Abstract
Revealing scaling rules is necessary for understanding the morphology, physiology and evolution of living systems. Studies of animal brains have revealed both general patterns, such as Haller's rule, and patterns specific for certain animal taxa. However, large-scale studies aimed at studying the ratio of the entire neuropil and the cell body rind in the insect brain have never been performed. Here we performed morphometric study of the adult brain in 37 insect species of 26 families and ten orders, ranging in volume from the smallest to the largest by a factor of more than 4,000,000, and show that all studied insects display a similar ratio of the volume of the neuropil to the cell body rind, 3:2. Allometric analysis for all insects shows that the ratio of the volume of the neuropil to the volume of the brain changes strictly isometrically. Analyses within particular taxa, size groups, and metamorphosis types also reveal no significant differences in the relative volume of the neuropil; isometry is observed in all cases. Thus, we establish a new scaling rule, according to which the relative volume of the entire neuropil in insect brain averages 60% and remains constant.
Similar content being viewed by others
Introduction
Large-scale studies of animal proportions supposedly started with the publication D'Arcy Wentworth Thompson's book Growth and Forms1. In fact, the first studies on the subject appeared long before the book (e.g.2), but it was Thomson's work that laid the foundations for this discipline, which, following the studies of Julian Huxley3,4, became a major fundamental and applied area of science5,6,7,8. Allometry and scaling of living systems are being studies within that area to this day. Studies of brain allometry are important for understanding the functional principles and evolution of animal nervous systems9,10,11. They have revealed both general patterns, such as Haller's rule, according to which the relative size of the brain decreases with decreasing body12, and patterns that hold true only for particular groups of animals.
Certain patterns of evolutionary and static allometry of the insect nervous system have been shown both for the entire central nervous system and brain and for particular synapse-rich neuropils of the brain. Increasing relative size with decreasing body size (according to Haller's rule) has been shown both for the brains of insects12,13,14,15,16,17,18,19,20,21,22,23,24,25,26 and for their entire central nervous systems12,16,18,25,27,28. Exceptions to this rule include particular lines in cultures of Trichogramma29 and Nasonia30. The sizes of particular synapse-rich neuropils of the brain can differ considerably between different insects and even within one species; they depend on many factors, such as the body size14,17,20,21,22,23, caste26,31,32,33,34,35,36,37,38,39,40,41, sex35,42,43,44,45,46,47,48, sociality49,50, ecology24,45,46,51, circadian rhythm type52, migratory activity46,53, and even age of the individual17,39. Ontogenetic allometry of the central nervous system, the brain, and synapse-rich neuropils has been described in insects with different types of development54,55,56,57 and others.
The ganglia and brain of arthropods have the same general organization and consist of neuropil formed by the processes of cells and of the cell body rind (cortex) formed by the bodies of these cells58. There is not much data on the total volume of the neuropil of the brain in insects, since in the majority of studies only volumes or relative volumes for a few brain regions are reported. For the few data available, the ratio of neuropil volume to cell body rind volume are similar across insects20,21,22,23, but no large-scale analysis of this ratio was performed. The purpose of this study is to analyze the ratio and allometry of the neuropil and the cell body rind in the brains of a wide range of insects.
Results and discussion
Analysis of our data and all available published data (Table 1) showed that adult insects generally have the same ratio of the total neuropil volume (NV) to brain volume (RNV) and it averages 60.5% ± 5.7. Allometric analysis for insects in general showed that the volume of the neuropil changes isometrically (the slope of the ratio of the NV to the volume of the brain (BV) or to the cell body rind volume (CV) does not differ significantly from 1; Table 2, Fig. 1). Exploratory analysis of particular taxonomic groups, size groups, and types of metamorphosis, based on samples of limited sizes, also revealed isometry and showed no significant differences between groups in RNV, slope or elevation (Table 2).
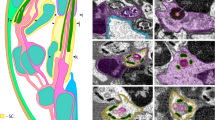
Scaling of neuropil in insect brains. (A) neuropil (green) and cell body rind (red) on a histological cross section of head of featherwing beetles Acrotrichis grandicollis (Coleoptera: Ptiliidae); (B) dependence of neuropil volume (NV) on brain volume (BV) in insects in general; (C) dependence of NV on cell body rind volume (CV) in major insect orders; (D) dependence of relative neuropil volume (RNV) on CV in major insect orders. All scales are logarithmic, except Y-axis in (D). For results of allometric analysis, see Table 2.
The only exception is the social bees, in which the average values of RNV and elevation in allometric analysis are significantly higher than in other insects (Table 2). However, these data need to be verified, because all data on social insects are taken from a single study, and they differ from data obtained earlier; for instance, for the honeybee (Apis mellifera) RNV is 64.9% in that latest study59 and 61.3% in an earlier study31. The small sample size also does not allow making final conclusions about the supposedly unusual RNV of bees. Among all the RNV values, there is one that is somewhat out of the general sample: 45% for the moth Antheraea pernyi55, but these data were obtained long ago and it is possible that using modern methods, especially 3D modeling, will correct these measurements. It is also possible that this is an interesting exception from the general rule, but lack of data on other lepidopterans makes it impossible to discuss this at present. A very low RNV value has been reported in the drone of Apis mellifera: 46.6%31, and this phenomenon requires further study. Interestingly, the eyeless mutant of Drosophila, in which the brain is almost two thirds as large as in the wild type, retains the same RNV as in the wild type57. It was repeatedly shown previously that different methods of sample preparation can change the size of structures, including the brain60, which can introduce significant variance in morphometric data. Apparently, the neuropil and body rind have similar deformation parameters in cases of different sample preparation methods, since our analysis of the data obtained by different methods shows no large deviations.
In the parasitic wasp Nasonia vitripennis (Hymenoptera: Pteromalidae) RNV in a sample consisting of the largest individuals is higher than in a sample consisting of the smallest individuals of the same species and averages 60.4 and 54.5%, respectively30. But artificial selection of individuals from opposite extremes of the body size range (as used in that study), especially for parasitoids kept in a culture, in which the characteristics of the host and population density of the parasitoid strongly affect the body size of the latter61,62, considerably expanding the reaction norm compared to natural populations63,64, produces data that could be difficult to compare with those obtained from natural populations. There are some known examples of artificial selection affecting the allometry characteristics of structures, but when artificial selection stops, allometry returns to its initial state65.
It is especially interesting that the same RNV is retained in miniature insects, which often exhibit considerable changes in the structure of the brain: asymmetry, displacement into other segments, huge relative volume, multiple reduction in the number of neurons and their sizes20,21,22,23,66. A significant decrease in the size of the cell bodies of neurons in microinsects compared with larger representatives of related groups of insects leads to changes in the nuclear-cytoplasmic ratio, a decrease in the number and size of organelles in the cell, and an increase in the level of chromatin compaction20,66,67. We showed earlier that it is the size of the cell bodies of neurons, limited by the minimum size of the nucleus, that limits the miniaturization of the central nervous system, which in turn is the most important factor limiting the minimum body size of insects67. It could be assumed that a neuropil consisting of processes of cells with a small number of organelles could tolerate miniaturization better than the cell body rind and could reduce its relative volume in miniature forms. But this is not the case: even the smallest insects have the same RNV as large insects. This is probably due to the fact that the efficiency of neurons depends on the diameter of their processes. As calculated earlier, the noise effects of ion channels make it impossible to transmit impulses along axons with a diameter of less than 80 nm68, and these physical limitations probably limit the decrease in the neuropil volume.
A special place is occupied by the parasitic wasp Megaphragma (Hymenoptera: Trichogrammatidae), in which about 98% of the brain volume is occupied by the neuropil, due to the fact that the central nervous system of the adult is almost anucleate in all studied species of this genus69,70,71. Because of these fundamental differences in brain organization, Megaphragma was excluded from our analysis in this study.
The same ratio of the neuropil and the cell body rind that we describe for the insect brain is also found in measurements of the total central nervous system of insects and other arthropods. The relative neuropil volume of the entire central nervous system (RNVcns) and of particular thoracic ganglia separately for the parasitic wasp Trichogramma telengai is no different from RNV, and only the abdominal ganglia have a slightly lower relative neuropil volume72. In the moth Antheraea pernyi, the relative volume of the neuropil of the mesothoracic ganglion is 65%, and that of abdominal ganglion 4 is 53%55. In the house cricket (Acheta domesticus), the relative volume of neuropil is 66–73% in the thoracic ganglia, and 63% in the last abdominal ganglion56. In the collembolan Orchesella villosa, by the age of the start of breeding, RNV is about 70%, but with subsequent molts it can reach 84% by the time of death73. In the spider Eratigena atrica, the relative volume of the neuropil is 61.1%74. In the spider Argiope aurantia it is 71.3%75. Unfortunately, at present there is not enough data for a comprehensive analysis of RNV and RNVcns in arthropods in general, but it is possible that a large-scale study will eventually reveal common patterns.
Interestingly, although RNV remains constant, the relative volumes of particular synapse-rich neuropils of the brain can vary considerably between different insect species or even within the same species, and the sizes of particular synapse-rich neuropils depend on many factors (for review, see “Introduction”). Furthermore, an increase in the relative sizes of the synapse-rich neuropils of one modality occurs at the expense of a decrease in the sizes of neuropils of other modalities or the size of undifferentiated neuropil46,51,52,76 and others. It is probably due to such compensations that RNV remains constant.
The structural plans of the brains of insects and vertebrates are fundamentally different and it is difficult to make direct comparisons. However, interestingly, the “wire fraction” (percentage of axons and dendrites) in different parts of the mouse brain is 3/5, and this is consistent with mathematical calculations of wiring optimization77. At the same time, the percentage of the cerebral cortex, which is occupied by the neuropil in humans and chimpanzees, differ considerably between different regions of the brain, 63–71% in chimpanzees and 77–84% in humans78. There are also a number of studies in which the volumes of the white and gray matters are evaluated. The relative volume of the gray matter decreases significantly with increasing body size and increasing number of neurons, and slopes and elevations differ between groups79,80,81. The relative volume of the gray matter in vertebrates varies between species within a very wide range, from 93% in the mouse Mus musculus to 66% in humans and to 50% in the elephant Loxodonta africana81. Thus, it can be assumed that the vertebrate brain shows a fundamentally higher diversity in the ratio of the neuropil to cellular regions than the insect brain. However, there is still not enough data for a large-scale analysis of different groups of animals.
Conclusion
Thus, our large-scale analysis reveals a new scaling rule, according to which the ratio of the neuropil to the cell body rind of the brain of adult insects is the same (3:2) and the relative volume of the entire neuropil is constant and averages 60% of the brain volume.
Methods
To analyze the relative volumes of the neuropil of the brain (RNV, the ratio of the total volume of the neuropil to the volume of the brain), 3D reconstructions of the brain made in the Bitplane Imaris program based on a series of histological sections were used. For these sections, the material was fixed in FAE (formaldehyde, acetic acid, and ethanol) and embedded in Araldite. The resulting blocks were used to make complete series sections 0.5–2 µm thick with a Leica RM2255 microtome. For 3D computer modeling, the series of sections were photographed under a Motic BA410 microscope. After, followed by the alignment of the resulting stack with FEI Amira. All structures were outlined manually and automatically recalculated as three-dimensional withusing Bitplane Imaris. The volumes of the brain and neuropil wereas calculated using 3D reconstructions in the Bitplane Imaris statistical module. The detailed methodology for processing the material and obtaining volumetric data has been described earlier22,25,66. The data on the adults of 24 species based on our original models are analyzed and published data are used for 13 other species (Table 2). A total of 37 species of 26 families and ten orders are analyzed, ranging in sizes from the smallest to the largest by a factor of over 4,000,000 by body volume and by a factor of 20,000 by brain volume. We used the classical definition of the brain as the supraesophageal ganglion (= supraesophageal zone82). Data analysis was performed in R using ANOVA to compare average values for samples and the smatr package83 for allometric analysis, using the standardized major axis (SMA). All analyzes were performed for all insects and in all four groups of samples (size group, orders, type of development, and sociality); in the groups of samples the values were compared between samples within the group (Table 2).
References
Thompson, D. A. W. On Growth and Form (Cambridge University Press, Cambridge, 1917).
Snell, O. Die Abhängigkeit des Hirngewichtes von dem Körpergewicht und den geistigen Fähigkeiten. Arch. Psychiatr. Nervenkr. 23, 436–446 (1892).
Huxley, J. S. Constant differential growth-ratios and their significance. Nature 114, 895–896 (1924).
Huxley, J. S. Problems of Relative Growth (Methuen and Co., Ltd., London, 1932).
McMahon, T. & Bonner, J. On Size and Life (Scientific American Books; W. H. Freeman & Co, New York, 1983).
Schmidt-Nielsen, K. Scaling: Why Is Animal Size So Important? (Cambridge Univ. Press, London, 1984).
Shmalhauzen, I. I. Pocт и диффepeнциpoвкa (Growth and differentiation). (Naukova Dumka, Kyiv, 1984).
Calder, W. A. Size, Function, and Life History (Dover Publications Inc., New York, 1996).
Striedter, G. F. Principles of Brain Evolution (Sinauer Associates is an imprint of Oxford University Press, Sunderland, 2005).
Chittka, L. & Niven, J. Are bigger brains better?. Curr. Biol. 19, R995–R1008 (2009).
Montgomery, S. H., Mundy, N. I. & Barton, R. A. Brain evolution and development: Adaptation, allometry and constraint. Proc. R. Soc. B 283, 20160433 (2016).
Rensch, B. Histological changes correlated with evolutionary changes of body size. Evolution 2, 218–230 (1948).
Beutel, R. G., Pohl, H. & Hünefeld, F. Strepsipteran brains and effects of miniaturization (Insecta). Arthropod. Struct. Dev. 34, 301–313 (2005).
Mares, S., Ash, L. & Gronenberg, W. Brain allometry in bumblebee and honey bee workers. Brain Behav. Evol. 66, 50–61 (2005).
Wehner, R., Fukushi, T. & Isler, K. On being small: Brain allometry in ants. Brain Behav. Evol. 69, 220–228 (2007).
Polilov, A. A. Anatomy of the smallest coleoptera, featherwing beetles of the tribe nanosellini (Coleoptera, Ptiliidae), and limits of insect miniaturization. Zool. Zh. 87, 181–188 (2008). (Entomol. Rev. 88(1): 26–33).
Riveros, A. J. & Gronenberg, W. Brain allometry and neural plasticity in the bumblebee Bombus occidentalis. Brain Behav. Evol. 75, 138–148 (2010).
Eberhard, W. G. & Wcislo, W. T. Grade changes in brain-body allometry. Adv. Insect Physiol. 40, 155–214 (2011).
Seid, M., Seid, M. A., Castillo, A. & Wcislo, W. T. The allometry of brain miniaturization in ants. Brain Behav. Evol. 77, 5–13 (2011).
Makarova, A. A. & Polilov, A. A. Peculiarities of the brain organization and fine structure in small insects related to miniaturization. 1. The smallest Coleoptera (Ptiliidae). Zool. Zh. 92, 523–533 (2013). (Entomol. Rev. 93: 703–713).
Makarova, A. A. & Polilov, A. A. Peculiarities of the brain organization and fine structure in small insects related to miniaturization. 2. The smallest Hymenoptera (Mymaridae, Trichogrammatidae). Zool. Zh. 92, 695–706 (2013). (Entomol. Rev. 93: 714–724).
Makarova, A. A. & Polilov, A. A. Peculiarities of the brain organization and fine structure in small insects related to miniaturization. 3. Barklice (Psocoptera, Liposcelididae). Zool. Zh. 96, 275–288 (2017). (Entomol. rev. 97(3): 288–301).
Makarova, A. A. & Polilov, A. A. Peculiarities of the brain organization and fine structure in small insects related to miniaturization. 4. Thrips (Thysanoptera, Thripidae). Zool. Zh. 96, 410–417 (2017). (Entomol. rev. 97(3): 302–309).
Bulova, S., Purce, K., Khodak, P., Sulger, E. & O’Donnell, S. Into the black and back: The ecology of brain investment in Neotropical army ants (Formicidae: Dorylinae). Sci. Nat. 103, 31 (2016).
Polilov, A. A. & Makarova, A. A. The scaling and allometry of organ size associated with miniaturization in insects: A case study for Coleoptera and Hymenoptera. Sci. Rep. 7, 43095 (2017).
O’Donnell, S., Bulova, S., Barrett, M. & von Beeren, C. Brain investment under colony-level selection: Soldier specialization in Eciton army ants (Formicidae: Dorylinae). BMC Zool. 3, 3 (2018).
Goossen, H. Untersuchungen an Gehirnen verschieden großer jeweils verwandter Coleopteren- und Hymenopterenarten. Zool. Jahrb. Abt. Allg. Zool. Physiol. Tiere 62, 1–64 (1949).
Polilov, A. A. & Beutel, R. G. Miniaturisation effects in larvae and adults of Mikado sp. (Coleoptera: Ptiliidae), one of the smallest free-living insects. Arthropod. Struct. Dev. 38, 247–270 (2009).
van der Woude, E., Smid, H. M., Chittka, L. & Huigens, M. E. Breaking Haller’s rule: Brain-body size isometry in a minute parasitic wasp. Brain Behav. Evol. 81, 86–92 (2013).
Groothuis, J. & Smid, H. M. Nasonia parasitic wasps escape from Haller’s rule by diphasic, partially isometric brain-body size scaling and selective neuropil adaptations. Brain Behav. Evol. 90, 243–254 (2017).
Witthöft, W. Absolute anzahl und verteilung der zellen im him der honigbiene. Z. Morphol. Oekol. Tiere 61, 160–184 (1967).
Farris, S. M., Robinson, G. E. & Fahrbach, S. E. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the adult worker honeybee. J. Neurosci. 21, 6395–6404 (2001).
Ismail, N., Robinson, G. E. & Fahrbach, S. E. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc. Natl. Acad. Sci. USA 103, 207–211 (2006).
Groh, C., Lu, Z., Meinertzhagen, I. A. & Rossler, W. Age-related plasticity in the synaptic ultrastructure of neurons in the mushroom body calyx of the adult honeybee Apis mellifera. J. Comp. Neurol. 520, 3509–3527 (2012).
Streinzer, M., Kelber, C., Pfabigan, S., Kleineidam, C. J. & Spaethe, J. Sexual dimorphism in the olfactory system of a solitary and a eusocial bee species. J. Comp. Neurol. 521, 2742–2755 (2013).
Molina, Y. & O’Donnell, S. Mushroom body volume is related to social aggression and ovary development in the paperwasp Polistes instabilis. Brain Behav. Evol. 70, 137–144 (2007).
Gronenberg, W. & Liebig, J. Smaller brains and optic lobes in reproductive workers of the ant Harpegnathos. Naturwissenschaften 86, 343–345 (1999).
Gronenberg, W. & Holldobler, B. Morphologic representation of visual and antennal information in the ant brain. J. Comp. Neurol. 412, 229–240 (1999).
Julian, G. E. & Gronenberg, W. Reduction of brain volume correlates with behavioral changes in queen ants. Brain Behav. Evol. 60, 152–164 (2002).
Riveros, A. J., Seid, M. A. & Wcislo, W. T. Evolution of brain size in class-based societies of fungus-growing ants (Attini). Anim. Behav. 83, 1043–1049 (2012).
O’Donnell, S., Bulova, S. J., DeLeon, S., Barrett, M. & Fiocca, K. Caste differences in the mushroom bodies of swarm-founding paper wasps: Implications for brain plasticity and brain evolution (Vespidae, Epiponini). Behav. Ecol. Sociobiol. 71, 116 (2017).
El Jundi, B., Huetteroth, W., Kurylas, A. E. & Schachtner, J. Anisometric brain dimorphism revisited: Implementation of a volumetric 3D standard brain in Manduca sexta. J. Comp. Neurol. 517, 210–225 (2009).
Kondoh, Y., Kaneshiro, K. Y., Kimura, K. & Yamamoto, D. Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc. R. Soc. Lond. B 270, 1005–1013 (2003).
Ehmer, B. & Gronenberg, W. Mushroom body volumes and visual interneurons in ants: Comparison between sexes and castes. J. Comp. Neurol. 469, 198–213 (2004).
Farris, S. M. & Roberts, N. S. Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc. Natl. Acad. Sci. USA 102, 17394–17399 (2005).
Ott, S. R. & Rogers, S. M. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. R. Soc. B 277, 3087–3096 (2010).
Kuebler, L. S., Kelber, C. & Kleineidam, C. J. Distinct antennal lobe phenotypes in the leaf-cutting ant (Atta vollenweideri). J. Comp. Neurol. 518, 352–365 (2010).
Mysore, K. et al. Caste and sex specific olfactory glomerular organization and brain architecture in two sympatric ant species Camponotus sericeus and Camponotus compressus (Fabricius, 1798). Arthropod. Struct. Dev. 38, 485–497 (2009).
O’Donnell, S., Donlan, N. & Jones, T. Developmental and dominance-associated differences in mushroom body structure in the paper wasp Mischocyttarus mastigophorus. Dev. Neurobiol. 67, 39–46 (2006).
O’Donnell, S. et al. Distributed cognition and social brains: Reductions in mushroom body investment accompanied the origins of sociality in wasps (Hymenoptera: Vespidae). Proc. R. Soc. B 282, 20150791 (2015).
Montgomery, S. H., Merrill, R. M. & Ott, S. R. Brain composition in Heliconius butterflies, posteclosion growth and experience-dependent neuropil plasticity. J. Comp. Neurol. 524, 1747–1769 (2016).
Stöckl, A. et al. Differential investment in visual and olfactory brain areas reflects behavioural choices in hawk moths. Sci. Rep. 6, 26041 (2016).
Heinze, S., Florman, J., Asokaraj, S., El Jundi, B. & Reppert, S. M. Anatomical basis of sun compass navigation II: The neuronal composition of the central complex of the monarch butterfly. J. Comp. Neurol. 521, 267–298 (2013).
Power, M. E. A quantitative study of the growth of the central nervous system of a holometabolous insect, Drosophila melanogaster. J. Morphol. 91, 389–411 (1952).
Panov, A. A. Growth of the ganglia of the central nervous system in the Chinese tussar moth (Antheraea pernyi Guér., Lepid) during individual development. Zool. Zh. 40, 694–706 (1961).
Panov, A. A. Postembryonic growth of ganglia of the central nervous system in the house cricket (Gryllus domesticus L., Orthoptera, Insecta). Dokl. Akad. Nauk SSSR 139, 230–233 (1961).
Hinke, W. Das relative postembryonale wachstum der hirnteile von Culex pipiens, Drosophila melanogaster und Drosophila-mutanten. Z. Morphol. Oekol. Tiere 50, 81–118 (1961).
Bullock, T. H. & Horridge, G. A. Structure and Function in the Nervous Systems of Invertebrates (W. H. Freeman a. Comp. Ltd., New York, 1965).
Gowda, V. & Gronenberg, W. Brain composition and scaling in social bee species differing in body size. Apidologie 50, 779–792 (2019).
Healy, S. D. & Rowe, C. A critique of comparative studies of brain size. Proc Biol Sci 274, 453–464 (2007).
Godfray, H. C. J. Parasitoids. Behavioural and Evolutionary Ecology (Princeton University Press, Princeton, 1994).
Rivers, D. B. & Denlinger, D. L. Fecundity and development of the ectoparasitic wasp Nasonia vitripennis are dependent on host quality. Entomol. Exp. Appl. 76, 15–24 (1995).
West, S. A., Flanagan, K. E. & Godfray, H. C. J. The relationship between parasitoid size and fitness in the field, a study of Achrysocharoides zwoelferi (Hymenoptera: Eulophidae). J. Anim. Ecol. 65, 631–639 (1996).
Ellers, J., Jacques, J. M. V. A. & Sevenster, J. G. A field study of size-fitness relationship in the parasitoid Asobara tabida. J. Anim. Ecol. 67, 318–324 (1998).
Bolstad, G. H. et al. Complex constraints on allometry revealed by artificial selection on the wing of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 112, 13284–13289 (2015).
Polilov, A. A. At the Size Limit—Effects of Miniaturization in Insects (Springer International Publishing, Berlin, 2016).
Polilov, A. A. Small is beautiful: Features of the smallest insects and limits to miniaturization. Annu. Rev. Entomol. 60, 103–121 (2015).
Faisal, A. A., White, J. A. & Laughlin, S. B. Ion-channel noise places limits on the miniaturization of the brain’s wiring. Curr. Biol. 15, 1143–1149 (2005).
Polilov, A. A. The smallest insects evolve anucleate neurons. Arthropod. Struct. Dev. 41, 29–34 (2012).
Polilov, A. A. Anatomy of adult Megaphragma (Hymenoptera: Trichogrammatidae), one of the smallest insects, and new insight into insect miniaturization. PLoS ONE 12, e0175566 (2017).
Polilov, A. A. First record of Megaphragma (Hymenoptera, Trichogrammatidae) in Columbia, and third animal species known to have anucleate neurons. J. Hymenopt. Res. 60, 181–185 (2017).
Makarova, A. A., Veko, E. N. & Polilov, A. A. Metamorphosis of the central nervous system Trichogramma telengai (Hymenoptera: Trichogrammatidae). Arthropod. Struct. Dev. 60, 101005 (2021).
Korr, H. Das postembryonale Wachstum verschiedener Hirnbereiche bei Orchesella villosa L. (Ins., Collembola). Z. Morphol. Oekol. Tiere 62, 389–422 (1968).
Napiorkowska, T. & Kobak, J. The allometry of the central nervous system during the postembryonic development of the spider Eratigena atrica. Arthropod. Struct. Dev. 46, 805–814 (2017).
Babu, K. S. Post embryonic development of the central nervous system of the spider Argiope aurantia (Lucas). J. Morphol. 146, 325–342 (1975).
O’Donnell, S. et al. Brain size and visual environment predict species differences in paper wasp sensory processing brain regions (hymenoptera: vespidae, polistinae). Brain Behav. Evol. 82, 177–184 (2013).
Chklovskii, D. B., Schikorski, T. & Stevens, C. F. Wiring optimization in cortical circuits. Neuron 34, 341–347 (2002).
Spocter, M. A. et al. Neuropil distribution in the cerebral cortex differs between humans and chimpanzees. J. Comp. Neurol. 520, 2917–2929 (2012).
Zhang, K. & Sejnowski, T. J. A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Natl. Acad. Sci. USA 97, 5621–5626 (2000).
Ventura-Antunes, L., Mota, B. & Herculano-Houzel, S. Different scaling of white matter volume, cortical connectivity, and gyrification across rodent and primate brains. Front. Neuroanat. 7, 3 (2013).
Mota, B. et al. White matter volume and white/gray matter ratio in mammalian species as a consequence of the universal scaling of cortical folding. Proc. Natl. Acad. Sci. USA 116, 15253–15261 (2019).
Ito, K. et al. A systematic nomenclature for the insect brain. Neuron 81, 755–765 (2014).
Warton, D. I., Wright, I. J., Falster, D. S. & Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259 (2006).
Neder, R. Allometrisches Wachstum von Hirnteilen bei drei verschieden grofien Schabenarten. Zool. Jahrb. Abt. Anat. Ontog. Tiere 77, 411–464 (1959).
Johansson, A. S. The nervous system of the milkweed bug, Oncopeltus fasciatus (Dallas) (Heteroptera, Lygaeidae). Trans. Am. Entomol. Soc. 83, 119–183 (1957).
Strausfeld, N. J. Atlas of an Insect Brain (Springer, Berlin, 1976).
Acknowledgements
This study was supported by the Russian Science Foundation (project no. 19-74-10019 to A.A.M., study of brain structure; project no. 19-14-00045 to A.A.P., data analysis and preparation of manuscript).
Author information
Authors and Affiliations
Contributions
A.A.P. designed the study; A.A.P. and A.A.M. collected and analyzed the data; A.A.P. wrote and edited the manuscript; A.A.P. and A.A.M. approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Polilov, A.A., Makarova, A.A. Constant neuropilar ratio in the insect brain. Sci Rep 10, 21426 (2020). https://doi.org/10.1038/s41598-020-78599-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78599-2
This article is cited by
-
Extremely small wasps independently lost the nuclei in the brain neurons of at least two lineages
Scientific Reports (2023)
-
Miniaturization does not change conserved spider anatomy, a case study on spider Rayforstia (Araneae: Anapidae)
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.