Abstract
Foot-and-mouth disease (FMD) endangers a large number of livestock populations across the globe being a highly contagious viral infection in wild and domestic cloven-hoofed animals. It adversely affects the socioeconomic status of millions of households. Vaccination has been used to protect animals against FMD virus (FMDV) to some extent but the effectiveness of available vaccines has been decreased due to high genetic variability in the FMDV genome. Another key aspect that the current vaccines are not favored is they do not provide the ability to differentiate between infected and vaccinated animals. Thus, RNA interference (RNAi) being a potential strategy to control virus replication, has opened up a new avenue for controlling the viral transmission. Hence, an attempt has been made here to establish the role of RNAi in therapeutic developments for FMD by computationally identifying (i) microRNA (miRNA) targets in FMDV using target prediction algorithms, (ii) targetable genomic regions in FMDV based on their dissimilarity with the host genome and, (iii) plausible anti-FMDV miRNA-like simulated nucleotide sequences (SNSs). The results revealed 12 mature host miRNAs that have 284 targets in 98 distinct FMDV genomic sequences. Wet-lab validation for anti-FMDV properties of 8 host miRNAs was carried out and all were observed to confer variable magnitude of antiviral effect. In addition, 14 miRBase miRNAs were found with better target accessibility in FMDV than that of Bos taurus. Further, 8 putative targetable regions having sense strand properties of siRNAs were identified on FMDV genes that are highly dissimilar with the host genome. A total of 16 SNSs having > 90% identity with mature miRNAs were also identified that have targets in FMDV genes. The information generated from this study is populated at http://bioinformatics.iasri.res.in/fmdisc/ to cater the needs of biologists, veterinarians and animal scientists working on FMD.
Similar content being viewed by others
Introduction
Foot-and-mouth disease virus (FMDV) causes a highly infectious disease in domestic and wild cloven-hoofed animals1. Although, vaccination has been used to protect these animals against FMDV, there are shortcomings in the efficacy of available vaccines1. In such scenario, RNA interference (RNAi) appears as a promising strategy to control virus replication2. RNAi is triggered by small non-coding RNA molecules (ncRNAs) including short interfering RNAs (siRNA) and microRNAs (miRNA), and use of RNAi-based methods have been demonstrated as an alternative method of controlling the transmission of FMDV3. The antiviral power of short-hairpin RNAs (shRNA) or siRNAs against FMDV has been demonstrated widely by showing that RNAi based therapeutics can negatively impact the progression of FMDV infection. In particular, de los Santos et al.4 have demonstrated a significant reduction in the virus yield with a reduced viral RNA and protein synthesis as compared to the control cells by expressing an shRNA specific to 2B nonstructural protein coding region of FMDV RNA in porcine cells during the infection. They have also reported that their strategy is effective against multiple FMDV serotypes.
miRNAs are involved with the post-transcriptional regulation of gene expression and also demonstrated to play vital roles in the intricate interaction network between virus and host5. Chang et al.6 constructed multiple miRNAs targeting the Internal Ribosome Entry Site (IRES) of FMDV and found IRES-specific miRNAs significantly inhibited FMDV infection. They found that a co-cistronic expression plasmid containing two miRNA hairpin structures considerably postponed the deaths of suckling mice infected with lethal dose of FMDV vaccine strains of three serotypes (O, A and Asia 1). Du et al.7 induced the protection against FMDV in cell culture and transgenic suckling mice by miRNA targeting the integrin αv receptor. In the recent past, Samir and Pessler8 reviewed the association of small non-coding RNAs (ncRNAs) with viral infectious diseases. They presented an insight into the preclinical studies on using small ncRNAs to combat viral diseases affecting the livestock population. Many scientists across the globe explored the effectiveness of host miRNAs against FMDV infection. For instance, Gutkoska et al.9 have identified two host miRNAs i.e., miR-203a-3p and miR-203a-5p that impaired the FMDV infection across multiple FMDV serotypes. Moreover, artificial microRNAs (amiRs) can be seen as a new hope against viral diseases, if their limitations could be addressed successfully. Gismondi et al.3 discussed the structural implications of target selection while using amiRs as antiviral strategy to FMDV. Thus, these RNAi effectors have to be explored and evaluated extensively by focusing on the limitations of their applicability.
First line of defense against the viral infections is innate immune system and hence, utilization of the innate responses for antiviral, therapeutic and vaccine-adjuvation strategies is becoming an effective way for controlling these infections. Thus, looking at the successful use and possibility of FMD therapeutics development based on RNAi, this study was designed by focusing on identifying (i) targets of miRBase miRNAs in FMDV, (ii) miRNA targets in Bos taurus to uncover the miRNAs that target both host and pathogen, (iii) miRNAs of Bos taurus that target FMDV to study host RNAi based mechanisms against FMDV, (iv) targets of confirmed anti-FMDV miRNAs, (v) regions in FMDV genes that are not significantly similar with Bos taurus genes, as the probable targets for artificial siRNAs, (vi) targets of simulated nucleotide sequences (SNSs) in FMDV that do not have targets in Bos taurus. Subsequently, a database was populated with the integrated information obtained from the study.
Materials and methods
Data collection and processing
Sequence data required for studying the RNAi mechanism in FMD was collected from public resources.
Target sequences
FMDV and Bos taurus genomic sequences were retrieved from National Center for Biotechnology Information (NCBI) repository (https://www.ncbi.nlm.nih.gov/). The downloaded data contained 7991 FMDV sequences and 91,682 Bos taurus sequences. Additionally, 10,973 sequences of 1000 nucleotides upstream (1 K upstream) to the Bos taurus genes (contains the regulatory regions) were also downloaded from University of California, Santa Cruz (UCSC) Genome browser (https://genome.ucsc.edu/).
miRNA sequences
The mature miRNA sequences were downloaded from miRBase (release 21; http://www.mirbase.org/). miRBase contains published and annotated miRNA sequences having predicted hairpin portion of miRNA transcripts (termed as mir) along with the information on location and sequence of the mature miRNA (termed as miR)10. The downloaded sequence data from miRBase contained 35,828 mature miRNA sequences from 285 different organisms.
Redundancy removal
Homology reduction at 100% was carried out to remove identical sequences using CD-HIT server11 from all the sequence datasets i.e., mature miRNAs and target sequences of both FMDV and Bos taurus. The target nucleotide sequences from host and pathogen were subjected to homology reduction separately. The 1 K upstream regions containing 10,973 sequences were not subjected to homology reduction. The sequence data (before and after homology reduction) is shown in Table 1.
Tools used for target identification
As miRNA targets were to be identified in both Bos taurus and FMDV, tools that deal with various types of target genomes were sought. Most of the available tools for miRNA target prediction have few fixed animal and plant genomes. Hardly, there exists any tool to predict miRNA targets in viruses. However, psRNATarget12 has the flexibility to incorporate desired genome data apart from the already included plant genome data to identify targets of small RNAs. Though there are many differences exist between miRNAs of plants and animals, they do have significant amount of similarities. The miRNAs of both plants and animals are evolutionarily related and post-transcriptionally regulate gene expression through interactions with their target mRNAs13. Thus, psRNATarget was chosen as miRNA target identification tool in this study. However, to cross validate the psRNATarget results, another tool, GUUGle14 was used. In addition, the offline version of Basic Local Alignment Search Tool (BLAST)15 was used to identify the targetable regions on FMDV genome. These tools are described below in detail.
psRNATarget
psRNATarget, a plant small RNA target analysis server, which features two important analysis functions: (i) reverse complementary matching between small RNA and target transcript using an established scoring schema, and (ii) target-site accessibility evaluation by calculating the Un-Paired Energy (UPE) required to 'open' secondary structure around small RNA's target site on mRNA. It distinguishes translational and post-transcriptional inhibition, and reports the number of small RNA-target site pairs that may affect small RNA binding activity to target transcript. The psRNATarget searches target gene based on both complementarity scoring analysis and secondary structure analysis12.
GUUGle
GUUGle is an algorithm and a utility program that accepts a set of target sequences and a set of query sequences with a length threshold k. It reports all matches under RNA rules between the target and reverse complement of the query sequence having k or more consecutive base pairs14. GUUGle has been used here as a confirmatory tool to check whether at least one target is predicted for any miRNA whose targets have been predicted by psRNATarget. Thus, minimum number of targets has been set as 1 in GUUGle.
BLAST
The offline version of BLAST15 was used to identify the regions in FMDV genes that are not significantly similar with Bos taurus genes as the probable targets for artificial siRNAs.
miRNA targets in FMDV and Bos taurus
The mature miRNA sequences were used as query to find their targets in FMDV and Bos taurus nucleotide sequence datasets. To achieve this, psRNATarget online server was used with default parameters and GUUGle was used offline with a command line user interface (CUI). The miRNAs not targeting any FMDV genes were discarded and remaining miRNAs were subjected to target identification in Bos taurus. The purpose was to identify the miRNAs having targets in FMDV but no targets or targets with higher UPE in Bos taurus. The UPE is generally used to evaluate mRNA site accessibility where lower energy values represent higher possibility to be an effective target site because the secondary structures may prevent small RNA and target site from contacting12.
Identification and experimental validation of anti-FMDV miRNAs of Bos taurus
miRNAs of Bos taurus were isolated from the complete set of miRNAs collected from miRBase. Their targets in FMDV were identified using both psRNATarget and GUUGle to understand the built-in anti-FMDV mechanism in the host. The Bos taurus miRNAs identified to have targets in FMDV were subjected to experimental validation. The detailed experimental validation is elaborated below with appropriate sub-sections.
Maintenance of BHK-21 cells
The Baby Hamster Kidney 21 (BHK-21) cell line (ATCC), was maintained in Glasgow's Minimum Essential Medium (GMEM) (Himedia, India) supplemented with 10% Fetal Bovine Serum (FBS), 60 µg per milliliter (μg/ml) penicillin, 100 μg/ml streptomycin and 100 μg/ml kanamycin, in a humidified incubator with 5% CO2 at 37 °C.
Cellular toxicity test
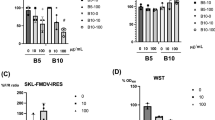
To check the cellular toxicity of miRNA mimics on BHK-21 cells, different nanomolar (nM) concentrations of miRNA mimics (5, 10, 25 and 50 nM) were used and transfection was performed in a 24-well plate, using HiPerFect transfection reagent (Qiagen), according to the manufacturer’s protocol. The live cell percentage/growth was determined by trypan blue staining of cells at 24 and 48 h post transfection.
Optimizing concentrations of miRNA mimics for transfection
Four concentrations of negative miRNA mimics (10, 15, 20 and 25 nM) were selected for the transfection to assess non-specific effect of miRNA mimics on virus replication. FMDV O/IND/R2/75 was infected at 0.001 multiplicity of infection (MOI), at 24 h post transfection. Cell supernatant was harvested at 12 h post infection and virus titer was calculated by 50% Tissue Culture Infectious Dose (TCID50) assay in BHK-21 cells16.
Transfection of miRNA mimics
The mimics of the considered Bos taurus miRNAs were custom prepared using Qiagen technology and the negative control miRCURY LNA miRNA mimic (UCACCGGGUGUAAAUCAGCUUG; not targeting either FMDV or any known vertebrate gene) from Qiagen (Cat#YM00479902) was used as the control. BHK-21 cells were transfected with miRNA mimics diluted to 10 nM in respective wells of a 24-well plate along with a mock control using HiPerFect transfection reagent (Qiagen), according to the manufacturer’s protocol. Further, to assess the antiviral activity of the miRNA mimics, FMDV O/IND/R2/75 was infected with 0.001 MOI, at 24 h post transfection. Cell supernatant was harvested at 12 h post infection and viral growth was monitored by titrating the progeny virus in the supernatant of the infected cell culture by TCID50 assay.
TCID50assay
TCID50/ml was determined from the cellular supernatant, to estimate the amount of progeny virus. Initially, 50 micro liter (μl) of tenfold diluted virus was added in quadruplet well containing 50 μl GMEM medium in 96-well cell culture plate. Then 50 μl (50,000 cells/well) of a suspension of BHK-21 cells was added. The cell plates were incubated at 37 °C under 5% CO2 conditions for 48 h. After incubation, each well was observed for the cytopathic effect (CPE) and TCID50/ml was calculated16.
Statistical analysis
Statistical analysis was carried out on the data obtained from wet-lab experiments that were repeated for three times. All values are expressed as "mean ± standard deviation". The difference between treatment means was tested using Student’s t-test to assess the statistical significance (p-value less than 0.05 was considered as significant difference between treatment means).
Prediction of plausible targets of confirmed anti-FMDV miRNAs
The information on anti-FMDV miRNAs were collected from VIRmiRNA17 database that is a resource for experimentally validated viral and anti-viral miRNAs with their corresponding targets. These miRNAs do not have any corresponding miRBase accessions. In addition to the reported targets, other plausible targets in FMDV were also predicted for these miRNAs using psRNATarget and GUUGle with default set of parameters.
Detection of targetable regions in FMDV
siRNAs seek perfect complementarity with the targets unlike miRNAs to knock down specific genes, with minor off-target exceptions18. Though, both miRNA and siRNA share same processing machinery, nature of the regulatory target is different for both. As miRNAs do not require exquisite sequence complementarity, they can impact the expression of multiple genes, not just similar ones unlike siRNAs. Further, the degree of sequence complementarity between miRNA and its intended target mRNA can have differential outcomes. Therefore, few biotechnology companies are currently focusing on siRNA-based treatments for various diseases along with other RNAi based therapeutics19. Various siRNAs have been designed against the gene encoding the VP1 protein of FMDV to inhibit the viral replication20,21,22. However, it is also necessary to consider that the designed siRNA should not have any off-target effect on the host genes. Of course, the evaluation of siRNAs for causing cytotoxicity to the cells has to be carried out before its practical application. In the light of above facts, we tried to identify the FMDV genes, which can be targeted by exogenous siRNAs, with the possible target regions that does not have significant similarity with any part of the host genes.
Initially, all FMDV sequences were locally aligned with the Bos taurus sequences using the standalone NCBI BLAST application. Genomic sequences of Bos taurus were set as a local database for the standalone BLAST whereas the FMDV sequences were subjected to similarity search against this local database. The FMDV sequences having no significant similarity with Bos taurus sequences were filtered out and split into fragments of 23 nucleotides that is equivalent to the length of dicer-processed siRNA i.e., 21–23 nucleotides23. Further, these gene fragments were realigned with Bos taurus sequences using the offline BLAST by adjusting the parameters for short nucleotide sequences. Finally, coordinates of the gene fragments which showed no hits against host sequences were reported as targetable regions of the corresponding FMDV genes. The target sequences are identical to sense strand of the respective siRNAs. Hence, properties like GC content24, melting temperature25,26, positional occurrence or non-occurrence of certain nucleotides in the sense strand27,28 responsible for siRNA activity were also computed for each targetable region. Positional preferences of specific nucleotides in the siRNA sense strand is well described in Zhou et al.27 and Reynolds et al.28. They suggested that occurrence of A at 3rd position, T at 10th position, T at 13th position, G or C at first position and A or T at 19th position and non-occurrence of G at 13th position on the siRNA sense strand is vital for potential siRNA selection, as these sense strand base preferences improve siRNA functionality.
Targets of SNSs in FMDV
SNSs are the nucleotide sequences of length 25 bases, created computationally from four nucleotide bases (A, T, G and C) by simple random sampling with replacement. The R-code used for generating a set of 150,000 SNSs is provided as Supplementary Fig. 1. SNSs containing the nucleotide repeats of length > 4 bases were discarded to avoid low complexity regions. Then targets of the remaining SNSs in FMDV were identified using GUUGle. The sequences targeting FMDV genes were subjected to target identification against Bos taurus genes. SNSs having targets only in FMDV and not in Bos taurus were selected and aligned against all downloaded mature miRNAs of miRBase using the offline BLAST program. SNSs having a high similarity with established set of miRNAs showed their possible miRNA-like properties.
Development of database
A collection of putative anti-FMDV miRNAs, SNSs (artificial miRNA like sequences) and probable targetable regions on FMDV genes were tabulated and stored in a MySQL database. A user friendly web interface for searching RNAi related information was created as part of "Foot and Mouth Disease Information System for Cattle (FMDISC)" with a three layered architecture. The Hypertext Markup Language (HTML) and Cascading Style Sheets (CSS) have been used as client side interface layer (front-end). The Personal Home Page: Hypertext Preprocessor (PHP) has been used as the server side application layer (middle Layer). The MySQL served as the database layer at the back end. Java script was used for client side customizations. A detailed flow diagram on identification of anti-FMDV miRNAs and development of the database on miRNAs related to FMD along with their targets is presented in the Fig. 1.
Results
The targets of miRBase miRNAs in FMDV and Bos taurus were predicted by using both psRNATarget and GUUGle. From the VIRmiRNA database, 4 anti-FMDV miRNAs were collected and examined in silico for their other possible targets in FMDV. The regions on FMDV genes were located that are not significantly similar with Bos taurus genes. These regions are expected to serve as the putative targets for artificial siRNAs. A number of SNSs that share similarity with miRBase miRNAs were also proposed here as the possible silencing agents for FMDV genes which can be further validated. The information obtained from this in silico analysis was documented and populated in the form of a database.
miRNAs targets in host and pathogen
Out of 35,828 miRNAs of miRBase, possible targets of 641 and 438 miRNA sequences in FMDV were identified through psRNATarget and GUUGle respectively (Table 2). For these miRNAs, more targets were observed in Bos taurus than in FMDV. Thus, a comparison of miRNA targets was made in host and pathogen based on UPE. In this context, 116 miRNAs were found with lesser UPE in FMDV than Bos taurus. Table 3 shows 14 miRNA accessions for which the UPE for FMDV targets is at least 5 units lesser than that of Bos taurus targets. The miRNA accession MIMAT0031768 targeting the FMDV accession FV536933.1 was observed with highest UPE difference of 10 units than its target in Bos taurus. It is also noticed that out of the 14 targets in Bos taurus, 7 are predicted sequences.
miRNAs of Bos taurus targeting FMDV genes
The miRNAs of Bos taurus from miRBase and VIRmiRNA were examined for their targets in FMDV to understand the probable host RNAi mechanism against FMD. A total of 284 targets (constituting the union of targets predicted by psRNATarget or GUUGle) in 98 distinct FMDV genomic sequences were identified for 8 mature miRNAs from miRBase and 4 from VIRmiRNA. Table 4 represents the number of FMDV targets of Bos taurus miRNAs. The highest number of targets has been predicted for the miRNA accession MIMAT0011924 for which GUUGle has also predicted one target. Both GUUGle and psRNATarget predicted same target (FV536931.1) for the miRNA accession MIMAT0024589, where the target region on the FV536931.1 also coincide (4572–4594 for psRNATarget and target start is 4574 in GUUGle).
Antiviral response of Bos taurus miRNAs against FMDV
Cellular toxicity was observed in the cells when transfected at 25 nM and higher miRNA mimic concentrations after 24 and 48 h, whereas at 5 and 10 nM the cell growth pattern was at par with mock (Supplementary Fig. 2a). This suggests that there is a limit to the miRNA mimic concentration that can be transfected into BHK-21 cells. Virus titration was done which also showed that higher mimic concentrations have significant inhibition effect against the virus in comparison to mock control (Supplementary Fig. 2b). Further, eight test miRNAs transfected at 10 nM concentration did not show cellular toxicity (Supplementary Fig. 2c). Therefore, 10 nM mimic concentration was used for actual transfection of the miRNAs.
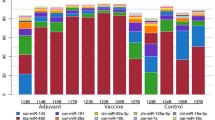
The miRNA mimics of 8 miRNAs viz., bta-miR-2287, bta-miR-2334, bta-miR-2366, bta-miR-2378, bta-miR-2399-3p, bta-miR-2408, bta-miR-2894, and bta-miR-6119-3p that were expressed transiently in BHK-21 cells and infected with FMDV showed variable antiviral effect, as indicated by altered progeny virus titer compared to control miRNA mimic. We observed significant reduction in the progeny virus titer in the culture supernatant at 12 h post infection where the predicted miRNAs possibly have restricted the viral replication to a varying extent by targeting different regions of invading FMD virus (Fig. 2). Compared to the negative control miRNA and mock control, bta-miR-2366, bta-miR-2378 bta-miR-2399-3p and bta-miR-2408 showed 1 Log10 virus reduction in BHK-21 cells.
Identification of targetable regions in FMDV
FMDV accessions along with their sequence coordinates were identified having no significant similarity with the Bos taurus genes. A total of 530 FMDV accessions were identified to have no significant similarity with the Bos taurus accessions. To accurately identify the targetable regions on these 530 FMDV accessions, their sequences were split into 23 nucleotides length fragments. The splitting resulted in 25,213 fragments, out of which only 3279 fragments were identified with no significant similarity when realigned to the Bos taurus sequences. Out of 3279 fragments, only 8 fragments were obtained with all favorable positional occurrences of nucleotides (A at 3rd position, T at 10th position, G not at 13th position, T at 13th position, G or C at first position and A or T at 19th position)24,25,26,27,28 in the sense strand of siRNA that is identical to the target region (Table 5). The mapping coordinates of these fragments on the respective FMDV genes were reported as the putative targetable regions.
The first 6 FMDV accessions (Table 5) are highly similar at the target regions that are from different isolates of same serotype (FMDV-type O). Thus, they have identical sequences (GTACAACGGTAGTTGCAGATACA) as well as same target coordinates, GC content and melting temperature. The seventh accession is also from the same serotype that have same GC content and melting temperature but different target coordinates. The last accession HM067705.1 (GTAACCCCGTTGTCGGCGTGGTC) is a polyprotein gene of FMDV-SAT2. Though only 8 fragments (targetable regions) have shown favorable properties, all the 3279 fragments (having no significant similarity with host genome) were populated as probable targetable regions in the database along with the properties like GC content, melting temperature, positional occurrence or non-occurrence of certain nucleotides.
Targets of SNSs in FMDV
Apart from publicly available natural miRNAs, artificial miRNAs also have been reported to confer antiviral effect3. Thus, identifying artificial miRNAs or miRNA-like agents through in silico sequence simulation and target prediction approaches may provide novel insights into FMD therapeutics. With this expectation, we have randomly generated 150,000 SNSs of length 25 nucleotides, out of which, only 60 SNSs (Supplementary Table 1) were found to have targets only in FMDV and not in Bos taurus. These 60 SNSs have 60 targets in 12 distinct FMDV accessions. Sixteen out of these 60 SNSs were observed to have similarity with 41 mature miRNAs. The maximum and minimum values for alignment length, identity and bit score are presented in Table 6.
The SNS sim_21145 (Supplementary Table 1) have shown maximum alignment length with the miRNA miR7516-5p possessing highest bit score and lowest e-value (0.31). The lower e-value indicates a higher significance of sequence similarity whereas a higher bit score designates a better sequence similarity15. These two scores together are used to compare the pair-wise alignments from different similarity searches. The SNSs having a high identity with the established set of miRBase miRNAs are reported in the study as miRNA like SNS and populated in the FMDISC.
Anti-FMDV repository
The anti-FMDV repository can be accessed from "miRNA" section of FMDISC (http://bioinformatics.iasri.res.in/fmdisc/) that has been developed and maintained at ICAR-Indian Agricultural Statistics Research Institute, New Delhi, India. The repository is enriched with the information on anti-FMDV miRNAs, putative targetable regions on FMDV genes, miRNA like SNSs with their targets in FMDV. The user friendly web interface provides appropriate search options for the miRNA of interest related to FMD. Web interfaces to search the information regarding anti-FMDV miRNAs, targetable regions on FMDV genomic sequences and FMDV targets of putative miRNA-like-SNS are described below with the appropriate snapshots.
miRNA targets on FMDV
The search interface for miRBase miRNA targets in FMDV provides the capability to search based on miRBase miRNA accession, miRNA symbol and FMDV target accession. It also provides interface for displaying the predicted targets by four options; (i) psRNATarget, (ii) GUUGle, (iii) Both, and (iv) Anyone (Fig. 3). By selecting “psRNATarget”, the targets only predicted by psRNATarget are displayed. Similarly, the targets predicted only by GUUGle are displayed upon selecting “GUUGle”. When the user selects “Both” or “Anyone”, intersection or union of the targets predicted by the above mentioned tools is displayed respectively.
The search interface for miRNA targets in FMDV is shown in Fig. 3a. The search results containing the list of miRNAs based on the search parameters is displayed in the Fig. 3b. The details on the miRNA of interest selected from the list (Fig. 3b) and its target are shown in the Fig. 3c with a link for finding its target in Bos taurus. The targets in Bos taurus are shown in the similar interface as displayed in the Fig. 3b,c.
miRNA targets compared in both host and pathogen based on UPE
This section of the repository gives information on the miRNAs that target both FMDV and Bos taurus with their corresponding UPEs. The details of these targets can be viewed by clicking on the respective target accessions. As a lower UPE represents a better target accessibility, the miRNAs with low UPE in FMDV and high UPE in Bos taurus may have vital importance in therapeutic developments for FMD. The snapshot of search interface for miRNAs targeting both host and pathogen is presented in the Fig. 4 with target UPE.
Host miRNA (miRBase and VIRmiRNA) targets in FMDV
A separate search interface was designed specifically for the cattle miRNAs targeting FMDV genes. The host miRNAs includes 8 miRNAs from miRBase and 4 from VIRmiRNA database. The miRNAs of VIRmiRNA database are the confirmed anti-FMDV miRNAs. The search interface (Fig. 5a) is designed with the option to select the host miRNAs which results a list of targets for the selected miRNA. For miRBase miRNAs, the target details are provided in a similar fashion as shown in Fig. 3c. The targets for miRNAs of VIRmiRNA database can be searched from both the links "Host miRNA Targets in FMDV" and "Anti-FMDV miRNAs". In the later one, additional details on the process, cell line, method of detection are given as shown in Fig. 5b.
Targetable regions
The targetable regions on the FMDV accessions can be searched by selecting an accession from the list given in search interface (Fig. 6a). The details of the targetable regions including the target accession, sequence of the targetable region, coordinates, left and right flanking sequences, GC content, melting temperature, positional occurrence/non-occurrence of nucleotides are provided in the result page (Fig. 6b).
Simulated nucleotides
The targets of SNSs can be searched by selecting the FMDV accessions from the list or by searching a keyword through the text box provided in the search interface (Fig. 7a). The results page shows a list of SNS with their corresponding targets for a particular search. Clicking on the "View" link against each SNS takes the user to another page where details of SNS, its target and its identity with miRBase miRNAs are provided (Fig. 7b).
Discussion
RNAi based therapeutics present a new strategy for inhibiting the replication of certain viruses2. Specifically, antiviral miRNAs and siRNAs against FMDV have demonstrated an alternative method of slowing down the viral transmission3. Thus, in silico approaches were explored in this study to understand applicability of RNAi based therapeutic solutions for FMD. Based on the available resources and cell line, a handful of host miRNAs were also experimentally validated at ICAR-Indian Veterinary Research Institute, Bangalore, India. Computationally, miRNA targets in both host and pathogen have been identified in this study as well as anti-FMDV miRNAs in the host have also been explored in silico and evaluated experimentally. The number of miRNA targets identified for Bos taurus is much more than the targets identified in FMDV because FMDV genome size is very small in comparison to Bos taurus genome. The FMDV genome ranges from 7000 to 8500 bp and varies depending on serotypes29 whereas the size of bovine genome is ~ 2.5 Gbp30 that is ~ 300,000 times higher than FMDV genome. The study of anti-FMDV miRNAs in Bos taurus revealed 12 mature miRNAs (8 from miRBase and 4 from VIRmiRNA databases) having a total of 284 targets in 98 distinct FMDV genomic sequences, which suggests the possible existence of miRNA based anti-FMDV mechanism in Bos taurus to defend the FMDV.
Viable miRNA targets have been identified by researchers worldwide with high reliability through miRNA profiling efforts that have been conducted with FMDV infected pigs and cattle31,32. This has inspired us to experimentally validate a handful of host miRNAs for their anti-FMDV properties. As 4 host miRNAs from VIRmiRNA database were reported to be anti-FMDV miRNAs, only the remaining 8 host miRNAs from miRBase were experimentally validated. The validation results showed that the mimics of these miRNAs targeted different genomic regions of the invading FMD virus and restricted its replication. The negative control miRNA mimic did not impact the viral replication. However, the magnitude of the antiviral effect of the 8 miRNA mimics was observed to be varying. This could be attributed to the difference in the degree of sequence-specific miRNA interactions with viral elements as reported previously31,33 and depends on the concentrations of miRNAs and targets. Further, the results of the experimental validation of all the considered 8 host miRNAs also supported the in silico predictions by more or less reducing the viral replication. However, further investigation is required to evaluate the efficacy of these miRNAs in vivo for establishing their potentiality in the development of novel therapeutic interventions during the FMDV infection.
The comparison of miRNA targets in host and pathogen based on UPE revealed 14 miRNA accessions for which the UPE for FMDV targets is at least 5 units lesser than that of Bos taurus targets. A lesser UPE represents higher possibility to be an effective target site12. Thus, these 14 miRNAs may be subjected to experimental validation in the context of FMDV therapeutics. In addition, the miRNA MIMAT0031768 targeting the FMDV accession FV536933.1 was observed with highest UPE difference of 10 units then its target in Bos taurus. The FV536933.1 is an 8170 bp long linear DNA of FMDV defined as "modified microbial nucleic acid". The sequence of a "modified microbial nucleic acid" constitute 3 out of 4 nucleotide bases and does not contain methylated or other cytosine alterations, instead, all cytosines are converted to uracil. Online BLAST results revealed that FV536933.1 shares more than 70% identity at 99% query coverage with 4 different strains of FMDV serotype SAT3 genomic sequences (AY593850.1, MG372727.1, KR108950.1 and KM268901.1) where the target regions (3843.0.3866) belong to the 1D (VP1) gene (3220.0.3873). It is worth mentioning that VP1 is one of the surface protein on the FMD virus particle that has an important role in determining virus antigenicity and receptor-mediated entry into the host cell34.
The genome comparison of both host and pathogen revealed only 530 out of 6305 FMDV sequences are not significantly similar with that of Bos taurus. Probably, this could be the reason for obtaining a good number of miRNAs having targets in both FMDV and Bos taurus. In order to identify targetable regions in FMDV genomic sequences, these 530 FMDV sequences were split into fragments of 23 nucleotides in length that resulted in 25,213 subsequences. Only 3279 out of 25,213 subsequences were found to be dissimilar with the Bos taurus genomic sequences. Further, only 8 out of 3279 subsequences were obtained with all favorable positional occurrences of nucleotides as in the sense strand (identical to the target region). The coordinates of these fragments are noticed to be mapped with the capsid gene of FMDV serotype O and polyprotein genes of both FMDV serotypes O and SAT2. The experimentally validated targetable regions are expected to contribute in the development of RNAi based therapeutics for FMD of cattle.
The main purpose of constructing a number of SNSs and finding their corresponding targets in FMDV was to identify anti-FMDV miRNA-like nucleotide fragments sharing similarity to miRBase miRNAs. Out of 150,000, only 16 SNS were observed to have targets in FMDV being > 90% identical with 41 mature miRNAs. The identity summary given in Table 6 confirms the homology of such SNS with the established set of miRNAs. Thus, miRNAs or siRNAs based on these artificial nucleotide sequences are expected to potentially contribute to the management of FMD, but must be successfully validated both in vitro and in vivo.
RNAi approach has been widely used for drug development and several phase I and II clinical trials are under way35. Despite enthusiastic studies in a large number of animal models, including systemic delivery to non-human primates36 there are a number of obstacles and concerns that remain. Some of these hurdles include but are not limited to: off-target effects, triggering of type I interferon responses, and an effective means of in vivo delivery. These issues need to be resolved before widespread application of RNAi-based therapeutics37. In addition, the success rate of therapeutic applications of siRNAs in both in vivo and in vitro conditions depends on various factors like, size of siRNA molecule, use of appropriate vectors and many more. Specifically, the effectiveness of RNAi as an antiviral strategy against RNA viruses like FMD encompasses additional limitations like emergence of resistant mutants, viral suppressors, cytotoxicity, high genetic variability of FMDV, large number of susceptible animals and economical feasibility38,39. To overcome these hurdles, multiple delivery vehicles are now in development for improving the introduction of RNAi-based constructs into intracellular environment40. A good number of RNAi-based therapeutics are currently in clinical trials and positive results from these trials have also facilitated strengthening of further attempts to develop clinically relevant RNAi therapies41. Application of RNAi therapeutics in combination with potential vaccine candidates might be explored to induce long-lasting protection in livestock population. However, the availability of huge amount of experimental data from rigorous research is required to further access the potentiality of RNAi in the management of FMD outbreaks.
References
Orsel, K., Bouma, A., Dekker, A., Stegeman, J. A. & De Jong, M. C. M. Foot and mouth disease virus transmission during the incubation period of the disease in piglets, lambs, calves, and dairy cows. Prev. Vet. Med. 88(2), 158–163 (2009).
Tan, E. L. & Hann Chu, J. J. RNA interference (RNAi)—An antiviral strategy for enteroviruses. J. Antivir. Antiretrovir. https://doi.org/10.4172/jaa.S9-002 (2011).
Gismondi, M. I., Ortiz, X. P., Currá, A. P., Asurmendi, S. & Taboga, O. Artificial microRNAs as antiviral strategy to FMDV: Structural implications of target selection. J. Virol. Methods 199, 1–10 (2014).
de los Santos, T., Wu, Q., de Avila Botton, S. & Grubman, M. J. Short hairpin RNA targeted to the highly conserved 2B nonstructural protein coding region inhibits replication of multiple serotypes of foot-and-mouth disease virus. Virology 335(2), 222–231 (2005).
Wen, B. P., Dai, H. J., Yang, Y. H., Zhuang, Y. & Sheng, R. MicroRNA-23b inhibits enterovirus 71 replication through downregulation of EV71 VPl protein. Intervirology 56, 195–200 (2013).
Chang, Y. et al. Multiple microRNAs targeted to internal ribosome entry site against foot-and-mouth disease virus infection in vitro and in vivo. Virol. J. 11(1), 1 (2014).
Du, J. et al. Induction of protection against foot-and-mouth disease virus in cell culture and transgenic suckling mice by miRNA targeting integrin αv receptor. J. Biotechnol. 187, 154–161 (2014).
Samir, M. & Pessler, F. Small non-coding RNAs associated with viral infectious diseases of veterinary importance: Potential clinical applications. Front. Vet. Sci. 3, 1–12 (2016).
Gutkoska, J., LaRocco, M., Ramirez-Medina, E., de Los Santos, T. & Lawrence, P. Host microRNA-203a is antagonistic to the progression of foot-and-mouth disease virus infection. Virology 504, 52–62. https://doi.org/10.1016/j.virol.2017.01.019 (2017).
Kozomara, A. & Griffiths-Jones, S. MiRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, 68–73 (2014).
Li, W. & Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22(13), 1658–1659 (2006).
Dai, X. & Zhao, P. X. PsRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 39, 155–159 (2011).
Millar, A. A. & Waterhouse, P. M. Plant and animal microRNAs: Similarities and differences. Funct. Integr. Genom. 5(3), 129–135 (2005).
Gerlach, W. & Giegerich, R. GUUGle: A utility for fast exact matching under RNA complementary rules including G-U base pairing. Bioinformatics 22, 762–764 (2006).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215(3), 403–410 (1990).
Reed, L. J. & Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497. https://doi.org/10.1093/oxfordjournals.aje.a118408 (1938).
Qureshi, A., Thakur, N., Monga, I., Thakur, A. & Kumar, M. VIRmiRNA: A comprehensive resource for experimentally validated viral miRNAs and their targets. Database (Oxford) 2014, 1–10 (2014).
Sahu, T. K., Singh, N., Meher, P. K., Wahi, S. D. & Rao, A. R. Epigenetics in sustaining the livelihood. Biotechnol. Bioinform. Comput. Biol. 6, 437–462 (2014).
Castanotto, D. & Rossi, J. J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 457(7228), 426–433. https://doi.org/10.1038/nature07758 (2009).
Chen, W. et al. RNA interference targetingVP1inhibitsfoot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J. Virol. 78(13), 6900–6907 (2004).
Kim, S. M. et al. Therapeutic application of RNA interference against foot-and-mouth disease virus in vitro and in vivo. Antivir. Res. 80(2), 178–184. https://doi.org/10.1016/j.antiviral.2008.06.001 (2008).
Lv, K. et al. Transient inhibition of foot-and-mouth disease virus replication by siRNAs silencing VP1 protein coding region. Res. Vet. Sci. 86, 443–452 (2009).
Siolas, D. et al. Synthetic shRNAs as potent RNAi triggers. Nat. Biotechnol. 23, 227–231 (2005).
Wang, L. & Mu, F. Y. A web-based design center for vector-based siRNA and siRNA cassette. Bioinformatics 20(11), 1818–1820 (2004).
Wallace, R. B. et al. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: The effect of single base pair mismatch. Nucleic Acids Res. 6(11), 3543–3557 (2006).
Gong, Y., Ma, Z., Gao, C., Wang, W. & Shen, J. Specially elaborated thermally induced phase separation to fabricate poly (l-lactic acid) scaffolds with ultra large pores and good interconnectivity. J. Appl. Polym. Sci. 101(5), 3336–3342 (2006).
Zhou, H., Zeng, X., Wang, Y. & Ray, B. A three-phase algorithm for computer aided siRNA design. Informatica 30, 357–364 (2006).
Reynolds, A. et al. Rational siRNA design for RNA interference. Nat. Biotechnol. 22(3), 326–330 (2004).
Ullah, H., Siddique, M. A., Sultana, M. & Hossain, M. A. Complete genome sequence of foot-and-mouth disease virus type A circulating in Bangladesh. Genome Announc. 2(3), e00506-e514 (2014).
Zimin, A. V. et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 10(4), R42 (2009).
Basagoudanavar, S. H. et al. Host serum microRNA profiling during the early stage of foot-and-mouth disease virus infection. Arch. Virol. 163, 2055–2063. https://doi.org/10.1007/s00705-018-3824-8 (2018).
Stenfeldt, C. et al. Proof-of-concept study: Profile of circulating microRNAs in Bovine serum harvested during acute and persistent FMDV infection. Virol. J. 14(1), 71. https://doi.org/10.1186/s12985-017-0743-3 (2017).
Santhakumar, D. et al. Combined agonist-antagonist genome-wide functional screening identifies broadly active antiviral microRNAs. Proc. Natl. Acad. Sci. U.S.A. 107(31), 13830–13835. https://doi.org/10.1073/pnas.1008861107 (2017).
Ashkani, J. & Rees, D. J. G. The critical role of VP1 in forming the necessary cavities for receptor-mediated entry of FMDV to the host cell. Sci. Rep. 6, 27140 (2016).
Tiemann, K. & Rossi, J. J. RNAi based therapeutics—Current status, challenges and prospects. EMBO Mol. Med. 1(3), 142–151 (2009).
Zimmermann, T. S. et al. RNAi-mediated gene silencing in non-human primates. Nature 441, 111–114 (2006).
Aagaard, L. & Rossi, J. J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 59(2–3), 75–86. https://doi.org/10.1016/j.addr.2007.03.005 (2007).
Grubman, M. J. & de los Santos, T. ,. Rapid control of foot-and-mouth disease outbreaks: Is RNAi a possible solution?. Trends Immunol. 26(2), 65–68. https://doi.org/10.1016/j.it.2004.12.002 (2005).
Bayry, J. & Tough, D. F. Is RNA interference feasible for the control of foot-and-mouth disease outbreaks?. Trends Immunol. 26(5), 238–241. https://doi.org/10.1016/j.it.2005.03.003 (2005).
Reischl, D. & Zimmer, A. Drug delivery of siRNA therapeutics: Potentials and limits of nanosystems. Nanomedicine 5(1), 8–20. https://doi.org/10.1016/j.nano.2008.06.001 (2009).
Bobbin, M. L. & Rossi, J. J. RNA interference (RNAi)-based therapeutics: Delivering on the promise. Annu. Rev. Pharmacol. Toxicol. 56, 103–122 (2016).
Acknowledgements
TKS acknowledges the receipt of fellowship from PG School, IARI, New Delhi, during his PhD study. All authors wish to acknowledge the grants received from CABin Scheme Network project on Agricultural Bioinformatics and Computational Biology (F.No. Agril.Edn.14/2/2017-A&P dated 02.08.2017), Indian Council of Agricultural Research (ICAR), New Delhi. Authors are also thankful to Mr. Srikanth Bairi for providing technical support during the study. Authors also acknowledge the timely help and support rendered by Dr. J.K. Jena, DDG(FS) for making the wet-lab study possible.
Author information
Authors and Affiliations
Contributions
T.K.S. and A.R.R. conceived and designed the study. T.K.S., A.K.S.G., P.K.M., G.P.R. collected the data. T.K.S., A.K.S.G., P.K.M., C.V. and A.R.R. performed the data analysis. T.K.S., A.K.S.G., A.R.R., S.M. and A.R. developed the database. N.G., S.H.B. and A.S. planned and conducted the wet lab experiment for the anti-FMDV miRNA validation. T.K.S., P.K.M. and A.R.R. drafted and finalized the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahu, T.K., Gurjar, A.K.S., Meher, P.K. et al. Computational insights into RNAi-based therapeutics for foot and mouth disease of Bos taurus. Sci Rep 10, 21593 (2020). https://doi.org/10.1038/s41598-020-78541-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78541-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.