05 determined using Student’s t test to compare mean A570nm by each strain on collagen-coated vs. uncoated wells. No difference in biofilm formation was observed when the reference C. difficile 630, BI-1 and VPI strains were cultured on wells with or without collagen. Overall, RT106 strains displayed denser biofilms on collagen-coated wells (One-sample one-tailed T-test; Halt: mean ΔA570nm > 0; H0: mean ΔA570nm = 0; Pvalue = 0.02038).
Biofilm densities of the non-RT106 toxigenic C. difficile strains BI1, 630 and VPI did not increase in the presence of collagen (Fig. 6b). However, eleven RT106 strains displayed collagen-dependent increase in biofilm formation when cultured on wells coated with both type I and type III human collagen. Overall, RT106 strains, as a group, have increased likelihood of displaying collagen-dependent biofilm formation.
We also interrogated the ability of the strains to form biofilms on either human type I or type III collagen individually. Although some RT106 strains showed increased biofilm formation on either collagen type (6 to human type I collagen; 5 to human type III collagen) (Supplemental Fig. S3), the RT106 strain group did not show collagen-dependent biofilm formation when only one collagen type was used for collagen coating. Curiously, GV426, GV453, and GV457 showed synergistic increase in biofilm formation to human types I and III collagen (Fig. 6).
We also tested the ability of the 21 RT106 strains to form biofilms on rat type 1 collagen and found that ten strains formed denser biofilms on rat collagen (Supplemental Fig. S4). GV425, GV426, GV432, GV453 and GV457 consistently formed denser biofilms on human and rat type I collagen compared to uncoated wells.
RT106 strains are variably motile
Flagella-dependent motility influences virulence of many pathogens49. All RT106 isolates tested, except GV375, GV415 and GV426, were motile (Fig. 7). We analyzed the genome of the non-motile RT106 strains for mutations in flagella-associated genes. In C. difficile 630 strain, flagella-associated genes are found in the F1 and F3 loci50,51. F1 and F3 loci are highly conserved in RT106; therefore, the nonmotile phenotype observed for GV375, GV415 and GV426 may possibly result from alterations in expression and/or post-translational modifications.
Most RT106 strains are robust toxin-producers
Toxigenic C. difficile produce up to two related glucosylating toxins, toxin A (TcdA) and toxin B (TcdB), which are encoded on the pathogenicity locus (PaLoc)52,53. Genome analysis of RT106 isolates revealed that all strains harbor the complete PaLoc and the gene for the TcdB1, instead of the highly toxigenic TcdB2 variant associated with select ribotypes including RT02754,55. We quantified secreted toxin, and observed that all RT106 strains, except GV457 and GV423, produced detectable TcdA/TcdB levels (Fig. 8). Nine RT106 isolates expressed TcdA/TcdB at levels comparable to the reference strain 630, while ten RT106 strains had similar (4/10) or higher (6/10) TcdA/TcdB levels compared to the RT027 strain BI-1.
Most clinical RT106 isolates are robust toxin producers. All RT106 samples, except for GV457 and GV423, secrete similar or greater TcdA/TcdB levels compared to C. difficile 630 strain. Four strains (GV753, GV377, GV364, GV453) produced similar TcdA/TcdB levels as the BI-1 reference strain. Six strains (GV524, GV425, GV371, GV421, GV599, GV375) secrete more TcdA/TcdB compared to BI-1. TcdA/TcdB levels secreted after 72-h culture in BHI broth were normalized to mg of total secreted proteins. Mean A450nm/mg of secreted protein and standard deviation are shown. Image is representative of two independent TcdA/TcdB ELISA assays with three sample replicates per condition.
Discussion
Consistent with broader trends in the United States, RT106 has emerged as the second leading ribotype from healthcare-associated cases in Southern Arizona15,16,17,18,19,20,21. Our genotypic and phenotypic characterization of multiple RT106 strains, along with the recent studies by Kociolek et al., represents an initial foray into defining key virulence properties of this clade27,29.
The factors contributing to the emergence and expansion of RT106 strains are presently undefined, but they appear to be distinct from those postulated for the healthcare- and US-dominant RT027 clade. First, the enhanced ability of RT027 strains to utilize trehalose, a sugar increasingly used in food products since the early 2000s, may have provided a selective advantage for this clade, although this has recently been disputed56,57. None of the 94 sequenced RT106 genomes harbor the Leu-1721-Ile substitution in the TreR repressor or the four-gene insertion sequence that allow RT027 and RT078 strains, respectively, to grow on low levels of trehalose56. Still, our studies do not rule out unique sugar- or carbon source-utilization capabilities of RT106 strains.
Second, DNA gyrase mutations conferring fluoroquinolone resistance may have contributed to the emergence and spread of RT027 strains42. While RT106 isolates from the United Kingdom were highly resistant to moxifloxacin, those from North American surveillance studies, including ours, were mostly susceptible to fluoroquinolones (Table 1)12,58,59,60. Thus, fluoroquinolone resistance does not explain their emergence and spread in the United States.
Third, the PaLoc region of RT027 strains displays several key differences relative to the historic strain 630 (RT012)61. These include a point mutation in tcdC (though not in all isolates) that results in a truncated version of the anti-sigma factor TcdC, and expression of a variant of toxin B (TcdB2). TcdB2 has enhanced ability to enter host cells, is more cytotoxic, and exhibits wider tissue tropism54,55. In contrast to RT027 strains, the PaLoc of RT106 strains is 100% identical to 63062,63,64,65; thus, these strains encode full-length TcdC and express the TcdB1 toxin variant. Both RT027 and RT106 isolates produce variable amounts of TcdA/TcdB. Also, unlike 630 and RT106 strains, RT027 strains encode the binary toxin. Thus, toxin variations seem to be an unlikely driving force for the spread of RT106 strains.
Detailed genome sequence analyses, however, suggest that the acquisition of novel genetic islands may be a contributor to RT106 emergence. All sequenced RT106 strains harbor a unique 46 kb genomic island (GI1) with a distinct GC content suggestive of horizontal acquisition. GI1 possesses several gene attributes that may confer competitive advantage to the RT106 clade. It harbors a vanZ allele (Locus ID FE556_11215), distinct from vanZ1 (Locus ID FE556_05915; 49% identity) present elsewhere in RT106 genome and in other C. difficile strains including the well-studied 63066 (Supplemental Table S1). In 630, VanZ1 was previously shown to confer low level resistance to the glycopeptide antibiotic, teicoplanin, but not to vancomycin66. The presence of a second VanZ allele may contribute to the modest increase in teicoplanin resistance of RT106 strains. The potential selective advantage of this phenotype cannot be ruled out; while teicoplanin is not FDA-approved for use in the US, it is widely used in Europe, Asia and South America.
In addition to the strain 630 cd2831 SrtB-anchored collagen-binding adhesin homolog (99% protein identity)67, all RT106 strains encode a paralog within GI1 (locus ID FE556_11350; 33% protein identity with CD2831); this gene was earlier reported as an ‘RT106-associated accessory gene’29. A subset of RT106 strains (13/94; 6 strains assayed for biofilm formation) also contains an additional paralog within GI3 (locus ID FE556_02390; 79% protein identity with GI1 locus ID FE556_11350). The robust collagen-dependent biofilm formation observed in the RT106 isolates may be due to the presence of any one or combination of these genes. Further investigation is required to parse the contribution of these genes to virulence. Since toxigenic C. difficile can breach the intestinal epithelium via cell junction disruption and/or epithelial cell death, thereby exposing the components of the extracellular matrix including collagen, strong vegetative cell and biofilm adhesion to collagen is a possible mechanism promoting C. difficile colonization of, and persistence in, the host.
GI1 also harbors genes for anti-restriction modification (ardA; Locus ID FE556_11265, FE556_11270), multi-drug resistance (mfs; Locus ID FE556_11225), methylglyoxal detoxification (gloA; Locus ID FE556_11330), and cation transport (Locus ID FE556_11135) containing a FieF domain (NCBI Conserved Domain cl30791) associated with iron-cobalt-zinc-cadmium resistance. Homologs of these genes are linked to virulence of other pathogens68,69,70. Finally, GI1 has features of a conjugative mobile element and contains genes for DNA excision/integration (Locus IDs FE556_11090 and FE556_11095) and encodes homologs of several proteins involved in Tcp conjugation machinery of C. perfringens including TcpE (YP_009063349.1; 46.24% similar to Locus ID FE556_11260), TcpG/TcpI hydrolase (YP_009063351.1; 51.49% similar to Locus ID FE556_11245), TcpF (YP_009063350.1; 49.35% similar to Locus ID FE556_11255), and TcpA (YP_009063346.1; 41.07% similar to Locus ID FE556_11305). It is presently unknown whether the entire 46 kb genomic island can mobilize to other C. difficile strains.
Fragments of GI1 were found in different C. difficile sequence types. Molecular clock analysis suggests that the complete island is a composite of sequences sequentially acquired from progenitor ST strains. The molecular clock based on the conserved GI1 segment is asynchronous with the one based on housekeeping genes (Fig. 4 and Supplemental Fig. S2). Further, consistent with higher GC content of GI1 relative to rest of the C. difficile genome, it is likely that the progenitor ST strains acquired the DNA segments from non-clostridial organisms via horizontal gene transfer. While the complete island is yet to be found in any other microbial genome or plasmid, two gene segments (8.4 kb and 13.7 kb) were detected in other enteric bacteria (Fig. 5). For the 8.4 kb gene segment, the most closely related sequences occur in Roseburia intestinalis M50/1 strains (92.2% identity). The 13.7 kb segment displays 99% identity to sequence within a 36 kb genomic island in E. faecium. While the 8.4 kb and 13.7 kb segment in GI1 may have been derived from E. faecium, the candidate donors of the other gene segments in GI1 are presently unknown. The presence of these genetic segments in disparate enteric organisms may suggest that they confer some selective advantage within the intestinal environment.
Conclusions
Clostridioides difficile RT106 is virulent in a hamster model of infection, and all sequenced isolates within this clade harbor a unique 46 kb GI1. Consistent with the presence of genes encoding a VanZ family protein and a SrtB-anchored collagen-binding adhesin within GI1, RT106 strains had increased teicoplanin resistance and robust collagen-dependent biofilm formation, respectively. Further investigation is required to implicate GI1 genes to RT106 virulence.
Methods
Clostridioides difficile surveillance
This study, approved by the University of Arizona Institutional Review Board, utilized to-be-discarded stool specimens from diarrheic patients at the Banner University Medical Center (BUMC) in Tucson, Arizona between August 1, 2015 and July 31, 2018. Samples were collected and stored at − 80 °C. From August 2015 to February 2017, tcdB-positive stool samples tested by BUMC via polymerase chain reaction (PCR) were included in the study. On March 2017, BUMC implemented the glutamate dehydrogenase (GDH) and toxin enzyme immunoassay for C. difficile testing. All GDH + samples were collected. We screened for the presence of tcdB in the GDH + /toxin− samples via PCR using the following primers: B1C (5′-GAAAATTTTATGAGTTTAGTTAATAGAAA-3′) and B2N (5′-CAGATAATGTAGGAAGTAAGTCTATAG-3′)71. For samples received during March 2017 to July 2018, only GDH+/toxin+ or GDH + /toxin− and tcdB-PCR-positive samples were analyzed in this study.
Ribotyping of clinical C. difficile isolates
Stool samples plated on taurocholate cycloserine cefoxitin fructose agar (TCCFA) were cultured anaerobically at 37 °C. Isolated colonies were lysed with G-Biosciences Toothpick-PCR, and supernatants were used as templates for ribotyping PCR using the following primers: 16S (5′-GTGCGGCTGGATCACCTCCT-3′) and 23S (5′-CCCTGCACCCTTAATAACTTGACC-3′)72,73. Isolated colonies were also submitted to the University of Arizona Genomics Core for genomic extraction using QIAGEN DNeasy column-based extraction kit and ribotyping PCR using the same 16S and 23S primers. PCR products were resolved via capillary electrophoresis using an AB Prism 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA) and amplicon length evaluated using Marker 1.85 (SoftGenetics, State College, PA). Ribotype identification from electropherograms was determined using Webribo (https://webribo.ages.at/)72.
DNA extraction
Genomic DNA samples were extracted using the protocol by Pospiech and Neumann74, with modifications. Briefly, 50 mL overnight cultures of C. difficile were pelleted and resuspended in 5 mL of SET buffer (75 mM NaCl, 25 mM EDTA, 20 mM Tris, pH 7.5). Cell lysis was facilitated by adding lysozyme (5 mg/mL final concentration) and incubating samples at 37 °C for 30 min. 500 μL of 10% SDS and 25 μL of 100 mg/mL proteinase K were added, and samples were incubated at 55 °C for 2 h. 2.5 mL of 5 M NaCl and 5 mL of chloroform was added, and samples mixed with frequent inversions. Samples were centrifuged at 3000×g for 15 min, and aqueous phase was collected. DNA was precipitated using 1 volume of isopropanol. DNA was then spooled, transferred to a microfuge tube, rinsed with 70% ethanol, and vacuum dried.
Whole genome sequencing
DNA from 38 RT106 samples were submitted to the Office of Knowledge Enterprise Development (OKED) Genomics Core at Arizona State University (Tempe, Arizona, USA) for whole genome sequencing. Illumina-compatible genomic DNA libraries were generated on BRAVO NGS liquid handler (Agilent Technologies, Santa Clara, CA) using Kapa HyperPlus KK8514 library kit (Kapa Biosystems, Wilmington, MA). DNA was enzymatically sheared to approximately 600 bp fragments, end-repaired and A-tailed as described in the Kapa HyperPlus protocol. Illumina-compatible adapters with unique indexes (IDT #00989130v2; IDT technologies, Skokie, IL) were ligated individually on each sample. The adapter-ligated molecules were cleaned using Kapa pure beads (KK89002, Kapa Biosystems), and amplified with Kapa HiFi DNA Polymerase (KK2502, Kapa Biosystems). Fragment size of each library was analyzed using Agilent Tapestation, and quantified via qPCR using KAPA Library Quantification Kit (KK4835, Kapa Biosystems) and Applied Biosystems Quantstudio 5 Real-time PCR System before multiplex pooling and sequencing in a 2 × 250 flow cell on the MiSeq platform (Illumina, San Diego, CA) at the ASU OKED Genomics Core. Genomic libraries were split in 3 MiSeq runs. De novo genome assembly was performed using CLC Genomics Workbench 11 (QIAGEN Bioinformatics, Redwood City, CA). Depth of coverage ranges between 17X-608X (Supplemental Table S6). Contigs were annotated via Rapid Annotation using Subsystem Technology (RAST) Version 2.075,76,77. Sequences for the 38 RT106 genomes were deposited through the National Center for Biotechnology Information (NCBI) Bankit (https://www.ncbi.nlm.nih.gov/WebSub/?tool=genbank) under the GenBank accession numbers listed on Supplemental Table S6.
Composition vector tree analysis
The 38 RT106 strains were mapped against a collection of all complete or draft C. difficile genomes sequences (1425 total sequences) available from the NCBI genome database (January 2019 download date). The composition vector tree was generated without sequence alignment by using a Composition Vector approach and CVtree Version 3.030. Interactive Tree of Life v4.3 (https://itol.embl.de/)78 was used to visualize and annotate the phylogenetic tree.
In silico multilocus sequence typing (MLST) and in silico ribotyping
Sequence types (ST) of C. difficile strains that claded with RT106 in the phylogenetic tree were determined based on the allelic patterns of 7 housekeeping genes28 using the C. difficile MLST database (http://pubmlst.org/cdifficile). In silico ribotyping PCR analysis was performed on the uncharacterized strains using NCBI Primer-Blast79 and the same 16S and 23S primers listed above. DH/NAP11/106/ST42 (Refseq assembly no. GCF_002234355.1), a complete closed genome, was used as a reference strain for the RT106 PCR fragment pattern.
Identification of RT106 genomic islands
A series of BLASTN searches (https://blast.ncbi.nlm.nih.gov/Blast.cgi)80 was performed to identify the unique genetic elements associated with RT106. GV364 sequence was first compared to the complete closed genome sequence of C. difficile 630 strain (Refseq assembly no. GCF_000009205.2). Large genetic elements (> 10 kb) not found in C. difficile 630 were then compared to all 94 RT106 strain sequences to identify genetic elements associated only with RT106. The resulting genetic elements were verified to be unique to RT106 by performing BLASTN searches against 1425 publicly available C. difficile genome sequences at the NCBI database. Genome circular map of a representative strain GV 364 was generated using Artemis DNA Plotter. GC content (%) and relative positions of GI1, GI2, and GI3 are indicated in the map (Fig. 2a).
Maximum likelihood (ML) trees of core genomes
ML trees were constructed for two groups of genomes; (1) 94 strains identified to clade together in the composition vector tree and found to contain a complete GI1, and (2) 265 strains that contain complete and partial (> 7.7 kb and 98% identity) segments of GI1. Panseq81 was used to determine the core SNPs. MEGA-X36 was used to infer phylogenies by using the Maximum Likelihood method and Tamura-Nei model82. Trees with the highest log likelihood were shown in Figs. 2c and 3. The bootstrap consensus tree inferred from 1000 replicates was taken to represent the evolutionary history of the taxa analyzed83.
Molecular clock analysis
The 7.1 kb genetic region common to 265 C. difficile strains was used to deduce the possible evolutionary formation of genetic island 1 on RT106. Mega-X36 was used to construct a timetree inferred by applying the RelTime method84,85 to the a phylogenetic tree whose branch lengths were calculated using the Maximum Likelihood (ML) method and the Tamura-Nei substitution model82. CD105KSE6 branched most distantly from RT106 (genetic distance of CD105KSE6 and GV973 = 0.024010404) based on the alignment of the 7.1 kb consensus region in GI1 and was used as the root for the tree. The bootstrap consensus tree inferred from 1000 replicates was taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches.
The independence of the acquisition of gene segments forming GI1 was tested by comparing the ML tree to a minimum spanning tree (MST) of MLST allele data profiles in the C. difficile MLST database (http://pubmlst.org/cdifficile). MST was created using PhyloViz v2.086.
Likelihood ratio test of tree topologies
Tree topologies were analyzed using IQ-TREE287 based on seven likelihood ratio-based tests (bootstrap proportion using RELL method test, one-sided Kishino-Hasegawa test, Shimodaira-Hasegawa test, weighted Kishino-Hasegawa test, weighted Shimodaira-Hasegawa test, expected likelihood weight, approximately unbiased test)88,89,90,91,92 to compare ML trees based on core genome SNPs (designated as T1 in this analysis) and seven MLST genes (designated as T2) against the ML tree based on the 7.1 kb shared region within GI1 (designated as T0) with the following hypotheses:
HO: T0 and T1, or T0 and T2, would explain the sequence diversity of the 7.1 kb shared region within GI1 equally well (T0 = T1 or T0 = T2).
HA: T1 and/or T2 does not explain the sequence diversity of the 7.1 kb shared region of GI1 (T0 ≠ T1 or T0 ≠ T2).
All tests were performed with 10,000 resamplings using the RELL method.
Antibiotic susceptibility testing
Overnight cultures of C. difficile strains were diluted to a McFarland scale of 0.5 (approximate OD600nm = 0.1). 100 μL of the culture was plated onto Brucella blood agar. E-test strips (BioMerieux, Durham, NC) for the following antibiotics were applied on the agar: cefotaxime, vancomycin, erythromycin, clindamycin, levofloxacin, metronidazole, moxifloxacin, tetracyline and teicoplanin. Minimum inhibitory concentration, defined as the lowest concentration of the agent that inhibited bacterial growth, was determined. Antibiotic susceptibility was based on Clinical and Laboratory Standard Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints. There are no set standard breakpoints for teicoplanin. To test whether prior incubation with a sub-inhibitory concentration of teicoplanin promotes increased resistance, overnight cultures of C. difficile strains were diluted to a McFarland scale of 0.5 (approximate OD600nm = 0.1). Five mL aliquots of the diluted culture were added into two new culture tube; Teicoplanin was added to one of the tubes to a final concentration of 0.0125 mg/mL. After 24 h of culture, antibiotic susceptibility testing was performed as indicated above.
Antibiotic resistance gene identification and profiling
Whole sequence genomes and proteomes were searched for antimicrobial resistance (AMR) genes using NCBI’s AMRFinderPlus93 and the Comprehensive Antibiotic Resistance Database’s Resistance Gene Identifier Software Version 5.1.1 and Antibiotic Resistance Ontology Version 3.1.094. Nonsynonymous SNPs in the AMR genes that may confer resistance to antibiotics used in susceptibility testing were compiled and tabulated in Supplemental Table S1.
Toxin ELISA
Relative levels of TcdA and TcdB toxins were determined using Alere Wampole A/B Toxin ELISA kit (Alere, Atlanta, GA). Overnight cultures of C. difficile strains were inoculated in 10 mL BHI at 1:100 dilution. Samples were cultured anaerobically for 72 h. Cultures were pelleted by centrifugation, and supernatants processed for Toxin ELISA following manufacturer’s protocol and using BioTek Synergy automated plate reader. Total protein present in the supernatants were quantified using Pierce BCA protein assay kit. Relative amounts of toxin were normalized to total proteins.
Motility assay
Motility agar plates were prepared by adding 20 mL of BHI with 0.3% agar per well of a 6-well plate. C. difficile strains were cultured in BHI overnight. Approximately 5 μL of the culture was collected and stabbed into the motility agar. Plates were sealed and incubated in a humid, anaerobic chamber for 72 h, and then imaged using Bio-Rad ChemiDoc Touch Imaging System.
Biofilm assay
Twenty-four well plates were coated with: human or rat tail collagen type I (88 ng per well), human collagen type III (88 ng per well) or a combination of human collagen type I and type III (88 ng of each collagen type per well). Overnight cultures of C. difficile strains were diluted in BHI containing 100 mM glucose (OD600nm = 0.1). One mL of the culture was added per well of the uncoated or collagen-coated plate and incubated anaerobically for 72 h at 37 °C. Supernatants were removed gently by tilting plates onto a collection basin. Biofilms were washed twice by gently submerging plates in glass basins of PBS. Excess PBS was removed by inverting plates onto tissue paper. Biofilms were fixed for 20–40 min at 37 °C, and then stained with 1 mL of 0.2% filter-sterilized crystal violet for 30 min. Biofilms were washed twice with PBS as described above. For quantification of biofilm growth, 1 mL of 4:1 ethanol/acetone solution was added to each sample. 100 μL aliquots were transferred to a 96-well plate, and absorbance at 570 nm (A570nm) was determined using BioTek Synergy automated plate reader. Relative changes in biofilm densities (ΔA570nm) were determined by comparing A570nm of crystal violet-stained biofilms formed on collagen-coated vs. on uncoated plastic wells.
Clostridioides difficile infection of Golden Syrian hamsters
The Golden Syrian hamsters model95 was employed to test GV599 virulence. A detailed protocol is included in the Supplementary Material. This animal study was approved by the Institutional Animal Care and Use Committee of the University of Arizona.
Ethical declarations
All methods were carried out in accordance with relevant guidelines and regulations. The C. difficile surveillance study was approved by the University of Arizona Institutional Review Board (Approval Number/ID 1707612129) as non-human subjects research. Informed consent was not required since to-be-discarded and de-identified stool samples were used.
Abbreviations
- CDI:
-
Clostridioides difficile Infections
- CDC:
-
Center for Disease Control and Prevention
- BUMC:
-
Banner University Medical Center
- MLST:
-
Multi-locus sequence typing
- CLSI:
-
Clinical and Laboratory Standard Institutes
- PaLoc:
-
Pathogenicity locus
- RT:
-
Ribotype
References
Bartlett, J. G. Antibiotic-associated diarrhea. N. Engl. J. Med. 346, 334–339 (2002).
McFarland, L. V. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 3, 563–578 (2008).
Nasiri, M. J. et al. Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: a systematic review and meta-analysis. Anaerobe 50, 32–37 (2018).
Schroeder, M. S. Clostridium difficile-associated diarrhea. Am. Family Physician 71, 921–928 (2005).
Ricciardi, R., Rothenberger, D. A., Madoff, R. D. & Baxter, N. N. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch. Surg. 142, 624–631 (2007).
Freeman, J. et al. The changing epidemiology of Clostridium difficile infections. Clin. Microbiol. Rev. 23, 529–549 (2010).
Lessa, F. C. et al. Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 825–834 (2015).
Ho, J. et al. Disease burden of Clostridium difficile infections in adults, Hong Kong, China, 2006–2014. Emerg. Infect. Dis. 23, 1671–1679 (2017).
McDonald, L. C. et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353, 2433–2441 (2005).
O’Connor, J. R., Johnson, S. & Gerding, D. N. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 136, 1913–1924 (2009).
Guh, A. Y. et al. Trends in incidence of long-term-care facility onset Clostridium difficile infections in 10 US geographic locations during 2011–2015. Am. J. Infect. Control. 46, 840–842 (2018).
Brazier, J. S. et al. Distribution and antimicrobial susceptibility patterns of Clostridium difficile PCR ribotypes in English hospitals, 2007–08. Euro Surveill. 13, 19000 (2008).
Bauer, M. P. et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377, 63–73 (2011).
Wilcox, M. H. et al. Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin. Infect. Dis. 55, 1056–1063 (2012).
Cheknis, A. et al. Molecular epidemiology of Clostridioides (Clostridium) difficile strains recovered from clinical trials in the US, Canada and Europe from 2006–2009 to 2012–2015. Anaerobe 53, 38–42 (2018).
Center for Disease Control and Prevention. 2012 Annual Report for the Emerging Infections Program for Clostridium difficile Infection Infection (Center for Disease Control and Prevention, Atlanta, 2012).
Centers for Disease Control and Prevention. 2013 Annual Report for the Emerging Infections Program for Clostridium difficile Infection Infection (Centers for Disease Control and Prevention, Atlanta, 2013).
Centers for Disease Control and Prevention. 2014 Annual Report for the Emerging Infections Program for Clostridium difficile Infection Infection (Centers for Disease Control and Prevention, Atlanta, 2014).
Centers for Disease Control and Prevention. 2015 Annual Report for the Emerging Infections Program for Clostridium difficile Infection Infection (Centers for Disease Control and Prevention, Atlanta, 2015).
Center for Disease Control and Prevention. 2016 Annual Report for the Emerging Infections Program for Clostridium difficile Infection Infection (Center for Disease Control and Prevention, Atlanta, 2016).
Centers for Disease Control and Prevention. 2017 Annual Report for the Emerging Infections Program for Clostridioides difficile Infection Infection (Centers for Disease Control and Prevention, Atlanta, 2017).
Carlson, T. J., Blasingame, D., Gonzales-Luna, A. J., Alnezary, F. & Garey, K. W. Clostridioides difficile ribotype 106: a systematic review of the antimicrobial susceptibility, genetics, and clinical outcomes of this common worldwide strain. Anaerobe 62, 102142 (2020).
Knetsch, C. W. et al. Whole genome sequencing reveals potential spread of Clostridium difficile between humans and farm animals in the Netherlands, 2002 to 2011. Euro Surveill. 19, 20954 (2014).
Eyre, D. W. et al. Comparison of control of Clostridium difficile infection in six English hospitals using whole-genome sequencing. Clin. Infect. Dis. 65, 433–441 (2017).
Wang, X. et al. Molecular typing of Clostridium difficile: concordance between PCR-ribotyping and multilocus sequence typing (MLST). Open Forum. Infect. Dis. 5, S176–S176 (2018).
Kurka, H. et al. Sequence similarity of Clostridium difficile strains by analysis of conserved genes and genome content is reflected by their ribotype affiliation. PLoS ONE 9, e86535 (2014).
Kociolek, L. K. et al. Whole-genome analysis reveals the evolution and transmission of an MDR DH/NAP11/106 Clostridium difficile clone in a paediatric hospital. J. Antimicrob. Chemother. 73, 1222–1229 (2018).
Griffiths, D. et al. Multilocus sequence typing of Clostridium difficile. J. Clin. Microbiol. 48, 770–778 (2010).
Kociolek, L. K., Gerding, D. N., Hecht, D. W. & Ozer, E. A. Comparative genomics analysis of Clostridium difficile epidemic strain DH/NAP11/106. Microbes Infect. 20, 245–253 (2018).
Zuo, G. & Hao, B. CVTree3 web server for whole-genome-based and alignment-free prokaryotic phylogeny and taxonomy. Genom. Proteom. Bioinf. 13, 321–331 (2015).
Bertelli, C. et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 45, W30–W35 (2017).
Waack, S. et al. Score-based prediction of genomic islands in prokaryotic genomes using hidden Markov models. BMC Bioinform. 7, 142 (2006).
Hsiao, W., Wan, I., Jones, S. J. & Brinkman, F. S. IslandPath: aiding detection of genomic islands in prokaryotes. Bioinformatics 19, 418–420 (2003).
Hudson, C. M., Lau, B. Y. & Williams, K. P. Islander: a database of precisely mapped genomic islands in tRNA and tmRNA genes. Nucleic Acids Res. 43, D48–D53 (2015).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Knyaz, C., Stecher, G., Li, M., Kumar, S. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Ravenhall, M., Škunca, N., Lassalle, F. & Dessimoz, C. Inferring horizontal gene transfer. PLOS Comput. Biol. 11, e1004095 (2015).
Boc, A., Philippe, H. & Makarenkov, V. Inferring and validating horizontal gene transfer events using bipartition dissimilarity. Syst. Biol. 59, 195–211 (2010).
Farrow, K. A., Lyras, D. & Rood, J. I. The macrolide-lincosamide-streptogramin B resistance determinant from Clostridium difficile 630 contains two erm(B) genes. Antimicrob. Agents Chemother. 44, 411–413 (2000).
Schmidt, C., Loffler, B. & Ackermann, G. Antimicrobial phenotypes and molecular basis in clinical strains of Clostridium difficile. Diagn. Microbiol. Infect. Dis. 59, 1–5 (2007).
Dridi, L., Tankovic, J., Burghoffer, B., Barbut, F. & Petit, J.-C. gyrA and gyrB mutations are implicated in cross-resistance to ciprofloxacin and moxifloxacin in Clostridium difficile. Antimicrob. Agents Chemother. 46, 3418–3421 (2002).
Drudy, D., Kyne, L., Mahony, R. & Fanning, S. gyrA mutations in fluoroquinolone-resistant Clostridium difficile PCR-027. Emerg. Infect. Dis. 13, 504–505 (2007).
Spigaglia, P. et al. Fluoroquinolone resistance in Clostridium difficile isolates from a prospective study of C. difficile infections in Europe. J. Med. Microbiol. 57, 784–789 (2008).
Huang, H. et al. Clostridium difficile infections in a Shanghai hospital: antimicrobial resistance, toxin profiles and ribotypes. Int. J. Antimicrob. Agents 33, 339–342 (2009).
Lin, Y.-C. et al. Antimicrobial susceptibilities and molecular epidemiology of clinical isolates of Clostridium difficile in Taiwan. Antimicrob. Agents Chemother. 55, 1701–1705 (2011).
Arthur, M., Depardieu, F., Molinas, C., Reynolds, P. & Courvalin, P. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 154, 87–92 (1995).
Vuotto, C., Donelli, G., Buckley, A. & Chilton, C. Clostridium difficile biofilm. Adv. Exp. Med. Biol. 1, 97–115 (2018).
Graham, M. F. et al. Collagen content and types in the intestinal strictures of Crohn’s disease. Gastroenterol. 94, 257–265 (1988).
Chaban, B., Hughes, H. V. & Beeby, M. The flagellum in bacterial pathogens: for motility and a whole lot more. Semin Cell Dev Biol 46, 91–103 (2015).
Stabler, R. A. et al. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J. Bacteriol. 188, 7297–7305 (2006).
Stabler, R. A. et al. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol. 10, R102 (2009).
Hammond, G. A. & Johnson, J. L. The toxigenic element of Clostridium difficile strain VPI 10463. Microb. Pathog. 19, 203–213 (1995).
Braun, V., Hundsberger, T., Leukel, P., Sauerborn, M. & von Eichel-Streiber, C. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181, 29–38 (1996).
Lanis, J. M., Heinlen, L. D., James, J. A. & Ballard, J. D. Clostridium difficile 027/BI/NAP1 encodes a hypertoxic and antigenically variable form of TcdB. PLoS Pathog. 9, e1003523 (2013).
Hunt, J. J., Larabee, J. L. & Ballard, J. D. Amino acid differences in the 1753-to-1851 region of TcdB influence variations in TcdB1 and TcdB2 cell entry. mSphere 2, e00268-e1217 (2017).
Collins, J. et al. Dietary trehalose enhances virulence of epidemic Clostridium difficile. Nature 553, 291–294 (2018).
Saund, K., Rao, K., Young, V. B. & Snitkin, E. S. Genetic determinants of trehalose utilization are not associated with severe Clostridium difficile infection outcome. Open Forum. Infect. Dis. 7, 1 (2020).
Sundram, F. et al. Clostridium difficile ribotypes 027 and 106: clinical outcomes and risk factors. J. Hosp. Infect. 72, 111–118 (2009).
Solomon, K. et al. PCR ribotype prevalence and molecular basis of macrolide–lincosamide–streptogramin B (MLSB) and fluoroquinolone resistance in Irish clinical Clostridium difficile isolates. J. Antimicrob. Chemother. 66, 1976–1982 (2011).
Tenover, F. C., Tickler, I. A. & Persing, D. H. Antimicrobial-resistant strains of Clostridium difficile from North America. Antimicrob. Agents Chemother. 56, 2929–2932 (2012).
Hunt, J. J. & Ballard, J. D. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol. Mol. Biol. 77, 567–581 (2013).
Wüst, J., Sullivan, N. M., Hardegger, U. & Wilkins, T. D. Investigation of an outbreak of antibiotic-associated colitis by various typing methods. J. Clin. Microbiol. 16, 1096 (1982).
Sebaihia, M. et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38, 779–786 (2006).
Rupnik, M. & Janezic, S. An update on Clostridium difficile toxinotyping. J. Clin. Microbiol. 54, 13–18 (2016).
Quemeneur, L. et al. Clostridium difficile toxoid vaccine candidate confers broad protection against a range of prevalent circulating strains in a nonclinical setting. Infect Immun. 86, e00717–e00742 (2018).
Woods, E. C., Wetzel, D., Mukerjee, M. & McBride, S. M. Examination of the Clostridioides (Clostridium) difficile VanZ ortholog, CD1240. Anaerobe 53, 108–115 (2018).
Hensbergen, P. J. et al. Clostridium difficile secreted Pro-Pro endopeptidase PPEP-1 (ZMP1/CD2830) modulates adhesion through cleavage of the collagen binding protein CD2831. FEBS Lett. 589, 3952–3958 (2015).
Chakraborty, S., Gogoi, M. & Chakravortty, D. Lactoylglutathione lyase, a critical enzyme in methylglyoxal detoxification, contributes to survival of Salmonella in the nutrient rich environment. Virulence 6, 50–65 (2015).
McMahon, S. A. et al. Extensive DNA mimicry by the ArdA anti-restriction protein and its role in the spread of antibiotic resistance. Nucleic Acids Res. 37, 4887–4897 (2009).
Pitondo-Silva, A., Gonçalves, G. B. & Stehling, E. G. Heavy metal resistance and virulence profile in Pseudomonas aeruginosa isolated from Brazilian soils. APMIS 124, 681–688 (2016).
Rupnik, M. et al. Characterization of polymorphisms in the toxin A and B genes of Clostridium difficile. FEMS Microbiol. Lett. 148, 197–202 (1997).
Indra, A. et al. Characterization of Clostridium difficile isolates using capillary gel electrophoresis-based PCR ribotyping. J. Med. Microbiol. 57, 1377–1382 (2008).
Bidet, P., Barbut, F., Lalande, V., Burghoffer, B. & Petit, J.-C. Development of a new PCR-ribotyping method for Clostridium difficile based on ribosomal RNA gene sequencing. FEMS Microbiol. Lett. 175, 261–266 (1999).
Pospiech, A. & Neumann, B. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11, 217–218 (1995).
Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genom. 9, 75 (2008).
Overbeek, R. et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res. 42, D206-214 (2014).
Brettin, T. et al. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 5, 8365 (2015).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242-245 (2016).
Ye, J. et al. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 13, 134 (2012).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Laing, C. et al. Pan-genome sequence analysis using Panseq: an online tool for the rapid analysis of core and accessory genomic regions. BMC Bioinform. 11, 461 (2010).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791 (1985).
Tamura, K. et al. Estimating divergence times in large molecular phylogenies. Proc. Natl. Acad. Sci. 109, 19333–19338 (2012).
Tamura, K., Tao, Q. & Kumar, S. Theoretical foundation of the RelTime method for estimating divergence times from variable evolutionary rates. Mol. Biol. Evol. 35, 1770–1782 (2018).
Francisco, A. P. et al. PHYLOViZ 2.0: providing scalable data integration and visualization for multiple phylogenetic inference methods. Bioinformatics 33, 128–129 (2016).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Kishino, H., Miyata, T. & Hasegawa, M. Maximum likelihood inference of protein phylogeny and the origin of chloroplasts. J. Mol. Evol. 31, 151–160 (1990).
Kishino, H. & Hasegawa, M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J. Mol. Evol. 29, 170–179 (1989).
Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51, 492–508 (2002).
Shimodaira, H. & Hasegawa, M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16, 1114–1114 (1999).
Strimmer, K. & Rambaut, A. Inferring confidence sets of possibly misspecified gene trees. Proc. R. Soc. B. 269, 137–142 (2002).
Feldgarden, M. et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob. Agents Chemother. 63, e00419–e00483 (2019).
Alcock, B. P. et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48, D517-d525 (2020).
Vedantam, G. et al. An engineered synthetic biologic protects against Clostridium difficile infection. Front. Microbiol. 9, 1 (2018).
Acknowledgements
This work was supported by funding from the National Institutes of Health [R33AI121590531(GV) and the US Dept. of Veterans Affairs [IK6BX003789(GV); I01BX001183(GV)]. Ribotyping work was performed with the support of the University of Arizona Genetics Core, University of Arizona, Tucson, AZ.
Author information
Authors and Affiliations
Contributions
B.P.R. performed the comparative genomic analyses. J.L.R. performed phenotypic characterization and statistical analysis, analyzed data and drafted the manuscript. R.C.W., A.S.M. and F.A. isolated C. difficile from stool samples and performed ribotyping analysis. A.H. performed moxifloxacin and teicoplanin MIC determination and biofilm assay experiments. A.W., J.L. and S.J. conducted the pilot hamster experiment. SPE and KWS contributed in the conceptualization of the research and analyzed data. G.V. and V.K.V. conceptualized and funded the studies, finalized the manuscript and provided full project oversight. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roxas, B.A.P., Roxas, J.L., Claus-Walker, R. et al. Phylogenomic analysis of Clostridioides difficile ribotype 106 strains reveals novel genetic islands and emergent phenotypes. Sci Rep 10, 22135 (2020). https://doi.org/10.1038/s41598-020-79123-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79123-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.