Abstract
Recent studies have shown that long-term alcohol intake from food can lead to numerous mental disorders in humans, and the social and health effects of excessive intake of alcohol currently represent serious problems for governments and families worldwide. However, to date, it has not been determined how alcohol affects the hypothalamic–pituitary–adrenal (HPA) axis. The zebrafish offers a good model for studying the toxicology of food-grade ethanol. In the present study, using zebrafish larvae exposed to 1% ethanol, we performed zebrafish behavioral analysis. Samples were collected for enzyme-linked immunosorbent assay (ELISA) and quantitative real time-polymerase chain reaction (qRT-PCR) experiments, and statistical analysis was performed. We found that ethanol decreased the locomotor activity of zebrafish larvae, which showed a more intense reaction to external stimuli. Ethanol also increased the level of HPA axis hormones in zebrafish larvae, influenced the level of neurotransmitters, and altered the expression of key genes in neurotransmitter metabolism. Ethanol exposure affects zebrafish behavior, increases the level of HPA axis hormones in zebrafish larvae, affects the level of neurotransmitters, and affects the expression of key genes in dopamine and serotonin metabolism. These findings may help to elucidate the effects of ethanol on HPA axis activity.
Similar content being viewed by others
Introduction
Ethanol is a substance consumed daily by humans that can cause considerable harm to human health, especially in minors1. Indeed, the social and health effects of alcoholism are among the most serious problems faced by governments and families every year2. Recent studies have found that long-term consumption of ethanol can lead to numerous physical2, behavioral3, and mental disorders4. Furthermore, drinking during pregnancy can have a serious impact on fetal development, leading to fetal alcohol syndrome5. The main manifestations of this syndrome are skull deformity, cardiovascular dysplasia, and permanent nervous system damage6. Long-term drinking can also increase the risk of stroke, dementia, and hypertension, increase blood lipid concentration, and lead to a higher incidence of various cardiovascular and cerebrovascular diseases7. Because the liver and kidney are the primary organs of alcohol metabolism, acute alcoholism can lead to alcoholic liver and kidney diseases5. The most common pathological changes in alcoholic liver are a decrease in the rough endoplasmic reticulum, swelling and deformation of the mitochondria, and the occurrence of liver cirrhosis8. Epidemiological studies have also demonstrated that alcoholics are more likely to develop liver cancer. More recent studies have revealed that long-term drinking is associated with major mental disorders, such as depression9.
Numerous studies on alcohol toxicity have been based on the zebrafish model10. The zebrafish (Danio rerio), as a vertebrate, has a genome with a high degree of homology with that of humans, which makes it a valuable model for developmental, neurological, and toxicological research11. For example, Lockwood et al.12 found that embryonic exposure to ethanol increased the susceptibility of larval zebrafish to chemically induced seizures11. Similarly, Matsui13 found that high concentrations of ethanol can lead to abnormal development of the visual system, while even low concentrations of ethanol can lead to behavioral disturbances12. Carva et al.14 found that the learning ability of adult and juvenile zebrafish treated with ethanol decreased significantly14. Accordingly, zebrafish is a good model for studying the toxicology of ethanol. While ethanol has been observed to cause depressive phenotypes in behavioral experiments, the underlying mechanism of this effect has not been elucidated.
The hypothalamic–pituitary–adrenal (HPA) axis is a bodily system that involves the hypothalamus, pituitary gland, and adrenal gland and is activated in response to environmental stress15. Specifically, the hypothalamus secretes corticotropin releasing-hormone (CRH) which, in turn, acts on the pituitary gland to release adreno-cortico-tropic-hormone (ACTH). ACTH then acts on the adrenal gland to secrete glucocorticoids (GCs), which regulate HPA axis activity through glucocorticoid receptors (GR or nr3c1)16. Cortisol is the main glucocorticoid in zebrafish, while corticosterone is the main glucocorticoid in rodents17. The HPA axis is an important aspect of the neuroendocrine system, as it controls the stress response and regulates numerous physical activities 16. Numerous neurotransmitters are involved in regulating hypothalamic activity which, in turn, regulates the activity of the entire HPA axis, including specific changes in mood18. Clinical studies have shown that many patients with depression exhibit decreased GR expression and increased HPA axis activity19. On day 4 postfertilization (dpf), zebrafish show a fully functional HPA axis, and the HPA axis of zebrafish has remarkably similar functions. Indeed, zebrafish have been successfully utilized in studies on the HPA axis. Thus, zebrafish offer a good model for studying the function of the HPA axis20.
To determine whether the HPA axis is a target of ethanol, in this study, we exposed zebrafish larvae to 1% ethanol and examined their subsequent behavior, hormone levels, neurotransmitter levels, and gene expression levels. We attempted to determine the effects of ethanol as a stress factor on zebrafish behavior related to the HPA axis.
Results
Exposure to ethanol affects zebrafish behavior
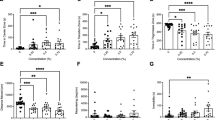
To investigate whether ethanol affects the autonomous behavior of zebrafish, we selected 120 hpf control and ethanol-exposed zebrafish larvae for behavioral analysis. The results showed that under identical conditions, the swimming distance of zebrafish exposed to 1% ethanol was significantly shorter than that of the control fish (Fig. 1A), while the swimming speed per unit time was significantly lower (Fig. 1B). Furthermore, the average swimming time per unit time of the ethanol group was significantly lower than that of the control group (Fig. 1C). Taken together, these results suggest that exposure to ethanol decreases the locomotor activity of zebrafish larvae.
Effects of ethanol exposure on zebrafish locomotor activity. After exposure to alcohol, we investigated the behavior of 120 hpf zebrafish larvae. (A) Total swimming distance in 10 min. (B) Swimming speed in 1 min. (C) Swimming time in 1 min. (D) Swimming distance in LD time intervals. (E) Total swimming distance in LD time intervals. Each group, n = 24. Student’s t-test was conducted. *p < 0.05; **p < 0.01; ***p < 0.001.
To test the exposed zebrafish’s ability to respond to external stimuli, we observed their responsiveness to alternating stimulation of light and dark (30 min of light and 30 min of darkness). Compared to the control group, the ethanol group showed significantly greater activity under light conditions (Fig. 1D,E) but a significantly reduced swimming distance under dark conditions (Fig. 1D,E). These findings indicate that the ethanol-exposed zebrafish had a more intense reaction to external stimuli.
Exposure to ethanol affects the level of HPA axis hormones
To investigate whether ethanol exposure affects zebrafish HPA axis activity, we used ELISA to detect the levels of related hormones. The results showed that the level of CRH was significantly elevated after exposure to ethanol at 3, 4, 5, and 6 dpf (Fig. 2A), as was the level of ACTH (Fig. 2B) and the level of cortisol (Fig. 2C). Taken together, these results suggest that ethanol increases the level of HPA axis hormones in zebrafish larvae.
Effects of ethanol on HPA axis hormone levels in zebrafish larvae. Samples were taken at 3, 4, 5, and 6 dpf. (A) CRH levels from 3 to 6 dpf. (B) ACTH levels from 3 to 6 dpf. (C) Cortisol levels from 3 to 6 dpf. Three independent experiments were conducted. Student’s t-test was performed. *p < 0.05; **p < 0.01; ***p < 0.001.
Exposure to ethanol affects the expression of HPA axis genes
To further investigate how ethanol affects HPA axis activity, we performed qRT-PCR analysis to investigate HPA axis gene expression. We found that the expression of crha exhibited no significant change at 3 and 4 dpf, but there was a significantly greater upregulation at 5 and 6 dpf in the ethanol group compared with the control group (Fig. 3A). Furthermore, the expression of the crhb gene showed a significant increase at 4, 5, and 6 dpf compared with the control group (Fig. 3B); there was no significant difference at 3 dpf (Fig. 3B). The expression of pomca and pomcb increased significantly with development time. Specifically, the expression of pomca showed clear upregulation at 4, 5, and 6 dpf, but there was no significant difference at 3 dpf (Fig. 3C). The expression of pomcb did not show a significant difference at 3 and 4 dpf, but it did show significant upregulation at 5 and 6 dpf in the ethanol group (Fig. 3D). The expression of nr3c1 increased with the development time, although there was a slight decrease in expression at 6 dpf, and the ethanol group did not show significant differences from the control group (Fig. 3E). Taken together, these results indicate that the HPA axis gene expression patterns of zebrafish larvae were notably affected by exposure to ethanol.
HPA axis gene expression patterns in zebrafish larvae after ethanol exposure. Samples were taken at 3, 4, 5, and 6 dpf. The expression patterns of (A) crha, (B) crhb, (C) pomca, (D) pomcb, and (E) gr. Three independent experiments were conducted. Student’s t-test was performed. *p < 0.05; **p < 0.01; ***p < 0.001.
Exposure to ethanol affects the level of neurotransmitters
Numerous monoamine neurotransmitters play important roles in the regulation of the HPA axis, particularly the regulation of an individual’s behavior and mood. To investigate whether ethanol exposure influences the level of neurotransmitters, particularly dopamine and serotonin, we used ELISA. The results showed that the level of dopamine showed a significantly stronger increase at 3, 5, and 6 dpf in the ethanol group than in the control group, although it decreased at 4 dpf (Fig. 4A). Serotonin, on the other hand, showed significantly stronger increases at all four time points in the ethanol group compared to the control group (Fig. 4B). These results indicate that exposure to ethanol influences the level of neurotransmitters.
Level of neurotransmitters in zebrafish larvae after ethanol exposure. Samples were taken at 3, 4, 5, and 6 dpf. (A) Dopamine concentration from 3 to 6 dpf. (B) 5-HT concentration from 3 to 6 dpf. Three independent experiments were conducted. Student’s t-test was performed. *p < 0.05; **p < 0.01; ***p < 0.001.
Exposure to ethanol affects the expression of key genes in dopamine and serotonin metabolism
Key genes act as rate-limiting enzymes in the synthesis and metabolism of certain neurotransmitters. We selected three key genes that served as rate-limiting enzymes for the above two neurotransmitters and performed qRT-PCR analysis. The results showed that compared with the control group, the expression of monoamine oxidase-a(maoa) significantly increased at 4, 5, and 6 dpf in the ethanol group; however, there was no significant difference at 3 dpf (Fig. 5A). Furthermore, the expression of tyrosine hydroxylase(th) was significantly greater in the ethanol group at 5 and 6 dpf but showed no significant difference at 3 and 4 dpf (Fig. 5B). Finally, the expression of dopamine β-hydroxylase(dbh) was significantly downregulated at 5 dpf in the ethanol-treated group compared with the control group (Fig. 5C). Thus, exposure to ethanol affects the expression of key genes during neurotransmitter metabolism.
Neurotransmitter metabolism gene expression patterns in zebrafish larvae after ethanol exposure. Samples were taken at 3, 4, 5, and 6 dpf. The expression patterns of (A) maoa, (B) th, and (C) dbh were observed. Three independent experiments were conducted. Student’s t-test was performed. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Zebrafish are a good model organism for studying neuroendocrine and behavioral effects20. There are numerous established behavioral and physiological tests and methods for generating genetic mutations in zebrafish, and numerous studies have utilized zebrafish to study ethanol toxicology21. In this study, we employed behavioral experiments, hormone measurements, and gene expression analyses to explore the effects of ethanol and found that ethanol affected zebrafish larvae activity, increased HPA axis activity, and disrupted neurotransmitter metabolism.
Exposure to 1% ethanol led to changes in the swimming behavior of zebrafish, including decreased swimming distance and speed. Furthermore, the light–dark alteration experiment showed that ethanol-exposed zebrafish showed significantly more activity in the light and lower activity under dark conditions than did the zebrafish in the control group. In the natural environment, sudden darkness is a dangerous environment for animals and can cause animals to increase their activity22. Thiago Acosta et al. reported that alcohol impairs predation risk response and communication in zebrafish27. This finding suggests that under light conditions, compared with the control zebrafish, 1% ethanol-treated zebrafish had significantly higher activity,while a significantly reduced swimming distance under dark conditions.
As noted above, the HPA axis is the primary system by which animals respond to changes in their external environment and stress, and the HPA axes of zebrafish and humans are highly similar24. Past studies have shown that the shallow water test leads to a clear increase in cortisol level and HPA activity25. Previous studies revealed that low concentrations of alcohol decrease cortisol production26, but our results show that 1% alcohol increases cortisol production27. In contrast to previous results, alcohol exposure reduces HPA axis activity22,23,24, and we think two reasons may explain this difference. First, for the exposure time, we exposed zebrafish larvae at 24 hpf; at this time, the HPA axis matured early, indicating that early-stage alcohol exposure can affect HPA axis activity. Second, in our experiment, ethanol exposure lasted for a long time, which was one of the reasons for the difference in results from the previous study.
GR is the core molecule in the HPA axis that regulates downstream gene expression. GR expression is usually downregulated in patients with depression28. In zebrafish, deletion of the gr gene can also lead to mood disorder29. In our study, we saw a trend to decrease in the expression of the gr gene. In addition, we found no difference in HPA axis activity at 3 and 4 dpf, possibly because the zebrafish larvae did not form a fully functional HPA axis before 4 dpf30. In addition, numerous compounds encountered during pregnancy can lead to epigenetic changes in gr31 which, along with development, have an effect on adolescents’ personality and mood32. Whether embryo or maternal alcohol exposure can lead to differences in the epigenetic regulation of zebrafish gr and mood changes in later developmental stages warrants further investigation.
The HPA axis is a neuroendocrine regulation axis linked to the level of neurotransmitters associated with the development of mental illness33,34,35, particularly dopamine36 and serotonin33,35. We found significant increases in the levels of both neurotransmitters after exposure to ethanol along with increased expression of the maoa and th genes and decreased expression of the dbh gene. The th37 is a rate-limiting enzyme in serotonin synthesis, dbh38 converts dopamine into norepinephrine, and maoa34,36 genes encode rate-limiting enzymes in dopamine metabolism. Thus, our results suggest that ethanol exposure increases the levels of dopamine and serotonin, perhaps partly due to decreased dbh. Previous studies have also found that in the hypothalamus, dopaminergic and serotonergic neurons can project to ACTH neurons to regulate the HPA axis37; however, the detailed mechanism of this projection has not been elucidated. Whether ethanol exposure leads to changes in neurotransmitters that, in turn, cause behavioral changes or whether ethanol exposure directly affects behavioral abnormalities caused by HPA axis activity is a matter for further research.
Conclusion
Ethanol exposure was observed to reduce zebrafish locomotor activity, increase HPA axis activity, and lead to significant changes in the levels of dopamine and serotonin. These results provide a new understanding of the effects of ethanol on the HPA axis.
Methods and materials
All experimental procedures were approved by the Yantai University Animal Care and Use Committee and were in accordance with governmental regulations of China.
Fish maintenance
Adult zebrafish of the AB line were raised in a recirculating water system under a 14/10-h light/dark (L/D) cycle at 28 °C. The zebrafish were fed three times per day. To produce embryos, two male and two female adult zebrafish were paired in one 2-L tank in the evening, and spawning occurred the next day within 1 h after the lights were switched on. The embryos were placed in 10-cm Petri dishes with egg water containing methylene blue (0.3 ppm) and were raised in a light-controlled (14/10-h L/D) incubator at 28 °C.
Ethanol treatment procedure
According to data obtained in a previous experiment27, 1% ethanol significantly alters zebrafish larvae behavior and gene expression; therefore, at 24 h postfertilization (hpf), 50 larvae were exposed to 25 ml 1% ethanol (directly dissolved in E3 solution) in each petri dish. A total of 500 embryos were treated. A control group consisting of sibling larvae raised in E3 solution (34.8 g NaCl/L; 1.6 g KCl/L; 5.8 g CaCl2·2H2O/L; 9.78 g MgCl2·6H2O/L) was also applied. Each treatment was repeated three times, and three independent experiments were performed. For each group, the ethanol solution was refreshed daily until the sampling stage was reached. At 3, 4, 5, and 6 dpf, 60 larval fish were collected for the enzyme-linked immunosorbent assay (ELISA), and 30 larval fish were collected for quantitative real time-polymerase chain reaction (qRT-PCR) experiments.
Zebrafish behavioral analysis
Locomotor activity analysis was performed as described in a previous study with several modifications38. At 5 dpf, a single larva was placed in each well of a 48-well plate (24 control and 24 ethanol-exposed larvae), thereby enabling simultaneous tracking of the larvae. The locomotor activities of the larvae were monitored for 30 min using an automated video-tracking system (Videotrack, ViewPoint LifeSciences, Lyon, France), while their movement was recorded and analyzed using Zebralab 3.10 Software (Videotrack, ViewPoint Life Sciences, Lyon, France). The 48-well plates were placed inside the Zebrabox Observation Chamber, where they were exposed to continuous infrared light or 10 min light ON and 10 min light OFF. Instruments were placed in the chamber to maintain a constant temperature of 28.5 °C. The Videotrack quantization parameters were set as in the previous study38. The test was performed three times. Data were further analyzed using Microsoft Excel.
RNA extraction and qRT-PCR
For the qRT-PCR analysis, total RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. Following DNase treatment, RNA (1 µg) was reverse-transcribed with oligo (dT) 18 primers and M-MLV reverse transcriptase. qRT-PCR analysis was then performed in the StepOne Real-Time PCR System using SYBR Premix Ex Taq (Takara Bio Inc., Dalian, Liaoning). At least three independent samples were examined in this analysis, with each being examined in triplicate. The mRNA level of the genes of interest was calculated using the 2−∆CT method and presented as relative (-fold) values of the control group after normalization to the β-actin mRNA levels.
The target gene levels were normalized to β-actin, and triplicates were run for each sample. In each reaction with the target gene primers, 1 µl of undiluted cDNA was used, whereas the cDNA used for β-actin amplification was diluted 1:10. The relative expression of the target genes was calculated using the 2(−ΔΔCT) method. The relative primers are listed in Table 1.
Enzyme-linked immunosorbent assay (ELISA)
Samples from the ethanol and control groups in the 3, 4, 5 and 6 dpf zebrafish were extracted. We analyzed HPA axis hormones and neurotransmitters using an ELISA kit obtained from Fanke Biotech (Shanghai, China) according to the manufacturer’s protocol.
Statistical analyses
The data are presented as the mean ± SD. Statistical differences between two or more groups were determined using Student’s t-test and one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test, respectively. All experiments were repeated three times, and the representative results are described in “Discussion”. A p-value < 0.05 was considered to indicate significance.
References
Sánchez-Marín, L. et al. Systemic blockade of lpa 1/3, lysophosphatidic acid receptors by ki16425 modulates the effects of ethanol on the brain and behavior. Neuropharmacology 133, 189–201 (2018).
Asatryan, L., Ostrovskaya, O., Lieu, D. & Davies, D. L. Ethanol differentially modulates p2x4 and p2x7 receptor activity and function in bv2 microglial cells. Neuropharmacology 128, 11–21 (2017).
Morais-Silva, G., Alves, G. C. & Marin, M. T. N-acetylcysteine treatment blocks the development of ethanol-induced behavioural sensitization and related Δfosb alterations. Neuropharmacology 110, 135–142 (2016).
Wang, X. et al. Minocycline protects developing brain against ethanol-induced damage. Neuropharmacology 129, 84–99 (2018).
Khoury, J. E., Jamieson, B. & Milligan, K. Risk for childhood internalizing and externalizing behavior problems in the context of prenatal alcohol exposure: A meta-analysis and comprehensive examination of moderators. Alcohol Clin. Exp. Res. 4, 1358–1377 (2018).
Tielbeek, J. J. et al. Genetic correlation of antisocial behaviour with alcohol, nicotine, and cannabis use. Drug Alcohol Depend. 187, 296–299 (2018).
Cheng, C.A. et al. Pregnancy increases stroke risk up to 1 year postpartum and reduces long-term risk. QJM-Mon. J. Assoc. Phys. 11(6), 355–360(2017).
Dreval, K. et al. Mir-1247 blocks sox9–mediated regeneration in alcohol- and fibrosis-associated acute kidney injury in mice. Toxicology 384, 40–49 (2017).
Arora, P. G. et al. Multi-tiered systems of support for school-based mental health: A systematic review of depression interventions. Sch. Ment. Health. 2, 1–25 (2019).
Getachew, B., Hudson, T., Heinbockel, T., Csoka, A. B. & Tizabi, Y. Protective effects of donepezil against alcohol-induced toxicity in cell culture: Role of caspase-3. Neurotox. Res. 34, 757–762 (2018).
Desmidt, A. A. et al. Zebrafish model for non‐syndromic x‐linked sensorineural deafness, dfnx1. Anat. Rec. 1–12(2019).
Lockwood, B., Bjerke, S., Kobayashi, K. & Guo, S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol. Biochem. Behav. 77(3), 647–654 (2004).
Arenzana, F. J. et al. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol. Teratol. 28(3), 342–348 (2006).
Carvan 3rd, M.J., Loucks, E., Weber, D.N.& Williams, F.E. Ethanol effects on the developing zebrafish: Neurobehavior and skeletal morphogenesis. Neurotoxicol. Teratol. 26, 757–768 (2004).
Salgado-Freiría, R., López-Doval, S. & Lafuente, A. Perfluorooctane sulfonate (pfos) can alter the hypothalamic-pituitary-adrenal (hpa) axis activity by modifying crf1 and glucocorticoid receptors. Toxicol Lett. 295, 1–9 (2018).
Yue, N. et al. Fine particulate matter constituents and stress hormones in the hypothalamus–pituitary–adrenal axis. Environ. Int. 119, 186–192 (2018).
Feder, A., Nestler, E. J. & Charney, D. S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 10, 446–457 (2009).
Esmaeili, M.H., Bahari, B. & Salari, A.A. ATP-sensitive potassium-channel inhibitor glibenclamide attenuates HPA axis hyperactivity, depression- and anxiety-related symptoms in a rat model of Alzheimer's disease. Brain Res. Bull. 137, 265–276 (2018).
Steenbergen, P. J., Richardson, M. K. & Champagne, D. L. The use of the zebrafish model in stress research. Prog. Neuro-Psychophys. 35, 1432–1451 (2011).
Peng, J. et al. Ethanol-modulated camouflage response screen in zebrafish uncovers a novel role for cAMP and extracellular signal-regulated kinase signaling in behavioral sensitivity to ethanol. J. Neurosci. 29, 8408–8418 (2009).
Mathur, P. & Guo, S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav. Brain Res. 219, 230–239 (2011).
Ramlan, N.F. et al. Time dependent effect of chronic embryonic exposure to ethanol on zebrafish: morphology, biochemical and anxiety alterations. Behav. Brain Res. 332, 40–49(2017).
Liu, C. et al. Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ. Sci. Technol. 45, 769–775 (2011).
Ramsay, J. M. et al. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 297, 157–162 (2009).
Tran, S., Chatterjee, D. & Gerlai, R. An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Danio rerio). Behav. Brain Res. 276, 161–170 (2015).
Baiamonte, M, Brennan, C.H., & Vinson, G.P. Sustained action of developmental ethanol exposure on the cortisol response to stress in zebrafish larvae and adults. PloS One 5, 1–13 (2015).
Werne, B. C. V. et al. Early life stress in depressive patients: hpa axis response to gr and mr agonist. Front. Psychiatry 5, 1–12 (2014).
Ziv, L. et al. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatr. 18, 681–691 (2013).
Zhao, J., Xu, T. & Yin, D. Q. Locomotor activity changes on zebrafish larvae with different 2,2’,4,4’-tetrabromodiphenyl ether (pbde-47) embryonic exposure modes. Chemosphere 94, 53–61 (2014).
Mulligan, C. J., D’Errico, N. C., Stees, J. & Hughes, D. A. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 7, 853–857 (2012).
Martin-Blanco, A. et al. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment, and clinical severity in borderline personality disorder. J. Psychiatr. Res. 57, 34–40 (2014).
Kordian, S. et al. Temporomandibular disorders related to stress and hpa-axis regulation. Pain Res. Manag. 1–7 (2018).
Marcilla, M., Muñoz, A. & Satué, K. Longitudinal changes in serum catecholamines, dopamine, serotonin, acth and cortisol in pregnant spanish mares. Res. Vet. Sci. 115, 29–33 (2017).
Zhang, W. et al. Timing of prenatal exposure to trauma and altered placental expressions of hpa-axis genes and genes driving neurodevelopment. J. Neuroendocrinol. 30, e12581 (2018).
Mandic, S. & Volkoff, H. The effects of fasting and appetite regulators on catecholamine and serotonin synthesis pathways in goldfish ( carassius auratus ). Comp Biochem Phys A. 223, 1–9 (2018).
Wu, L. S., Lee, C. S., Weng, T. Y., Wang, K. H. & Cheng, A. T. Association study of gene polymorphisms in gaba, serotonin, dopamine, and alcohol metabolism pathways with alcohol dependence in taiwanese han men. Alcohol Clin. Exp. Res. 40, 284–290 (2016).
Xu, Y., Sheng, H., Tang, Z., Lu, J. & Ni, X. Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav. Brain Res. 288, 71–78 (2015).
Wang, M. Y. et al. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environ. Toxicol. Chem. 30, 2073–2080 (2011).
Acknowledgements
We thank the support from Yantai University biotechnology platform.
Funding
This work was supported by key research and development plan in Shandong Province (2019GSFI08220) and Natural Science Foundation Program of china (31971546).
Author information
Authors and Affiliations
Contributions
W.D. did this experiment and wrote some parts of the article. X.C., M.S., F.B., Z.Z. proofread and wrote some parts of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, W., Chen, X., Shi, M. et al. Ethanol affects behavior and HPA axis activity during development in zebrafish larvae. Sci Rep 10, 21402 (2020). https://doi.org/10.1038/s41598-020-78573-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78573-y
This article is cited by
-
Removal of dead fish eggs by Asellus aquaticus as a potential biological control in aquaculture
Scientific Reports (2024)
-
Neu1 deficiency induces abnormal emotional behavior in zebrafish
Scientific Reports (2021)
-
The importance of individual variation for the interpretation of behavioural studies: ethanol effects vary with basal activity level in zebrafish larvae
Psychopharmacology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.