Abstract
We investigated the properties of the low molecular weight thermo-alkali-stable and mercury ion-tolerant xylanase production from Thermomyces dupontii KKU-CLD-E2-3. The xylanase was purified to homogeneity by ammonium sulfate, Sephadex G–100 and DEAE–cellulose column chromatography which resulted 27.92-fold purification specific activity of 56.19 U/mg protein and a recovery yield of 2.01%. The purified xylanase showed a molecular weight of 25 kDa by SDS–PAGE and the partial peptide sequence showed maximum sequence homology to the endo-1,4-β-xylanase. The optimum temperature and pH for its activity were 80 °C and pH 9.0, respectively. Furthermore, the purified xylanase can maintain more than 75% of the original activity in pH range of 7.0–10.0 after incubation at 4 °C for 24 h, and can still maintain more than 70% of original activity after incubating at 70 °C for 90 min. Our purified xylanase was activated by Cu2+ and Hg2+ up to 277% and 235% of initial activity, respectively but inhibited by Co2+, Ag+ and SDS at a concentration of 5 mM. The Km and Vmax values of beechwood xylan were 3.38 mg/mL and 625 µmol/min/mg, respectively. Furthermore, our xylanase had activity specifically to xylan-containing substrates and hydrolyzed beechwood xylan, and the end products mainly were xylotetraose and xylobiose. The results suggested that our purified xylanase has potential to use for pulp bleaching in the pulp and paper industry.
Similar content being viewed by others
Introduction
Xylan is the main carbohydrate found in hemicellulose consists of β-1,4-linked d-xylopyranose units for the main chain of a homo polymeric backbone, and short chain branches consisting of O-acetyl, α-L-arabinofuranosyl and α-D-glucuronyl residues1,2. Its complete hydrolysis requires the action of several different enzymes including endo-β-1,4-xylanase (EC 3.2.1.8) and β-xylosidase (EC 3.2.1.37) which released xylooligosaccharides and d-xylose as the main products, respectively3,4.
Xylanase has been widely used for application in several industries such as biorefinery, food, animal feed, pulp and paper, and textile industries. For the purpose of pulping and bleaching processes in pulp and paper industries, the xylanase should have high catalytic efficiency and must be stable and active both at high temperature and in alkaline condition, as well as tolerant to metal ions5,6. In addition, there is evidence that low molecular weight xylanase can effectively diffuse into the fibrous pulp and thus can efficiently hydrolyze xylan in biomass hydrolysis or pulp bleaching7. However, using xylanase in pulp bleaching process, there are still several properties needed to be considered. For example, enzyme should be free of cellulase and active at high temperature of, e.g., 50–90 °C and in alkaline pH of 8–10 for 30–300 min8. Recently, low-molecular-weight and thermo-alkali-stable xylanase have been observed in some species of thermophilic fungi. The thermophilic fungi are a good producer of extracellular xylanase which are thermostable, broad tolerance to pH variation, great resistance to denaturing agents, and have optimum activity at elevated temperatures9,10,11. However, the xylanase activity of these fungi was usually inhibited by metal ions, especially the mercury ion, Hg2+12,13,14,15. Therefore, it is important to search for new sources of xylanases production that have the required properties suitable for application in industry.
Previously, we reported the optimization of culture condition for the production of cellulase-free xylanase from the fungus, T. dupontii KKU–CLD–E2–3, whose crude xylanase activity was outstanding under alkaline and thermostable conditions16. These remarkable properties were interesting for application in pulp bleaching of paper industry. In order to achieve the pulp bleaching step, which is planned for the future, the xylanase produced by T. dupontii KKU–CLD–E2–3 was, therefore, purified and characterized. The partial sequence of amino acid consensus with endo-1,4-β-xylanase was also investigated in this present work. In addition, we are the first to report that the xylanase activity from this fungus was significantly activated by mercury ion (Hg2+). This novel finding suggested that our xylanase has a unique characteristic when compared to those of other xylanase-producing fungi whose activities are strongly inhibited by Hg2+.
Materials and methods
Materials
Beechwood xylan was purchased from Sigma Chemical Co., St. Louis, MO, USA. Sephadex G–100 gel filtration media was obtained from GE Healthcare in Uppsala, Sweden. Diethylaminoethyl (DEAE–cellulose) was purchased from Sigma-Aldrich. in St. Louis, MO, USA. AmershamTM ECLTM gel 4–20% Kit was purchased from Merck, Darmstadt, Germany (Silica Gel 60 F254). Xylose and xylooligosaccharide were purchased from Sigma Chemical (Wako Pure Chemical Industries. Ltd, USA.). All other chemicals were of analytical grade.
The thermophilic fungi
Thermomyces dupontii KKU–CLD–E2–3 (Accession number LC428093) was used as the inoculums and was isolated from elephant dung collected from Chulabhorn Dam, Chaiyaphum province, Thailand16. This fungus was obtained from the Mycorrhizal and Fungal Technology Laboratory, Department of Microbiology, Faculty of Science, Khon Kaen University, Thailand.
Xylanase production in solid-state fermentation (SSF) and enzyme extraction
In this study, the xylanase production in solid-state fermentation by the fungus T. dupontii KKU–CLD–E2–3 was performed by following the method described by Seemakram et al.16 in which fermentation conditions were optimized for the production process. The T. dupontii KKU–CLD–E2–3 was cultivated in 500 mL Erlenmeyer flask containing a 10 g of corn cob and 17 mL mineral solution (NH4Cl 1.77 g, KH2PO4 3.0 g, MgSO4 7H2O 0.5 g, CaCl2 0.5 g, glucose 5.0 g per 1000 mL distilled water). The initial pH and moisture content of the medium was set to be 10.74 and 74.56%, respectively. Then, all flasks were sterilized at 121 °C for 15 min. Ten agar plugs (0.5 cm diameter) of a 4-d-old mycelial culture of the strain were inoculated in each flask, and incubated at 44.72 °C (Seemakram et al.16) under static conditions for 8 d. After that, 100 mL of 0.05 M Tris–HCl buffer having pH 9.0 was added to the cultures. We then shake the sample at 150 rpm and 30 °C for 60 min. The solid materials and fungal biomass were separated by centrifugation (13,551×g, 20 min). The supernatant was used for enzyme assays.
Xylanase and protein assays
Xylanase activity was assayed by using beechwood xylan as substrate. A 0.5 mL of enzyme solution was added to 0.5 mL of 1% beechwood xylan solution in 0.05 M Tris–HCl buffer, pH 9.0. The mixture was incubated at 80 °C for 15 min, and the reducing sugar released was determined by Somogyi Nelson method17. One unit of xylanase activity was defined as the amount of enzyme required to liberate 1 μmol of reducing sugars from substrate per minute under the assay conditions. Protein was determined by the Lowry method18 using bovine serum albumin (BSA) as standard.
Xylanase purification
The purification of crude xylanase was carried out with three steps consisted of ammonium sulfate precipitation, gel filtration and ion exchange chromatography by following the method described by Seemakram et al.16. The crude enzyme was precipitated by ammonium sulphate ((NH4)2 SO4) at concentration of 20–80% for overnight at 4 ºC. The precipitated protein was separated by centrifugation at 6000 rpm for 15 min and dissolve in 0.05 M Tris–HCl, pH 9.0, then precipitated protein was dialyzed against the same previously used buffer. Thereafter, 1.0 mL of protein sample was purified by gel filtration with Sephadex G–100 column (100 cm × 1.0 cm) and equilibrated with 0.05 M Tris–HCl buffer (pH 9.0). The protein sample was eluted by 0.05 M Tris–HCl buffer pH 9.0 at a flow rate of 0.25 mL/min, and 2 mL fractions were collected. Then, all active fractions were pooled and applied to final of purification step by ion exchange chromatography with a DEAE–Cellulose column (20 cm × 1.5 cm) equilibrated with the same previously used buffer at a flow rate of 0.5 mL/min. The bound proteins were eluted with a NaCl gradient (2 M) in the same buffer at a flow rate of 0.5 mL/min, and 2 mL fractions were collected. The highly active fractions were gathered and stored at − 40 °C for further characterization.
SDS–polyacrylamide gel electrophoresis (SDS–PAGE)
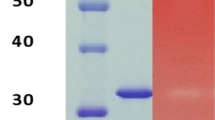
The purified enzyme was checked for their purity by SDS–polyacrylamide gel electrophoresis (PAGE). The SDS–PAGE was performed following a modified method of Laemmli19 using AmershamTM ECLTM gel 4–20% Kit. Proteins in the gel were visualized by Coomassie brilliant blue R–250 staining. The chromatin pre-stained protein ladder (10–175 kDa) was used as a molecular weight marker.
Protein identification by LC–MS/MS and data analysis
The protein was loaded in 14% acrylamide gel, and then visualized by Coomassie brilliant blue R–250. The specific band of target purified xylanase was cut by new clean scalpel blade and transferred into a microcentrifuge tube. The purified xylanase was analyzed for amino acid identification by LC–MS/MS system model API 2000 MDS SCIEX. The amino acid sequences were used for analyzing by the MASCOT program (www.matrixscience.com) and a database of annotated comparative protein structure models by the Protein Modeling 101 (https://www.proteinmodelportal.org) with the initial searching parameters; Enzyme: Trypsin; carbamidomethylation (C) as fixed modification, and oxidation (HW) and oxidation (M) as variable modification; peptide mass tolerance of 0.5 Da and fragment mass tolerance of 0.5 Da; a peptide charge state of + 1, + 2, + 3. The proteins were identified through the service of the Research Instrument Center, Khon Kaen University, Thailand.
Effect of pH on the activity and stability of xylanase
Investigation for determining the optimum pH of purified xylanase activity was determined in a pH ranging from 3.0 to 12.0 at 80 °C and performed by following the method described by Seemakram et al.16. We used various buffers including 0.1 M McIlvaine (pH 3.0–8.0), 0.05 M Tris–HCl (pH 8.0–9.0), 0.05 M Glycine–NaOH (pH 9.0–10.0), and 0.05 M Na2HPO4-NaOH (pH 10.0–12.0). The pH stability of purified xylanase was pre-incubated in these buffers at 4 °C for 24 h. The residual activity was measured under the standard assay conditions.
Effect of temperature on the activity and stability of xylanase
The optimum temperature for xylanase activity was determined at various temperatures (50–90 °C) in an optimum buffer and performed by following the method described by Seemakram et al.16. The thermal stability of purified xylanase in the optimum buffer was incubated at different temperatures of 50–90 °C for 90 min. After cooling, the residual xylanase activity was measured according to the standard assay conditions.
Determination for substrate specificity and kinetic parameters
The purified xylanase was measured substrate specificity using 1% (w/v) of xylan consist of Beechwood xylan, Birchwood xylan, Oat spelt xylan, Carboxymethyl cellulose (CMC), Cellulose powder and Avicel, respectively13. The relative activity of enzyme for each substrate were analyzed at optimum temperature for 15 min and measured according to the standard assay conditions with slightly modification described by Seemakram et al.15. The kinetic experiments were performed at different concentrations of each substrate ranged from 2.5–30 mg/mL14 in an optimum buffer and incubated with the purified xylanase at optimum temperature for 15 min. The values of Michaelis–Menten constant (Km) and maximal reaction velocity (Vmax) were analyzed by the linear regression method describe by Lineweaver and Burk20.
Effect of metal ions and reagents on xylanase activity
The purified xylanase was analyzed by pre-incubating the enzyme in solutions containing 1, 5 and 10 mM concentrations of different mineral salts (CuSO4, MgSO4, FeSO4, CoCl2, HgCl2, ZnCl2, AgNO3, MnSO4), ethylenediaminetetraacetic acid (EDTA) and sodium dodecyl sulfate (SDS) for 1 h at room temperature (25 ± 2 °C). Then, xylanase activity was compared to the control without adding metal ions and nonmetal reagents. The residual activity was then measured under the standard assay conditions.
Hydrolysis products of beechwood xylan
The hydrolysis products from purified xylanase were determined by incubating the beechwood xylan with purified enzyme under the optimum conditions assay for 12 h. The hydrolyzed products were measured at different time intervals using thin layer chromatography (TLC) on silica gel plates. Xylose and xylooligosaccharide were used as standards. The products on TLC plates were developed using methanol: glacial acetic acid: H2O (6 : 7 : 2, v/v/v) as the mobile phase which modified from the method described by Li et al.21. The hydrolysis products were detected by spraying with methanol/sulfuric acid mixture (95 : 5, v/v), followed by heating at 100 °C for 15 min.
Results
Purification of xylanase
Purification of crude xylanase was achieved by classical method including ammonium sulfate precipitation, Sephadex G–100 gel filtration and DEAE–cellulose ion-exchange column chromatography. The crude xylanase, having a total activity of 5102.22 U and specific activity of 2.01 U/mg protein, was precipitated using ammonium sulfate to 30% saturation, further purified by Sephadex G–100 gel filtration and DEAE–cellulose ion-exchange column chromatography. After three steps of purification, the specific activity of the crude enzyme increased from 2.01 to 56.19 U/mg protein with a recovery yield of 2.01% and the 27.92-fold apparent homogeneity (Table 1). In addition, the purified xylanase from T. dupontii KKU–CLD–E2–3 was subjected to determination for molecular weight and homogeneous by SDS–PAGE. The purified xylanase appeared as a single protein band with a molecular mass of approximately 25 kDa (Fig. 1).
Protein identification by LC–MS/MS and data analysis
The sample from T. dupontii KKU–CLD–E2–3 was analyzed with amino acid identification of purified analyze by LC–MS/MS. The obtained peptide sequences were used for analyzing by the MASCOT search (Table 2), which amino acid similar to those fungi previously reported (Table 3). This strain was related to members of the description endo-1,4-β-xylanase, where it showed maximum sequence identities of 90% and similarity of 1.452 at resolution 1.55 Å with the endo-1,4-β-xylanase from Thermomyces lanuginosus (Accession number gi|3915307).
Effect of pH on the activity and stability of xylanase
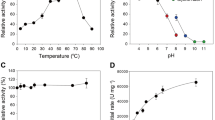
The purified endo-1,4-β-xylanase from T. dupontii KKU–CLD–E2–3 was active at pH 9.0 (Fig. 2a), a higher pH than those described in previous reports for other fungi (Table 4). The purified endo-1,4-β-xylanase retained over 75% of the original activity in pH range of 7.0–10.0 after incubation at 4 °C for 24 h (Fig. 2b).
Effect of temperature on the activity and stability of xylanase
The endo-1,4-β-xylanase from T. dupontii KKU-CLD-E2-3 was optimally active at 80 °C (Fig. 2c). For thermo-stability, xylanase activity was retained more than 70% of the original activity after heating at 70 °C for 90 min (Fig. 2d).
Substrate specificity and kinetic constants
The substrate specificity of the purified endo-1,4-β-xylanase was determined on several substrates (Table 5). The results showed that the highest activity was obtained in beechwood xylan, followed by birchwood xylan, and oat spelt xylan, respectively. In contrast, the xylanase did not have an activity towards Avicel, CMC and cellulose powder. The Km and Vmax of endo-1,4-β-xylanase was determined by beechwood xylan as a substrate. In this case, the Km and Vmax values of the purified endo-1,4-β-xylanase were 3.38 mg/mL and 625 µmol/min/mg, respectively.
Effect of metal ions and reagents on xylanase activity
The effect of different metal ions and reagents on the activity of the purified xylanase from T. dupontii KKU–CLD–E2–3 is summarized in Table 6. The addition of metal ions and nonmetal reagents at a concentration of 1 mM showed increased xylanase activity, except for Ag+ and SDS. The presence of Cu2+, Mg2+, Fe2+, Hg2+, Zn2+, Mn2+ and EDTA at a concentration of 5 mM increased xylanase activity, especially Cu2+ and Hg2+ that increased the xylanase activity up to 277% and 235%, respectively. On the other hand, Co2+, Ag+ and SDS at a concentration of 5 mM showed inhibition of enzyme activity. In addition, xylanase activity was significantly activated by Cu2+ and Mg2+ at a concentration of 10 mM up to 248% and 259% of activity, respectively.
Hydrolysis properties of the purified xylanase
The hydrolysis of beechwood xylan by purified endo-1,4-β-xylanase was studied using thin layer chromatography (TLC) (Fig. 3). The result revealed that the purified endo-1,4-β-xylanase liberated mainly xylotriose and xylotetraose from beechwood xylan.
Discussion
Among xylanase-producing thermophilic fungi, there are only a few reports on the properties of xylanase from Thermomyces dupontii. In this research, the thermo-alkali-stable and metal ions-tolerant xylanase from T. dupontii KKU–CLD–E2–3 was purified and characterized. The purification fold of xylanase purification in this study was higher than some previous studies using other thermophilic fungi. For instance, the observations of Kumar and Shukla22 showed that the xylanase from Thermomyces lanuginosus VAPS24 was purified 5.92-fold using acetone precipitation followed by ultrafiltration. According to Yang et al.23, the purified xylanase from Aspergillus fumigatus Yang FC2–2 was purified to 25.5-fold and apparent homogeneity of 13.9% recovery yield. Maalej et al.24 reported that the purified xylanase from Talaromyces thermophilus was purified 23-fold using diethylaminoethyl cellulose anion exchange chromatography, P–100 gel filtration, and Mono Q chromatography. Chanwicha et al.13 also reported purification of xylanase to 14.5-fold with 2.3% recovery from Thermoascus aurantiacus var. evisporus KKU–PN–I2–1. The purified xylanase from T. dupontii KKU–CLD–E2–3 was appeared as a single protein band on SDS-PAGE gel with a molecular mass of approximately 25 kDa, which was in agreement with the value of 25 kDa xylanase from T. thermophiles24 and 25.8 kDa xylanase from Paecilomyces thermophila J1810. The apparent molecular mass of the xylanase in this study was similar to the 26.2 kDa xylanase from Thermomyces lanuginosus CBS 288.545 and 27 kDa xylanase from T. aurantiacus var. levisporus KKU–PN–2–113.
Then, this protein band was identified against genome sequence assembly v1.0 database (MASCOT search) and the Protein Modeling 101 in order to obtain peptide sequences. The bands excised from the gel were analyzed by mass spectrometry and were compared to protein sequences available in the NCBI database revealed. The protein sequence matches with Endo-1,4-β-xylanase of Thermomyces spp. at 90% identity (Accession number: gi|3,915,307)25. In addition, these results showed that identities were similar to protein from Thermomyces lanuginosus (Accession number:gi|335,371,365) at 89% identity26. The relatively high yield of enzyme and the coverage of peptides from mass-spec was so sparse which the protein sequence coverage of 31% indicated that identity or extensive homology from mass-spectrometry has reliability. Because of the individual ions scores > 54 of indicating identity have the significance at p < 0.05 of mascot score histogram. Besides, the peptide sequence of xylanase has the glycans attached in the fragment after cutting with the specific enzyme27 that attached to the amide group of an asparagine residue within the consensus peptide sequence28, resulting in the observed ion (m/z) to high value has changed it is not matches the coverage of another peptide sequence in the database. The results of the experiments in Table 5 confirmed that this enzyme was xylanase.
According to previous literature, the purified xylanase from Humicola grisea var. thermoidea was most active at pH of 4.5–6.529. Maalej et al.24 reported that the optimum pH of the purified xylanase from T. thermophilus was 7.0. The optimum pH of xylanase from Thermomyces lanuginosus THKU–49 was 6.030. Furthermore, Li et al.31 found that the hybrid xylanase enzyme (TXynFM) from Talaromyces thermophilus F1208 had optimum activity at pH 6.5. Also, Ping et al.32 reported that the purified xylanase from Thermoascus aurantiacus M–2 was most active at pH 5.0. In addition, the highest xylanase activity from Myceliophthora heterothallica F.2.1.4 was observed at pH 6.033. Our results showed that the purified xylanase from T. dupontii KKU–CLD–E2–3 was most active at pH 9.0, which was higher pH than those reported for other thermophilic fungi. Because this fungus could be grown in alkaline pH conditions resulted in the enzyme production by this fungus had an optimal pH higher than the others fungi which similar to that from the report from Chanwicha et al.13.
The purified xylanase from strain KKU–CLD–E2–3 remained more than 75% of the original activity in pH range 7.0–10.0 after incubation at 4 °C for 24 h. This was in contrast to Vafiadi et al.34 who found that the hybrid xylanase from Sporotrichum thermophile was stable in a pH range of 4.0–8.0. The purified xylanase from T. lanuginosus THKU–49 was stable at pH 3.5–8.030. In addition, Amo et al.35 found that the purified xylanase from M. heterothallica F.2.1.4 was stable in pH range of 4.5–9.5.
In terms of optimum temperature of enzyme, the optimum temperature of the purified enzyme from strain KKU–CLD–E2–3 was found to be 80 °C. Interestingly, the optimum temperature of this xylanase was higher than those from the other xylanase-producing thermophilic fungi. Li et al.5 reported that xylanase from T. lanuginosus CBS 288.54 was optimally active at 70–75 °C, while Maalej et al.24 reported the optimum temperature of the purified xylanase from T. thermophiles to be 75 °C. Khucharoenphaisan et al.30 found that the optimum pH of xylanase from Thermomyces lanuginosus THKU–49 was 70 °C. Also, Chanwicha et al.13 reported that the purified xylanase from T. aurantiacus var. levisporus KKU–PN–I2–1 was optimally active at 60 °C. The purified xylanase from the thermophilic fungus M. thermophila BF1–7 was 50 °C14. In addition, the maximum activity of purified xylanase from T. aurantiacus M–2 was determined at 75 °C32. As for the thermo-stability of enzyme from our strain KKU–CLD–E2–3, xylanase activity was retained more than 70% of the original activity after heating at 70 °C for 90 min. This result was similar to values for the xylanase from other strains of thermophilic fungi13,30,32.
The highest hydrolytic activity of purified xylanase was exhibited toward beechwood xylan, followed by birchwood xylan and oat spelt xylan, respectively. Our results indicated that substrate specificity of xylanase depends on type of xylan substrates. The purified xylanase in this study did not act towards Avicel, carboxymethyl cellulose and cellulose powder. These results indicated that the purified xylanase from T. dupontii KKU–CLD–E2–3 was a true xylanase and belongs to the glycosylic family G11. The xylanases of family G11 have no activity on cellulose and are the low-molecular-weight enzymes24. The Km and Vmax values of the purified xylanase in this study were 3.38 mg/mL and 625.00 µmol/min/mg, respectively. This study then provided better value of both Km and Vmax in comparison to the value previously reported from T. thermophilus (Km 22.51 mol/L and Vmax 1.235 µmol/min/mg)24, T. aurantiacus var. levisporus KKU-PN-I2-1 (Km 40.9 mol/L and Vmax 6.2 µmol/min/mg)13, M. thermophila BF1-7 (Km 9.67 mol/L and Vmax 5.38 µmol/min/mg)14, and T. aurantiacus M-2 (Km 4.81 mol/L and Vmax 467.2 µmol/min/mg)32.
The purified xylanase activity in this study was stimulated up to 277% and 235%, respectively by Cu2+ and Hg2+ but inhibited by Co2+, Ag+ and SDS at a concentration of 5 mM. In addition, xylanase activity was significantly activated by Cu2+ and Mg2+ at a concentration of 10 mM up to 248% and 259%, respectively. According to Boonrung et al.14, the purified xylanase from M. thermophila BF1–7 was activated by Cu2+, Mg2+ and Ag+ at 1 mM. The observations of Chanwicha et al. 13 showed that the xylanase from T. aurantiacus var. levisporus KKU–PN–I2–1 was stimulated by Cu2+and Mg2+ but was inhibited by Ag+ at 1 mM. Maalej et al.24 reported that the purified xylanase from T. thermophilus was stimulated by Ag+ but was inhibited by Mg2+ at 10 mM. Also, Kumar and Shukla22 found that the xylanase from T. lanuginosus VAPS24 was strongly inhibited by Cu2+ at a concentration of 10 mM. While the Hg2+ has previously been reported to completely inhibit the activity of xylanase13,14,22,24,35,36 it should be noted that our purified xylanase showed good stability in the presence of Hg2+. The active effect of Hg2+ ions is possibly due to its binding to the free SH group of thiol acid, the interaction of carboxyl group and/or the imidazole group of amino acid37, which the thiol groups are essential for enzyme activity, because thiol groups are one of the main targets of heavy metals. However, HgCl2 is a known inhibitor of thermitase through its binding to the free SH group38. Besides, the effects of metal ions and reagent to enzyme inhibition such as Co2+ and Ag2+ are mixed inhibitors, which do not bind to the active site, but to another region of the enzyme, and thus to the formation of complex with the reactive groups of the enzyme, resulted in reducing of the enzyme availability function39. In addition, the enzyme activity is inhibited by SDS, which interferes in hydrophobic regions of the enzyme, resulted in alternate of its three-dimensional structure indicating that cause enzyme denaturation40.
The purified xylanase from T. dupontii KKU–CLD–E2–3 hydrolyzed beechwood xylan to yield mainly xylotetraose, xylotriose and xylose as end products. This result indicated that the purified xylanase of this fungus was endo-xylanase. These data were in agreement with the observations of Li et al.29 which found that the xylanase from Talaromyces thermophilus F1208 mainly liberated xylotriose, xylotetraose and xylopentaose from beechwood xylan as substrates. The purified xylanase from Aspergillus carneus M34 mainly liberated xylotriose and xylotetraose from beechwood xylan41.
Conclusions
In this study, we firstly reported the amino acid sequence of the low-molecular-weight thermo-alkali-stable xylanase of T. dupontii KKU–CLD–E2–3. Until now, no previous studies have been reported on the mercury ion (Hg2+) tolerant xylanase from thermophilic fungi. Interestingly, our purified xylanase was resistant to HgCl2 (Hg2+). In addition, these enzyme characteristics suggested the high potential in the pulp and paper industry. Since the optimum pH and the temperature of purified xylanase activity are relatively similar to the pH of the initial pulp and the temperature of bleaching activity in the factory processes. The enzyme was used in bleaching processes without pH and temperature alteration in the standard bleaching process of the factory production line. In addition, purified xylanase had high specific activity and low molecular weight, which ease to access into the fiber wall structure and can efficiently hydrolyze xylan in pulp bleaching without adverted effect on the structure of cellulose in pulp fiber, resulting in an improvement of pulp and paper quality. Therefore, this enzyme can be used in place of chemicals in bleaching process, which more environmentally-friendly than the chemical bleaching. To verify the potential use of purified xylanase from T. dupontii KKU–CLD–E2–3 in bleaching step, further experiment is needed to be carried out under the actual condition, which is planned for the future.
References
Pal, A. & Khanum, F. Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: Characterization of immobilized enzyme. Proc. Biochem. 46, 1315–1322 (2011).
Verma, D. & Satyanarayana, T. Molecular approaches for ameliorating microbial xylanases. Bioresour. Technol. 117, 360–367 (2012).
Kolenová, K., Vrŝanská, M. & Biely, P. Purification and characterization of two minor endo-β-1,4-xylanases of Schizophyllum commune. Enzyme Microb. Technol. 36, 903–910 (2005).
Hatanaka, K. Incorporation of fluorous glycosides to cell membrane and saccharide chain elongation by cellular enzymes. Top Curr. Chem. 308, 291–306 (2012).
Li, X.T., Jiang, Z.Q., Li, L.T., Yang, S.Q., Feng, W.Y., Fan, J.Y. & Kusakabe, I. Characterization of a cellulase-free, neutral xylanase from Thermomyces lanuginosus CBS 288.54 and its biobleaching effect on wheat straw pulp. Bioresour. Technol. 96, 1370–1379 (2005).
Dobrev, G. & Zhekova, B. Purification and characterization of endoxylanase Xln-2 from Aspergillus niger B03. Turk. J. Biol. 36, 7–13 (2012).
Walia, A., Mehta, P., Chauhan, A., Kulshrestha, S. & Shirkot, C. K. Purification and characterization of cellulase-free low molecular weight endo β-1,4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J. Microbiol. Biotechnol. 30, 2597–2608 (2014).
Techapun, C., Poonsaran, N., Watanabe, M. & Sasaki, K. Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocess. Proc. Biochem. 38, 1327–1340 (2003).
Kalogeris, E. et al. Performance of an intermittent agitation rotating drum type bioreactor for solid-state fermentation of wheat straw. Bioresour. Technol. 86, 207–213 (2003).
Li, L., Tian, H., Cheng, Y., Jiang, Z. & Yang, S. Purification and characterization of a thermostable cellulase-free xylanase from the newly isolated Paecilomyces themophila. Enzyme Microb. Technol. 38, 780–787 (2006).
Moretti, M. M. S. et al. Selection of thermophilic and thermotolerant fungi for the production of cellulases and xylanases under solid-state fermentation. Braz. J. Microbial. 43, 1062–1071 (2012).
Bajaj, B. K. & Abbass, M. Studies on an alkali-thermostable xylanase from Aspergillus fumigatusMA28. 3Biotech1 1, 161–171 (2011).
Chanwicha, N., Katekaew, S., Aimi, T. & Boonlue, S. Purification and characterization of alkaline xylanase from Thermoascus aurantiacus var. levisporus KKU-PN-I2–1 cultivated by solid-state fermentation. Mycoscience. 56, 309–318 (2015).
Boonrunga, S., Katekaew, S., Mongkolthanaruk, W., Aimi, T. & Boonlue, S. Purification and characterization of low molecular weight extreme alkaline xylanase from the thermophilic fungus Myceliophthora thermophila BF1-7. Mycoscience 57, 408–416 (2016).
Seemakram, W., Boonrung, S., Katekaew, S., Aimi, T. & Boonlue, S. Purification and characterization of low molecular weight alkaline xylanase from Neosartorya tatenoi KKU–CLB–3–2–4–1. Mycoscience. 57, 326–333 (2016).
Seemakram, W. et al. Optimization of culture conditions for xylanase production from cellulase-free xylanase-producing thermophilic fungus, Thermomyces dupontii KKU−CLD−E2−3. Chiang Mai J. Sci. 47, 391–402 (2020).
Nelson, N. A photometric adaptation of somogyi method for the determination of glucose. J. Bio. Chem. 153, 375–380 (1944).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with folin phenol reagent. J. Biol. Chem. 193, 265–275 (1959).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Lineweaver, H. & Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56, 658–666 (1934).
Li, XL., Zhang, ZQ., Dean, JFD., Eriksson, KEL. & Ljungdahl, LG. Purification and characterization of a new xylanase (APX-II) from the fungus Aureobasidium pullulans Y-2311–1. Appl. Environ. Microbiol. 59, 3212–3218. (1993).
Kumar, V. & Shukla, P. Extracellular xylanase production from T. lanuginosus VAPS24 at pilot scale and thermostability enhancement by immobilization. Process Biochem. 71, 53–60 (2018).
Yang, Q. et al. Identification of three important amino acid residues of xylanase AfxynA from Aspergillus fumigatus for enzyme activity and formation of xylobiose as the major product. Process Biochem. 50, 571–581 (2015).
Maalej, I., Belhaj, I., Masmoudi, N. F. & Belghith, H. Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus: Purification and characterization. Applied. Biochem. Biotechnol. 158, 200–212 (2009).
Schlacher, A., Holzmann, K., Hayn, M., Steine, R. W. & Schwa, H. Cloning and characterization of the gene for the thermostable xylanase XynA from Thermomyces lanuginosus. J. Biotechnol. 49, 211–218 (1996).
Shrivastava, S., Shukla, P., Deepalakshmi, P. D. & Mukhopadhyay, K. Characterization, cloning and functional expression of novel xylanase from Thermomyces lanuginosus SS-8 isolated from self-heating plant wreckage material. World J. Microbiol. Biotechnol. 29, 2407–2415 (2013).
Fonseca-Maldonado, R. et al. Engineering the pattern of protein glycosylation modulates the thermostability of a GH11 xylanase. J. Biol. Chem. 288, 25522–25534 (2013).
Helenius, A. & Aebi, M. Intracellular functions of N-linked glycans. Science. 291, 2364–2369 (2001).
Lucena-Neto, S. A. & Ferreira-Filho, E. X. Purification and characterization of a new xylanase from Humicola grisea var. thermoidea. Braz. J. Microbiol. 35, 86–90 (2004).
Khucharoenphaisan, K., Tokuyama, S. & Kitpreechavanich, V. Purification and characterization of a high-thermostable β-xylanase from newly isolated Thermomyces lanuginosus THKU-49. Mycoscience. 51, 405–410 (2010).
Li, Q. et al. Improving special hydrolysis characterization into Talaromyces thermophilus F1208 xylanase by engineering of N-terminal extension and site-directed mutagenesis in C-terminal. Int. J. Biol. Macromol. 96, 451–458 (2017).
Ping, L. et al. Production and characterization of a novel acidophilic and thermostable xylanase from Thermoascus aurantiacu. Int. J. Biol. Macromol. 109, 1270–1279 (2018).
Amo, G. S. et al. Heterologous expression, purification and biochemical characterization of a new xylanase from Myceliophthora heterothallica F.2.1.4. International J. Biol. Macromol. 131, 798–805 (2019).
Vafiadi, C., Christakopoulos, P. & Topakas, E. Purification, characterization and mass spectrometric identification of two thermophilic xylanases from Sporotrichum thermophile. Proc. Biochem. 45, 419–424 (2010).
Lv, Z., Yang, J. & Yuan, H. Production, purification and characterization of an alkaliphilic endo-b-1,4-xylanase from a microbial community EMSD5. Enzyme Microb. Technol. 43, 343–348 (2008).
Shi, P., Du, Y., Yang, H., Huang, H., Zhang, X., Wang, Y. & Yao, B. Molecular characterization of a new alkaline-tolerant xylanase from Humicola insolens Y1. Biomed Res. Int. 1–7 (2015).
Lucas, R., Robles, A., García, M. T., Cienfuegos, G. & Alvarez de Gálvez, A. 2001 Production, purification, and properties of an endoglucanase produced by the Hyphomycete Chalara (Syn.Thielaviopsis) paradoxa CH32. J. Agric. Food Chem. 49, 79–85 (2001).
Dauter, Z., Betzel, C., Höhne, W. E., Ingelman, M. & Wilson, K. S. Crystal structure of a complex between thermitase from Thermoactinomyces vulgarisand the leech inhibitor eglin. FEBS Lett. 236, 171–178 (1998).
Xiong, K., Yan, Z. X., Liu, J. Y., Pei, P. G., Deng, L., Gao, L. & Sun, B. G. Inter domain interactions influence the substrate affinity and hydrolysis product specificity of xylanase from Streptomyces chartreusis L1105. Ann. Microbiol. 70 (2020).
Zheng, H. et al. Isolation, purification, and characterization of a thermostable xylanase from a novel strain, Paenibacillus campinasensis G1–1. J. Microbiol. Biotechnol. 22, 930–938 (2012).
Fang, H. Y., Chang, S. M., Lan, C. H. & Fang, T. J. Purification and characterization of a xylanase from Aspergillus carneus M34 and its potential use in photoprotectant preparation. Process Biochem. 43, 49–55 (2008).
Gaffney, M., Carberry, S., Doyle, S. & Murphy, R. Purification and characterisation of a xylanase from Thermomyces lanuginosus and its functional expression by Pichia pastoris. Enzyme Microb. Technol. 45, 348–354 (2009).
Yegin, S. Single-step purification and characterization of an extreme halophilic, ethanol tolerant and acidophilic xylanase from Aureobasidium pullulans NRRL Y-2311-1 with application potential in the food industry. Food Chem. 221, 67–75 (2017).
Basit, A. et al. Characterization of two endo-β-1, 4-xylanases from Myceliophthora thermophila and their saccharification efficiencies, synergistic with commercial cellulase. Front. Microbiol. 9, 233 (2018).
Acknowledgements
This study was financially supported by Protein and Proteomics Research Centre for Commercial and Industrial Purposes in FY 2016 in ongoing project No ProCCI60001 and Centre of Excellence on Biodiversity (BDC), Office of Higher Education Commission under project code BDC-PG1-163002 for partial financial support. The work was partially supported by Chiang Mai University. We are grateful to Microbial Resources and Application Group.
Author information
Authors and Affiliations
Contributions
W.S. and S.B. planned the experiment, contributed in design and selection of methodology of the experiment. S.B., T.A. and S.L. participated in the designed the experiments and performed the research. J.E. provided critical feedback and helped to edit the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seemakram, W., Boonrung, S., Aimi, T. et al. Purification, characterization and partial amino acid sequences of thermo-alkali-stable and mercury ion-tolerant xylanase from Thermomyces dupontii KKU–CLD–E2–3. Sci Rep 10, 21663 (2020). https://doi.org/10.1038/s41598-020-78670-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78670-y
This article is cited by
-
Exploring the potential of a new thermotolerant xylanase from Rasamsonia composticola (XylRc): production using agro-residues, biochemical studies, and application to sugarcane bagasse saccharification
3 Biotech (2024)
-
Purification of xylanases from Aureobasidium pullulans CCT 1261 and its application in the production of xylooligosaccharides
World Journal of Microbiology and Biotechnology (2022)
-
Application of surfactants in papermaking industry and future development trend of green surfactants
Applied Microbiology and Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.