Abstract
Fluoroindate glasses co-doped with Pr3+/Er3+ ions were synthesized and their near-infrared luminescence properties have been examined under selective excitation wavelengths. For the Pr3+/Er3+ co-doped glass samples several radiative and nonradiative relaxation channels and their mechanisms are proposed under direct excitation of Pr3+ and/or Er3+. The energy transfer processes between Pr3+ and Er3+ ions in fluoroindate glasses were identified. In particular, broadband near-infrared luminescence (FWHM = 278 nm) associated to the 1G4 → 3H5 (Pr3+), 1D2 → 1G4 (Pr3+) and 4I13/2 → 4I15/2 (Er3+) transitions of rare earth ions in fluoroindate glass is successfully observed under direct excitation at 483 nm. Near-infrared luminescence spectra and their decays for glass samples co-doped with Pr3+/Er3+ are compared to the experimental results obtained for fluoroindate glasses singly doped with rare earth ions.
Similar content being viewed by others
Introduction
Fluoroindate glasses belong to the low-phonon Heavy Metal Fluoride Glass (HMFG) family, which have been extensively studied for their numerous optical applications. From literature data it is well-known that fluoroindate glasses containing rare earth ions with their lower phonon energies close to 510 cm−1 are promising materials for up-conversion luminescence applications. For example, the laser based on the orange-to-ultraviolet conversion in a Nd3+ doped fluoroindate glass powder has been well demonstrated1. These effects are practically not possible to obtain in Nd3+ doped oxide or oxyfluoride glass host matrices. Generally, the efficient up-conversion luminescence of Pr3+, Tm3+ and Ho3+ ions in fluoroindate glasses2,3,4 was successfully observed. Moreover, the up-conversion luminescence spectra of Pr3+, Ho3+ and Tm3+ ions in fluoroindate glasses5,6,7,8,9 are enhanced drastically in the presence of Yb3+. In the later system9, i.e. Tm3+/Yb3+ co-doped fluoroindate glass, the blue up-conversion luminescence of Tm3+ ions is highly increased with Yb3+ concentration. For the optimum Tm3+ concentration (0.5 mol%), the up-conversion emission intensity was increased by a factor about 100 by co-doping with 2.25 mol% of Yb3+. In particular, fluoroindate glasses singly doped with Er3+ ions and doubly doped with Er3+/Yb3+ ions have been examined for up-conversion luminescence10,11,12,13, which was measured under 790 nm, 980 nm or 1480 nm laser excitation. The up-conversion luminescence processes of Er3+ were analyzed with activator concentration, temperature, pumping wavelength and power of diode laser used as the excitation source. In general, the up-conversion results in a strong green emission and weaker blue and red emissions and the Yb3+ co-doping will certainly increase the efficiency of an up-conversion-based optical device. Further investigations confirmed this hypothesis. An intensity enhancement of 4.5 for the green up-conversion luminescence in Er3+/Yb3+ co-doped fluoroindate glass has been obtained by focusing the incoming beam with a 3.8 μm silica microsphere14. Also, these experimental results open a new method to improve the up-conversion emission intensity in biological samples with rare earth doped nanoparticles that can be used as nano-sensors. In another case, the generation of photocurrent in a commercial solar cell has been achieved under excitation at 1480 nm in fluoroindate glass samples co-doped with Er3+/Yb3+ ions15.
Fluoroindate glasses with their excellent spectroscopic properties offer the possibility of using these materials not only in the operation of erbium up-conversion lasers. Also, they belong to promising glass materials emitting near-infrared radiation. Nowadays there is a great interest in compact lasers operating in the near-infrared (1.5 μm) and mid-infrared (2.8 μm) for optical communications, medical and eye-safe light detecting and ranging applications. However, the near-infrared luminescence studies were limited practically to fluoroindate glasses containing Er3+ or Er3+/Yb3+ ions10,13. Luminescence spectra exhibit a highly intense signal at 1.5 μm due to 4I13/2 → 4I15/2 transition of Er3+. Decay measurements indicate that luminescence lifetime for the upper 4I13/2 laser state of Er3+ ions in fluoroindate glass is quite long and its value is close nearly to 10 ms at room temperature10. The mid-infrared luminescence results obtained for Er3+/Yb3+ ions in fluoroindate glass suggest that co-doping with Yb3+ favors only the up-conversion processes which depopulate efficiently the 4I11/2 state, reducing the emission intensity related to 4I11/2 → 4I13/2 transition of Er3+ ions at 2.8 μm13. These phenomena are important for diode-pumped solid-state lasers, which could provide a compact and efficient device with the advantage of easy coupling with fiber integrated optical systems. For diode-pumped lasers luminescence at near-infrared (1.5 μm) and mid-infrared (2.8 μm) spectral ranges, the trivalent Er3+ seems to be an excellent candidate due to 4I13/2 → 4I15/2 and 4I11/2 → 4I13/2 electronic transitions, respectively. From accumulated data it is quite well-known that the transmission capacity of WDM systems (wavelength division multiplexing) should be improved. Many research groups are looking for a new glass matrices and their fibers in order to obtain signal amplification beyond the conventional optical NIR window between 1530 and 1565 nm, commonly known as the C-band. In fact, spectral bandwidth for the commercial EDFA system (Erbium Doped Fiber Amplifiers) based on silicate glass is equal to 40 nm at C-band and broadband near-infrared transmission is limited, unfortunately16. For that reason, several glass matrices containing rare earth ions are still tested in order to obtain excellent materials with broader emission bands and longer lifetimes. These improved spectroscopic parameters are necessary for signal amplification in telecommunication and laser technology. Some recently published works for fluoroindate glass clearly indicate that a logical extension of Er3+-doped systems would be the addition of other rare earth ions such as Tm3+ or Ho3+ ions17,18. The interesting results for fluoroindate glasses co-doped with Ho3+/Tm3+ and Ho3+/Nd3+ ions19,20 emitting near-infrared and mid-infrared radiation at 2 μm and 3.9 μm under direct excitation (888 nm or 808 nm) have been also well presented and discussed in details. Among rare earths, the trivalent Pr3+ ions exhibit broadband near-infrared luminescence covering a wavelength range from 1.2 μm to 1.7 μm, which is really important for optical fiber amplifiers operating at O-band (1260–1360 nm), E-band (1360–1460 nm), S-band (1460–1530 nm), C-band (1530–1565 nm), and L-band (1565–1625 nm)21. The recent results indicate that glasses22,23,24,25 and crystals26 co-doped with Pr3+/Er3+ seems to be quite good candidates for broadband near-IR luminescence. The superbroadband near-IR luminescence is contributed mainly by the 1D2 → 1G4 (Pr3+) and 4I13/2 → 4I15/2 (Er3+) transitions which lead to emission lines located at about 1.48 and 1.53 μm25. To the best of our knowledge, these phenomena were not yet examined for fluoroindate glasses co-doped with Pr3+/Er3+.
Results and discussion
Fluoroindate glasses singly doped with Pr3+ and Er3+
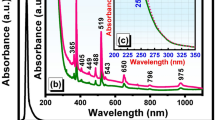
The optical absorption spectra of fluoroindate glasses singly doped with Pr3+ and Er3+ ions were recorded at room temperature. They are presented in Fig. 1.
In general, the absorption bands are inhomogeneously broadened and clearly resolved, characteristic for 4f2 (Pr3+) and 4f11 (Er3+) transitions of rare earths. They are attributed to the electronic transitions originating from the 3H4 (Pr3+) and 4I15/2 (Er3+) ground states to the higher-lying excited states of rare earths. From the optical absorption spectra the experimental oscillator strengths have been determined. The band intensities are estimated by measuring the areas under the absorption lines using the equations:
where: ∫ε(ν) represents the area under the absorption line, A indicates the absorbance, c is the concentration of the Ln3+ ion (Ln = Pr or Er) in mol l−1 and l denotes the optical path length. In the next step, the theoretical oscillator strengths for each transition of Pr3+ and Er3+ ions were calculated using the Judd–Ofelt theory27,28. The theoretical oscillator strength is defined as follows:
where m is the mass of the electron, c is the velocity of light, h is the Planck constant and λ is the mean wavelength of the each transition. In order to perform the analysis, the refractive index of the medium (n = 1.48 for fluoroindate glass) and Ut2 from Ref.29 representing the square of the matrix elements of the unit tensor operator Ut (connecting the initial J and final J' states) were used for calculations. Next, the experimental and theoretical oscillator strengths have been compared. They are listed in Table 1.
Transitions are from the 3H4 (Pr3+) and 4I15/2 (Er3+) ground states to the levels indicated. The three phenomenological intensity parameters Ωt (t = 2, 4, 6) are found to be (in 10–20 cm2 units): Ω2 = 2.01 ± 0.81, Ω4 = 5.25 ± 0.90, Ω6 = 5.10 ± 0.35, rms = 1.4 × 10–6 (for Pr3+) and Ω2 = 1.47 ± 0.22, Ω4 = 1.51 ± 0.30, Ω6 = 1.69 ± 0.11, rms = 0.7 × 10–6 (for Er3+), respectively.
The three phenomenological Judd–Ofelt intensity parameters Ωt (t = 2, 4, 6) for rare earth ions in fluoroindate glasses are found to be (in 10–20 cm2 units); Ω2 = 2.01 ± 0.81, Ω4 = 5.25 ± 0.90, Ω6 = 5.10 ± 0.35, rms = 1.4 × 10–6 (for Pr3+) and Ω2 = 1.47 ± 0.22, Ω4 = 1.51 ± 0.30, Ω6 = 1.69 ± 0.11, rms = 0.7 × 10–6 (for Er3+). The fit quality was expressed by the magnitude of the root-mean-square deviation, which is defined by rms = Σ (Pmeas – Pcalc)2.
In the next step, the intensity parameters Ωt (t = 2, 4, 6) were applied to calculate the radiative transition rates, luminescence branching ratios and radiative lifetimes. The radiative transition rates AJ for excited levels of Pr3+ and Er3+ ions from an initial level J to a final ground level J’ were calculated using the following relation:
The total radiative emission rate AT involving all the intermediate terms is the sum of the AJ terms. The radiative lifetime τrad of an excited level is the inverse of the total radiative emission rate (Eq. 5), whereas the luminescence branching ratio β is due to the relative intensities of transitions from excited level to all terminal levels (Eq. 6).
The calculated radiative transition rates AJ, luminescence branching ratios β and corresponding radiative lifetimes τrad for Pr3+ and Er3+ ions in fluoroindate glasses are presented in Table 2. The calculation results are limited to transitions originating from the 1D2 and 1G4 excited levels of Pr3+ as well as the 4I11/2 and 4I13/2 excited levels of Er3+, from which the main luminescence lines in the near-infrared spectral range (950–1650 nm) occur.
Measured lifetimes τm for the 1D2 and 1G4 (Pr3+) and the 4I11/2 and 4I13/2 (Er3+) excited levels are also given in Table 2 in order to determine quantum efficiencies η of rare earth ions in fluoroindate glasses using the following equation:
Figure 2 presents near-infrared luminescence spectra of fluoroindate glasses singly doped with Er3+ and Pr3+. The spectra were measured for glass samples in the 950–1650 nm ranges. For both Er3+ and Pr3+ doped glass samples, the activator concentration is equal to 0.1 mol%.
For Er3+ singly doped glass, the spectra consist of luminescence bands centered at 980 nm and 1535 nm, which correspond to transitions originating from the 4I11/2 and 4I13/2 excited levels to the 4I15/2 ground level, respectively. When glass sample is excited at higher-lying 4F7/2 (488 nm) or 2H11/2 (523 nm) level, luminescence band near 1230 nm associated to the 4S3/2 → 4I11/2 (Er3+) transition is also well observed.
The most intense luminescence band is related to the main 4I13/2 → 4I15/2 near-infrared laser transition of Er3+. Its luminescence intensity depends strongly on excitation wavelengths. Several spectroscopic parameters for the 4I13/2 → 4I15/2 near-infrared transition of Er3+ ions in fluoroindate glass at 1535 nm, necessary for optical and laser characteristics, were determined. The luminescence linewidth defined as the full width at half maximum (FWHM) for the 4I13/2 → 4I15/2 (Er3+) transition is equal to 72 nm. The measured luminescence lifetime for the upper 4I13/2 laser level of Er3+ ions in fluoroindate glass is close to 8.6 ms, whereas radiative lifetime calculated from the Judd–Ofelt framework seems to be 8.62 ms (Table 2). Thus, the quantum efficiency for the upper 4I13/2 laser level of Er3+ ions in fluoroindate glass is nearly ~ 100%. The same situation is also observed for the higher-lying 4I11/2 level of Er3+, for which the measured and calculated radiative lifetimes and the quantum efficiency are close to τm = 6.65 ms, τrad = 6.71 ms and η = ~ 99%. The quantum efficiency for the main 4I13/2 → 4I15/2 (Er3+) laser transition at 1535 nm in fluoroindate glass is significantly higher in comparison to the values obtained earlier for borate (η = 3%) and germanate (η = 71%) glasses30. This phenomenon is related directly to the lower phonon energy (hω = 510 cm−1) of the fluoroindate glass-host17.
The peak stimulated emission cross-section σem is one of the most important radiative parameters and should be also examined because the strong luminescence band at 1535 nm due to the 4I13/2 → 4I15/2 transition has been considered as a potential near-infrared laser emission of Er3+ ions in fluoroindate glass. It is generally accepted that an efficient laser transition is characterized by relatively large value of σem. The peak stimulated emission cross-section σem can be obtained from the calculated radiative transition rate AJ using the following relation:
where λp is the peak luminescence wavelength, Δλ is the effective linewidth (FWHM), n is the refractive index and c is the velocity of light. The maximum peak stimulated emission cross-section is close to nearly 5.4 × 10–21 cm2 at 1535 nm for fluoroindate glass and its value is typical for fluoride laser glasses containing Er3+ ions31.
Near-infrared luminescence spectra of Pr3+ ions in fluoroindate glasses are also presented on Fig. 2 (bottom). In general, three near-infrared luminescence bands are quite well observed under different excitation wavelengths. They correspond to 1D2 → 3F3,4 (1050 nm), 1G4 → 3H5 (1335 nm) and 1D2 → 1G4 (1450 nm) electronic transitions of Pr3+. However, the intensities of luminescence bands of Pr3+ are extremely low, when glass sample was excited at higher lying 3P2 (445 nm), 3P1 (469 nm) or 3P0 (480 nm) level, respectively. In this case, the 1G4 → 3H5 transition with its FWHM value equal to about 195 nm is dominant transition of trivalent Pr3+. The 1G4 measured lifetime of Pr3+ (τm = 0.365 ms) is considerably lower than corresponding calculated radiative lifetime (τm = 2.8 ms). Although the luminescence branching ratio for the 1G4 → 3H5 transition at 1335 nm is relatively high and its value is close to β = 65%, the quantum efficiency (η = 13%) for the 1G4 excited level of Pr3+ ions is rather low (Table 2). Completely different situation is observed for Pr3+ ions in fluoroindate glass under direct excitation of 1D2 state at 590 nm. The near-infrared luminescence bands originating to transitions from the 1D2 level of Pr3+ ions are highly intense compared to the 1G4 → 3H5 transition at 1335 nm. In particular, broadband near-infrared spectra covering a wavelength range from 1300 nm to about 1650 nm are great of interest and really important for optical telecommunication21. The main most intense near-infrared luminescence band near 1450 nm is assigned to the 1D2 → 1G4 transition of Pr3+ and its value of FWHM is close to 130 nm. Although the luminescence branching ratio (β = 9%), the measured (τm = 0.308 ms) and calculated radiative (τm = 0.410 ms) lifetimes are not too high, the quantum efficiency for the 1D2 level of Pr3+ is close to η = 75% (Table 2). Furthermore, the peak stimulated emission cross-section for the 1D2 → 1G4 transition of Pr3+ ions in fluoroindate glass was determined. From literature data it is well-known that inorganic glasses singly doped with praseodymium exhibit fascinating prospects in broadband near-infrared fiber amplifier, when the emission cross-section profile is large21. In our case, the peak stimulated emission cross-section for the 1D2 → 1G4 transition of Pr3+ ions is also relatively large. Its value is close to 0.5 × 10−20cm2. Finally, the stimulated emission cross-section (σem), the emission linewidth (FWHM) and the measured lifetime (τm) were applied to calculate the gain bandwidth (σem × FWHM product) and the figure of merit (FOM) given by σem × τm, respectively. It is interesting to see that the σem × FWHM product for the 1D2 → 1G4 transition of Pr3+ ions in fluoroindate glass is equal to 65 × 10−27 cm3, which is rather low compared to the following values: 176.4 × 10−27 cm3 for fluorotellurite glass32 and 203.5 × 10−27 cm3 for gallo-germanate glass with BaF233. This behavior is mainly due to the fact that luminescence linewidth for the 1D2 → 1G4 transition of Pr3+ ions in fluoroindate glass (FWHM = 130 nm) is considerably lower than values obtained for fluorotellurite glass (FWHM = 196 nm) and gallo-germanate glass (FWHM = 208.5 nm). On the other hand, the figure of merit (FOM) for the 1D2 → 1G4 transition of Pr3+ ions in fluoroindate glass is relatively larger (σem x τm = 154 × 10−26 cm2s) in comparison to the values 83.1 × 10-26cm2s for fluorotellurite glass32 and 107.5 × 10−26 cm2s for gallo-germanate glass modified by BaF233. Our experimental studies and theoretical calculations demonstrate that Pr3+ singly doped fluoroindate glasses are promising for broadband near-infrared amplifiers.
Fluoroindate glasses co-doped with Pr3+/Er3+
Figure 3 present absorption (top) and excitation (bottom) spectra of fluoroindate glasses co-doped with Pr3+/Er3+. The results are compared to the absorption spectra, which were measured for rare earth singly doped glass samples (inset).
The absorption spectrum for Pr3+/Er3+ co-doped glass sample consists of several bands corresponding to both electronic transitions of rare earths originating from the ground levels 3H4 (Pr3+) and 4I15/2 (Er3+) to the excited levels. In particular, two spectral ranges near 590 nm and between 480 and 488 nm are important from the spectroscopic point of view. In the first spectral range near 590 nm, the absorption band associated to 3H4 → 1D2 transition of Pr3+ is located. In the second spectral range, two absorption bands related to 3H4 → 3P0 transition of Pr3+ (480 nm) and 4I15/2 → 4F7/2 transition of Er3+ (488 nm) are quite well overlapped.
The excitation spectra measured for Pr3+/Er3+ co-doped glass samples under monitoring emission wavelength 1530 nm (4I13/2 Er3+) give interesting results. It is clearly shown in these two spectral ranges denoted as (*) that the intensity of band at 590 nm assigned to 3H4 → 3P0 transition of Pr3+ ions increase, whereas the relative band intensities of 3H4 → 3P0 (Pr3+) and 4I15/2 → 4F7/2 (Er3+) transitions located between 480 and 488 nm are drastically changed with increasing Pr3+ concentration. It also suggests that the energy transfer process between Pr3+ and Er3+ ions in fluoroindate glass occurs.
In the next step, luminescence spectra of fluoroindate glasses co-doped with Pr3+/Er3+ have been examined under different excitation wavelengths. In general, Pr3+/Er3+ co-doped glasses belong to amorphous systems emitting visible light and near-infrared radiation. It is quite well-known that several visible emission bands from the 3P0, 1D2 (Pr3+) and the (2H11/2,4S3/2), 4F9/2 excited levels of rare earth ions can be well observed for Pr3+/Er3+ co-doped glasses34. These aspects for fluoroindate glass are not presented and discussed here. Our investigations are limited to Pr3+/Er3+ co-doped fluoroindate glass emitting near-infrared radiation. Figure 4 shows near-infrared luminescence spectra of fluoroindate glasses co-doped with Pr3+/Er3+ under direct 488 nm (top) and 590 nm (bottom) excitation. The results are compared to the spectra, which were measured for rare earth singly doped glass samples.
Four emission bands at 980 nm, 1230 nm, 1335 nm and 1535 nm are observed for Pr3+/Er3+ co-doped fluoroindate glasses under direct excitation of 4F7/2 level of Er3+ at 488 nm. They correspond to 4I11/2 → 4I15/2 (Er3+), 4S3/2 → 4I11/2 (Er3+), 1G4 → 3H5 (Pr3+) and 4I13/2 → 4I15/2 (Er3+) transitions, respectively. In contrast to Er3+ singly doped glass, the intensity of emission band at 1535 nm due to the main 4I13/2 → 4I15/2 (Er3+) near-infrared laser transition is reduced, whereas the emission band intensities related to 4I11/2 → 4I15/2 and 4S3/2 → 4I11/2 (Er3+) transitions increase with increasing Pr3+ concentration in samples co-doped with Pr3+/Er3+. The emission linewidth for 4I13/2 → 4I15/2 (Er3+) near-infrared laser transition is nearly independent on Pr3+ concentration and its FWHM value is close to 75 ± 3 nm. The intensity of emission band at 1335 nm associated to the 1G4 → 3H5 (Pr3+) transition increase with increasing activator (Pr3+) concentration. The experimental results for Pr3+/Er3+ co-doped fluoroindate glasses clearly suggest (a) the presence of the energy transfer process from Er3+ to Pr3+ and (b) near-infrared emission of Pr3+ at 1335 nm under direct excitation of Er3+. Further investigations indicate that five near-infrared luminescence bands at about 980 nm, 1050 nm, 1335 nm, 1450 nm and 1535 nm are quite well observed for Pr3+/Er3+ co-doped fluoroindate glasses under direct excitation of 1D2 level of Pr3+ at 590 nm. Emission bands are due to the 4I11/2 → 4I15/2 (Er3+), 1D2 → 3F3,4 (Pr3+), 1G4 → 3H5 (Pr3+), 1D2 → 1G4 (Pr3+) and 4I13/2 → 4I15/2 (Er3+), respectively. The presence of 4I11/2 → 4I15/2 and 4I13/2 → 4I15/2 transitions of Er3+ in the luminescence spectra measured under direct excitation of Pr3+ confirms the energy transfer process from Pr3+ to Er3+ ions in fluoroindate glasses co-doped with Pr3+/Er3+. Firstly, broadband near-infrared luminescence at around 1000 nm in Pr3+/Er3+ co-doped glass samples is due to two overlapped 4I11/2 → 4I15/2 (Er3+) and 1D2 → 3F3,4 (Pr3+) transitions. These phenomena were studied previously for fluorotellurite glass co-doped with Pr3+/Er3+ ions35. Secondly, the near-infrared emission band at 1535 nm due to 4I13/2 → 4I15/2 (Er3+) transition is overlapped with 1G4 → 3H5 transition of Pr3+ in glass samples co-doped with Pr3+/Er3+. These effects are not observed for Pr3+ singly doped glass sample. Furthermore, the intensity of emission band at 1335 nm due to the 1G4 → 3H5 (Pr3+) transition enhance, whereas the intensity of emission band at 1450 nm corresponding to 1D2 → 1G4 (Pr3+) is reduced with increasing Pr3+ concentration in glass composition. Interestingly, luminescence linewidth for broadband near-infrared luminescence covering a wavelength range from 1300 nm to about 1650 nm and associated to two overlapped 1G4 → 3H5 and 1D2 → 1G4 transitions of Pr3+ enhance drastically in fluoroindate glasses co-doped with Pr3+/Er3+. In contrast to Pr3+ singly doped glass (FWHM = 130 nm), emission linewidth for glass samples co-doped with Pr3+/Er3+ increase to nearly 225 ± 5 nm and its value slightly depends on Pr3+ concentration. All near-infrared transitions indicated for Pr3+/Er3+ co-doped fluoroindate glasses excited directly at 488 nm or 590 nm are schematized on the energy level diagram (Fig. 5).
The mechanisms for Pr3+/Er3+ co-doped glass system involving possible energy transfer and radiative/nonradiative relaxation channels, i.e. energy transfer routes (ET), multiphonon relaxation (MPR) and cross-relaxation (CR) processes, have been proposed and discussed in details by Zhou et al.25.
When glass sample co-doped with Pr3+/Er3+ is directly pumped at 488 nm, the excited level 4F7/2 (Er3+) is well populated by the ground-state absorption process (GSA). The energy transfer process (ET) from the 4F7/2 (Er3+) level to the 3P0 (Pr3+) level is not observed and it is rather impossible, because the 3P0 level of Pr3+ is higher-lying than the 4F7/2 level of Er3+. There is also the main reason that near-infrared luminescence bands from the higher-lying 1D2 level of Pr3+ ions in fluoroindate glasses are not observed under 488 nm excitation of Er3+. The excitation energy transfers very fast nonradiatively to the 4S3/2 level by multiphonon relaxation (MPR) and then relaxes radiatively generating near-infrared emission at 1230 nm associated to the 4S3/2 → 4I11/2 transition of Er3+. The another possible way to depopulate 4F7/2 level is nonradiative relaxation to the 4I11/2 level by MPR process owing to small energy gaps between the 4F7/2 excited level and lower-lying levels or cross-relaxation process (CR): [4F7/2, 4I15/2 → 4I11/2, 4I11/2], when concentration of Er3+ ions in glass sample is relatively high. Next, part of excitation energy is transferred nonradiatively from the 4I11/2 level to the lower-lying 4I13/2 level by MPR process, and consequently two near-infrared luminescence bands centered at 980 nm and 1535 nm due to 4I11/2 → 4I15/2 and 4I13/2 → 4I15/2 transitions are well observed. Finally, the excitation energy is successfully transferred from the 4I11/2 (Er3+) level to the 1G4 (Pr3+) level (ET1) and the 4I13/2 (Er3+) level to the 3F3,4 (Pr3+) level (ET2) by energy transfer processes. In particular, the ET1 process plays the important role, leading to near-infrared luminescence at 1335 nm due to 1G4 → 3H5 transition of Pr3+ under direct excitation of Er3+.
When glass sample co-doped with Pr3+/Er3+ is directly pumped at 590 nm, the excited level 1D2 (Pr3+) is well populated by the ground-state absorption process (GSA). Part of excitation energy relaxes radiatively and two near-infrared luminescence bands at about 1050 nm and 1450 nm are observed, which correspond to transitions originating from the 1D2 excited level to the lower-lying 3F3,4 and 1G4 levels of Pr3+. Another part of excitation energy is transferred nonradiatively by well-known CR process: [1D2, 3H4 → 1G4, 3F3,4]36. At this moment, it should be also pointed that the second CR process [1D2, 3H4 → 3F3,4, 1G4] was also proposed, but it is still not sure which one is more dominant. For Pr3+/Er3+ co-doped samples, another cross-relaxation route that depopulate efficiently the 1D2 (Pr3+) level is also possible. This can be attributed to the following CR process: [1D2 (Pr3+), 4I15/2 (Er3+) → 1G4 (Pr3+), 4I13/2 (Er3+)]. Thus, part of the excitation energy is transferred from Pr3+ to Er3+ ions, giving important contribution to near-infrared emission at 1535 nm related to 4I13/2 → 4I15/2 transition of Er3+. However, the near-infrared luminescence at 980 nm due to 4I11/2 → 4I15/2 transition of Er3+ is also successfully observed under excitation of Pr3+ ions at 590 nm (Fig. 4). The back energy transfer process from the 1G4 (Pr3+) level to the 4I11/2 (Er3+) level is rather impossible, because the 4I11/2 level of Er3+ is higher-lying than the 1G4 level of Pr3+.
Based on the energy level diagram, the 4I11/2 level of Er3+ ions in Pr3+/Er3+ co-doped glass samples can be populated in two ways. First, the presence of the phonon-assisted energy transfer process from the 1D2 (Pr3+) level to the 4F9/2 (Er3+) is proposed but it should be ignored considering the relatively large energy gap between them (~ 1500 cm−1) and the absence of luminescence lines from the 4F9/2 level25, for example well-known 4F9/2 → 4I15/2 red transition of Er3+ ions at 670 nm. The second way is the possible cross-relaxation process: [1D2 (Pr3+), 4I15/2 (Er3+) → 3F3,4 (Pr3+), 4I11/2 (Er3+)]. The question how higher-lying 4I11/2 level of Er3+ is populated under direct 590 nm excitation of Pr3+ is still open and actual, and further experiments are necessary to understand the population mechanism and multichannel relaxation in Pr3+/Er3+ co-doped glass systems. The presence of cross-relaxation processes from the 1D2 (Pr3+) level in fluoroindate glasses co-doped with Pr3+/Er3+ was confirmed by luminescence spectra and decay measurements.
Luminescence decays from the 1D2 level of Pr3+ ions in fluoroindate glasses co-doped with Pr3+/Er3+ are presented in Fig. 6 (top). The luminescence decay curves for the 1D2 level of Pr3+ were measured under monitoring emission wavelength 1450 nm.
Based on decays, luminescence lifetimes for the 1D2 level of Pr3+ were determined. For Pr3+/Er3+ co-doped glass samples, the 1D2 luminescence lifetime is reduced from 197 µs (0.1Er-0.1Pr) to 127 µs (0.1Er-0.3Pr) and 92 µs (0.1Er-0.5Pr) with increasing Pr3+ concentration. This behavior may be attributed to the presence of cross-relaxation processes between neighboring pairs Pr3+–Pr3+ and Pr3+–Er3+ in fluoroindate glasses. Also, we compare results for Pr3+-doped fluoroindate glasses in the absence and presence of Er3+. Thus, the measured 1D2 lifetime is reduced from 308 µs (0.1Pr) to 197 µs (0.1Er–0.1Pr), whereas the quantum efficiency for the 1D2 excited level of Pr3+ decreases from 75% (0.1Pr) to 48% (0.1Er–0.1Pr).
Figure 6 (bottom) shows the visible-NIR emission spectra of fluoroindate glasses singly doped with Pr3+ and Er3+ and doubly doped with Pr3+/Er3+. In the 630–880 nm spectral range, two emission bands are well observed for Er3+ singly doped glass under 488 nm excitation. They correspond to the 4F9/2 → 4I15/2 red transition near 670 nm and the 4S3/2 → 4I13/2 NIR transition at about 840 nm. The 4F9/2 → 4I15/2 red transition at 670 nm is not observed for Pr3+/Er3+ co-doped glass samples under direct excitation of Pr3+ at 590 nm. It was also verified for fluorotellurite glass co-doped with Pr3+/Er3+ excited at 594 nm25. In comparison to the results obtained for Pr3+ singly doped glass, the emission band at about 690 nm related to the 1D2 → 3H5 (Pr3+) transition was registered in this spectral range for Pr3+/Er3+ co-doped glass samples. Unexpectedly, the additional NIR luminescence band at 800 nm corresponding to the 4I9/2 → 4I15/2 transition of Er3+ ions was also detected in Pr3+/Er3+ co-doped fluoroindate glasses under direct excitation 1D2 (Pr3+) level at 590 nm. For that reason, it is interesting to explain how the 4I9/2 (Er3+) level is populated in Pr3+/Er3+ co-doped glass samples excited at the 1D2 (Pr3+) level. We postulate the following mechanisms involving cross-relaxation (CR) and energy transfer up-conversion (ETU) processes applied to populate 4I9/2, 4I11/2 and 4I13/2 excited levels of Er3+. The excited level 1D2 (Pr3+) is depopulated through the cross-relaxation processes: [1D2 (Pr3+), 4I15/2 (Er3+) → 1G4 (Pr3+), 4I13/2 (Er3+)] and [1D2 (Pr3+), 4I15/2 (Er3+) → 3F3,4 (Pr3+), 4I11/2 (Er3+)]. Thus, both 4I11/2 and 4I13/2 levels are well populated and near-infrared emission bands at 980 nm and 1535 nm due to the 4I11/2 → 4I15/2 and 4I13/2 → 4I15/2 transitions of Er3+ occur. Additionally, other processes play significant role in changing the population of 4I11/2 and 4I13/2 excited levels of Er3+ ions. The energy transfer up-conversion process (ETU): [4I13/2, 4I13/2 → 4I9/2, 4I15/2] gives important contribution to efficient population of higher-lying 4I9/2 level of Er3+ ions37. Further studies for the Er3+/Tm3+/Pr3+ triply doped fluoride glass indicate that the ETU process [4I13/2, 4I13/2 → 4I9/2, 4I15/2] increases population of the 4I9/2 level and then the lower-lying 4I11/2 level can be populated by a fast multiphonon relaxation from the 4I9/2 level, which leads to an increase of mid-infrared emission at 2700 nm38. Owing to presence of ETU process, it is possible to detect near-infrared emission peaking at 800 nm for Pr3+/Er3+ co-doped fluoroindate glass, which corresponds to the 4I9/2 → 4I15/2 transition of Er3+. The origin of near-infrared luminescence of Er3+ at 800 nm has been examined in an excellent work published recently39. It was stated that there is no NIR emission found at 800 nm, when the samples are excited to the higher-lying levels (2H11/2 or 4F9/2) than the 4I9/2 level, and the nonradiative relaxation from the upper excited levels to the 4I9/2 emitting level of Er3+ ions is extremely inefficient. The 4I9/2 level of Er3+ is mainly populated by the adjacent lower-lying 4I11/2 level upon excitation at 980 nm39. It was also confirmed for Er3+ doped chalcogenide glasses and fibers, where the conversion of incoherent infrared light around 4400 nm into a near-infrared signal at 810 nm was obtained by simultaneously 982 nm pumping40.
Further spectroscopic analysis of Pr3+/Er3+ co-doped fluoroindate glasses excited at 488 nm (Fig. 4) clearly indicate that the intensity of near-infrared luminescence band at 1535 nm due to the main 4I13/2 → 4I15/2 (Er3+) laser transition is decreased with increasing Pr3+ concentration in comparison to the intensities of 4I11/2 → 4I15/2 and 4S3/2 → 4I11/2 (Er3+) transitions of Er3+. Zhou et al.25 suggested that the relative increase of the near-infrared luminescence of Er3+ at 1230 nm compared with of 1530 nm can be ascribed to the improved population inversion between the upper and lower levels. Moreover, the decrease of near-infrared luminescence at 1530 nm is due to the energy transfer process from the 4I13/2 (Er3+) level to the 3F3,4 (Pr3+) levels. At a consequence, near-infrared emission bands related to transitions originating from the 4I11/2 level to the lower-lying 4I13/2 (2700 nm) and 4I15/2 (980 nm) levels can be enhanced. The luminescence decay analysis for the 4I11/2 and 4I13/2 excited levels of Er3+ ions in Pr3+/Er3+ co-doped fluoroindate glasses confirms this hypothesis.
Figure 7 shows luminescence decay curves for the 4I13/2 and 4I11/2 excited states of Er3+ ions in fluoroindate glasses co-doped with Pr3+/Er3+. The decay curves were measured under monitoring emission wavelength 980 nm and 1530 nm, respectively. In both cases, luminescence decay curves measured for Pr3+/Er3+ co-doped glass samples are shortened with increasing Pr3+ concentration in comparison to glasses singly doped with Er3+. Based on decay curves, luminescence lifetimes for the 4I13/2 and 4I11/2 excited levels of Er3+ were determined. Based on measured lifetimes for glass samples with the absence and presence of Pr3+, the energy transfer efficiencies for the 4I13/2 and 4I11/2 levels of Er3+ ions were also calculated. Luminescence lifetimes and energy transfer efficiencies varying with Pr3+ concentration are schematically presented on Fig. 8.
The luminescence lifetime for the 4I13/2 level of Er3+ is decreased from 8.60 ms (0.1Er) to 3.40 ms (0.1Er–0.1Pr), 1.96 ms (0.1Er–0.3Pr) and 1.36 ms (0.1Er–0.5Pr) with increasing Pr3+ concentration. The reduction of luminescence lifetime for the 4I11/2 level of Er3+ ions is also observed. The measured lifetime is changed from 6.65 ms (0.1Er) to 5.91 ms (0.1Er–0.1Pr), 4.92 ms (0.1Er–0.3Pr) and 4.14 ms (0.1Er–0.5Pr) for glass samples with the presence of Pr3+. Trends in luminescence lifetimes 4I11/2 and 4I13/2 (Er3+) varying with Pr3+ concentration are similar, but changes in lifetimes and their corresponding values for glasses singly doped with Er3+ and co-doped with Pr3+/Er3+ are significant from the spectroscopic point of view. It is worthy to notice that luminescence lifetimes obtained for Pr3+/Er3+ co-doped glasses are generally lower for the 4I13/2 level than 4I11/2 level, in contrast to measured lifetime for Er3+ singly doped glass which is higher for the 4I13/2 level (8.60 ms) than 4I11/2 level (6.65 ms). This is also the experimental proof that the intensity of near-infrared emission band at 980 nm due to the 4I11/2 → 4I15/2 transition increase, whereas the intensity luminescence band at 1535 nm related to the 4I13/2 → 4I15/2 transition of Er3+ is reduced with increasing Pr3+ concentration in fluoroindate glasses co-doped with Pr3+/Er3+. It was confirmed by luminescence decay curve measurements for similar fluoride ZBLAN glasses co-doped with Pr3+/Er3+ ions41, where the influence of the Pr3+ codopant on the 4I13/2 and 4I11/2 emission lifetimes has been also studied. For ZBLAN glasses, a significant reduction in the 4I13/2 lifetime was observed following the addition of a small amount of Pr3+, while the 4I11/2 lifetime of Er3+ was affected to a lesser degree. Thus, the degree of energy transfer process from Er3+ to Pr3+ ions is found to be much higher for the 4I13/2 level (ET2) than for the 4I11/2 level (Fig. 5). It was also confirmed by calculation of the energy transfer efficiencies in fluoroindate glasses co-doped with Pr3+/Er3+, where the values of ηET are higher for the 4I13/2 level than for the 4I11/2 level. The energy transfer efficiency for the 4I13/2 level of Er3+ is increased from 60.5% (0.1Er-0.1Pr) to 77.2% (0.1Er-0.3Pr) and 84.2% (0.1Er-0.5Pr), whereas the value of ηET for the 4I11/2 level of Er3+ is changed from 13.3% (0.1Er-0.1Pr) to 27.9% (0.1Er-0.3Pr) and 39.3% (0.1Er-0.5Pr) with increasing Pr3+ concentration.
Figure 9 present near-infrared luminescence spectra of fluoroindate glasses co-doped with Pr3+/Er3+ under selective excitation wavelengths in the spectral range from 480 to 488 nm. This experiment motivated us to demonstrate unambiguously how the excitation wavelengths playing the crucial role in Pr3+/Er3+ co-doped system influence on broadband near-infrared luminescence covering a spectral range from 1250 to 1650 nm. The inset shows schematically values of emission linewidth (FWHM) depending on the selective excitation wavelengths.
The mechanisms for Pr3+/Er3+ co-doped glass system upon selective excitation wavelength at 488 nm (4F7/2 Er3+) involving several relaxation routes and two energy transfer processes ET1 (from the 4I11/2 level) and ET2 (from the 4I13/2 level) have been already discussed here.
Upon selective pumping at 480 nm, the 3P0 level of Pr3+ is well populated by the ground state absorption process (GSA) and then the excitation energy transfers by means of two ways. Part of the excitation energy is transferred from the 3P0 level of Pr3+ to the 4F7/2 level of Er3+ ions by the energy transfer process ET3. The second way to depopulate the 3P0 level is very fast non-radiative relaxation to the next lower-lying 1D2 level of Pr3+ ions through multiphonon relaxation (MPR) and cross-relaxation process (CR): 3P0, 3H4 → 1D2, 3H6. In the next step, several near-infrared emission bands originating to radiative transitions from the 1D2 and 1G4 levels to the lower-lying levels of Pr3+ are quite well observed and these aspects have been examined by us in this work. In particular, three near-infrared emission bands of Pr3+ and Er3+ located at spectral range denoted as (*) are the most interesting and important from the optical point of view. They are due to the 1G4 → 3H5 (Pr3+), 1D2 → 1G4 (Pr3+) and 4I13/2 → 4I15/2 (Er3+) transitions of rare earths in fluoroindate glass. Their relative intensities are changed drastically when the excitation wavelength varies from 480 to 488 nm. It is evidently to see that the luminescence band intensities of Pr3+ ions are reduced drastically, while the intensity of band due to the 4I13/2 → 4I15/2 transition of Er3+ ions is enhanced when the excitation wavelength is selectively changed from 480 to 488 nm. The inset of Fig. 9 shows values of FWHM depending on the excitation wavelength. When sample co-doped with Pr3+/Er3+ is excited at 480, 481 or 482 nm, the 1G4 → 3H5 transition of Pr3+ ions is dominant transition and emission linewidth seems to be 120–130 nm, which is similar to the value obtained for Pr3+ singly doped glass (FWHM = 130 nm). When glass sample co-doped with Pr3+/Er3+ is excited at spectral range 484/488 nm, the 4I13/2 → 4I15/2 transition of Er3+ ions is dominant transition and values of FWHM are close to about 75–85 nm, similar to the Er3+ singly doped glass (72 nm). Super-broadband near-infrared luminescence with corresponding value of FWHM = 278 nm is successfully observed, when Pr3+/Er3+ co-doped sample was excited selectively at 483 nm. The luminescence linewidth for Pr3+/Er3+ co-doped fluoroindate glass (278 nm) is similar to the values of FWHM equal to 236 nm and 296 nm, which were obtained for tellurite glasses doubly doped with Pr3+/Er3+ ions42 and triply doped with Pr3+/Er3+/Nd3+ ions43. Our experimental investigations confirm that the selective excitation wavelength 483 nm is an optimal pump source to obtain super-broadband luminescence in fluoroindate glass co-doped with Pr3+/Er3+. The previous results suggest that the Pr3+/Er3+ co-doped phosphate glasses with a 483 nm pump source exciting simultaneously both 3P0 (Pr3+) and 4F7/2 (Er3+) levels are promising amorphous materials for broadband optical amplifiers44. The energy level diagram and all transitions for fluoroindate glass co-doped with Pr3+/Er3+ excited selectively at blue spectral region are schematized on Fig. 10.
Based on previous scientific reports we confirm that our glass with Pr3+/Er3+ is a potential material for broadband fiber amplifiers45 and its near-IR emission property depends critically on the excitation wavelengths46. Similar to oxyfluoroaluminate and fluorozirconate systems47, fluoroindate glasses doped with rare earths have potential applications as laser materials.
To summarize, near-infrared luminescence spectra of fluoroindate glasses co-doped with Pr3+/Er3+ at 1200–1650 nm range have been examined under different excitation wavelengths and the results are compared to glass samples singly doped with Pr3+ and Er3+ (Fig. 11).
Pr3+-singly doped fluoroindate glass shows near-infrared emission centered at 1335 nm and 1450 nm corresponding to the 1G4 → 3H5 and 1D2 → 1G4 transitions and their relative emission band intensities depend critically on the excitation wavelengths (480 and 590 nm). Er3+-singly doped fluoroindate glass under 488 nm excitation shows near-infrared emission near 1535 nm associated to the main 4I13/2 → 4I15/2 laser transition. When fluoroindate glass co-doped with Pr3+/Er3+ is excited at 590 nm, the intensity of emission band at 1335 nm due to the 1G4 → 3H5 transition is increased in comparison to the 1D2 → 1G4 transition at 1450 nm, and the spectral linewidth is larger for Pr3+/Er3+ co-doped glass sample (FWHM = 225 nm) than Pr3+ singly doped glass (FWHM = 130 nm).
Superbroadband near-infrared luminescence in Pr3+/Er3+ co-doped fluoroindate glass under selective excitation wavelength (483 nm) is successfully observed. Broadband emission with its linewidth equal to FWHM = 278 nm covering a wavelength range from 1200 to 1650 nm corresponds to the overlapped 1G4 → 3H5 (Pr3+), 1D2 → 1G4 (Pr3+) and 4I13/2 → 4I15/2 (Er3+) transitions of rare earth ions. However, we cannot unambiguously exclude the presence of 3F3,4 → 3H4 (Pr3+) transition at about 1600 nm, which was quite well observed in selenide glasses doped with Pr3+ and co-doped with Pr3+/Er3+ ions48. Further luminescent studies for Pr3+/Er3+ co-doped fluoroindate glasses excited at 488 nm suggest that they are promising candidates as dual-wavelength fiber-optic amplifiers for 1335 nm and 1535 nm windows similar to the previous excellent results published for Ge-As-Ga-S glasses co-doped with Pr3+/Er3+ ions49. Moreover reported emission properties of Pr3+/Er3+ and Er3+50 doped glasses inclined to use them in optical fiber construction as a fluoroindate fibers started to be valuable for SC generation51 and lasers52,53,54 beyond 3 μm. Future research will be devoted for fiber development and its length optimization as we believe to obtain superbroadband emission.
Conclusions
Fluoroindate glasses co-doped with Pr3+/Er3+ have been investigated for near-infrared luminescence applications. Near-infrared luminescence properties have been examined under selective excitation wavelengths. The radiative and nonradiative relaxation channels involving several processes like multiphonon relaxation (MPR), cross-relaxation (CR), energy transfer up-conversion (ETU) and their mechanisms are proposed in Pr3+/Er3+ co-doped glass samples under direct excitation of Pr3+ and/or Er3+ ions and the energy transfer processes (ET) from Pr3+ to Er3+ and from Er3+ to Pr3+ were identified. In particular, near-infrared luminescence covering a wavelength range from 1200 to 1650 nm is really important for broadband optical amplifiers and these aspects for fluoroindate glasses have been analyzed in details. Broadband near-infrared emission (FWHM = 278 nm) corresponding to the 1G4 → 3H5 (Pr3+), 1D2 → 1G4 (Pr3+) and 4I13/2 → 4I15/2 (Er3+) transitions in fluoroindate glass co-doped with Pr3+/Er3+ is successfully observed under direct 483 nm excitation. Based on luminescence decay measurements, the measured lifetimes for the excited levels of rare earth ions and the energy transfer efficiencies were determined. Near-infrared luminescence spectra and their decays for glass samples co-doped with Pr3+/Er3+ are compared to the experimental results obtained for fluoroindate glasses singly doped with rare earth ions and theoretical calculations using the Judd–Ofelt framework.
Methods
Synthesis
Fluoroindate glasses singly and doubly doped with rare earth ions (Ln3+) with the following molar composition: 37.9InF3–20ZnF2–20SrF2–16BaF2–4GaF3–2LaF3–0.1LnF3, where Ln = Pr or Er (referred as 0.1Pr and 0.1Er), and (38–x–y)InF3–20ZnF2–20SrF2–16BaF2–4GaF3–2LaF3–xErF3–yPrF3, where x = 0.1; y = 0.1, 0.3, 0.5 (referred as 0.1Er–0.1Pr, 0.1Er–0.3Pr and 0.1Er–0.5Pr), were prepared by melting (covered platinum crucible) and quenching method in glove box in a nitrogen atmosphere (O2, H2O < 0.5 ppm). Ammonium bifluoride (NH4HF2) was added as afluorinating agentto the batch before melting. For the studied glass system, the concentration of ErF3 is relatively low (0.1 mol%). Thus, the energy transfer processes between Er3+ ions are negligibly small. With increasing ErF3 concentration the thermal stability reduces and strong luminescence quenching is observed due to nonradiative processes corresponding to the Er3+–Er3+ interaction increase. Also, previous investigations for fluoroindate glasses clearly indicate that the higher activator (Er3+) concentrations lead to partial crystallization of fluoroindate glasses50. The glass batches were firstly fluorinated at 270 °C for 2 h and then melted at 900 °C for 1 h. Finally, the glass was cast into a stainless steel plate and then annealed at 290 °C for 2 h slowly cooled to room temperature to minimize internal stress during the quenching process. Transparent glassy plates of 10 × 10 mm dimension were obtained. Each glass sample of 1 mm in thickness was polished for optical measurements.
Measurement and characterization
Similar to our previous works for fluoroindate glasses17,18, the appropriate laser equipment was used for measurements of luminescence spectra and decay curves. The laser system consists of PTI QuantaMaster QM40 spectrofluorimeter, optical parametric oscillator (OPO), Nd:YAG laser (Opotek Opolette 355 LD), double 200 mm monochromators and multimode UVVIS PMT (R928) and Hamamatsu H10330B-75 detectors, and PTI ASOC-10 [USB-2500] oscilloscope. The Varian Cary 5000 UV–VIS–NIR spectrophotometer was used for the absorption spectra measurements and the Metricon 2010 prism coupler for the refractive index at a wavelength of 632.8 nm. Resolution for spectral measurements was 0.1 nm, whereas an accuracy for decay curves was ± 0.5 μs.
Data availability
All data regarding the work presented here is available upon reasonable request to the corresponding author.
References
de Oliveira, M. A. S., de Araujo, C. B. & Messaddeq, Y. Upconversion ultraviolet random lasing in Nd3+ doped fluoroindate glass powder. Opt. Express 19, 5620–5626. https://doi.org/10.1364/OE.19.005620 (2011).
de Araujo, L. E. E. et al. Frequency upconversion of orange light into blue light in Pr3+-doped fluoroindate glasses. Phys. Rev. B 50, 16219–16223. https://doi.org/10.1103/PhysRevB.50.16219 (1994).
Kishimoto, S. & Hirao, K. Intense ultraviolet and blue upconversion fluorescence in Tm3+-doped fluoroindate glasses. J. Appl. Phys. 80, 1965–1969. https://doi.org/10.1063/1.363087 (1996).
Rakov, N., Maciel, G. S., de Araujo, C. B. & Messaddeq, Y. Energy transfer assisted frequency upconversion in Ho3+ doped fluoroindate glass. J. Appl. Phys. 91, 1272–1276. https://doi.org/10.1063/1.1430889 (2002).
Lozano, W. et al. Upconversion of infrared-to-visible light in Pr3+-Yb3+ codoped fluoroindate glass. Opt. Commun. 153, 271–274. https://doi.org/10.1016/S0030-4018(98)00268-5 (1998).
Oliveira, A. S. et al. Twentyfold blue upconversion emission enhancement through thermal effects in Pr3+/Yb3+-codoped fluoroindate glasses excited at 1.064 µm. J. Appl. Phys. 87, 4274–4278. https://doi.org/10.1063/1.373065 (2000).
Borrero-Gonzalez, L. J., Galleani, G., Manzani, D., Nunes, L. A. O. & Ribeiro, S. J. L. Visible to infrared energy conversion in Pr3+-Yb3+ co-doped fluoroindate glasses. Opt. Mater. 35, 2085–2089. https://doi.org/10.1016/j.optmat.2013.05.024 (2013).
Martin, I. R., Rodriguez, V. D., Lavin, V. & Rodriguez-Mendoza, U. R. Upconversion dynamics in Yb3+-Ho3+ doped fluoroindate glasses. J. Alloys Compd. 275–277, 345–348. https://doi.org/10.1016/S0925-8388(98)00336-3 (1998).
Martin, I. R., Mendez-Ramos, J., Rodriguez, V. D., Romero, J. J. & Garcia-Sole, J. Increase of the 800 nm excited Tm3+ blue upconversion emission in fluoroindate glasses by codoping with Yb3+ ions. Opt. Mater. 22, 327–333. https://doi.org/10.1016/S0925-3467(02)00292-6 (2003).
Catunda, T., Nunez, L. A. O., Florez, A., Messaddeq, Y. & Aegerter, M. A. Spectroscopic properties and upconversion mechanisms in Er3+-doped fluoroindate glasses. Phys. Rev. B 53, 6065–6070. https://doi.org/10.1103/PhysRevB.53.6065 (1996).
Maciel, G. S., de Araujo, C. B., Messaddeq, Y. & Aegerter, M. A. Frequency upconversion in Er3+-doped fluoroindate glasses pumped at 1.48 µm. Phys. Rev. B 55, 6335–6342. https://doi.org/10.1103/PhysRevB.55.6335 (1997).
Ribeiro, C. T. B., Zanatta, A. R., Nunes, L. A. O., Messaddeq, Y. & Aegerter, M. A. Optical spectroscopy of Er3+ and Yb3+ co-doped fluoroindate glasses. J. Appl. Phys. 83, 2256–2260. https://doi.org/10.1063/1.366965 (1998).
de Sousa, D. F. et al. Er3+:Yb3+ codoped lead fluoroindogallate glasses for mid infrared and upconversion applications. J. Appl. Phys. 85, 2502–2507. https://doi.org/10.1063/1.369612 (1999).
Perez-Rodriguez, C. et al. Upconversion emission obtained in Yb3+-Er3+ doped fluoroindate glasses using silica microspheres as focusing lens. Opt. Express 21, 10667–10675. https://doi.org/10.1364/OE.21.010667 (2013).
Hernandez-Rodriguez, M. A., Imanieh, M. H., Martin, L. L. & Martin, I. R. Experimental enhancement of the photocurrent in a solar cell using upconversion process in fluoroindate glasses exciting at 1480 nm. Solar Energy Mater. Solar Cells 116, 171–175. https://doi.org/10.1016/j.solmat.2013.04.023 (2013).
Bradley, J. D. B. & Pollnau, M. Erbium-doped integrated waveguide amplifiers and lasers. Laser Photonics Rev. 5, 368–403. https://doi.org/10.1002/lpor.201000015 (2011).
Kochanowicz, M. et al. Near-IR and mid-IR luminescence and energy transfer in fluoroindate glasses co-doped with Er3+/Tm3+. Opt. Mater. Express 9, 4772–4781. https://doi.org/10.1364/OME.9.004772 (2019).
Kochanowicz, M. et al. Structure, luminescence and energy transfer of fluoroindate glasses codoped with Er3+/Ho3+. Ceram. Int. 46, 26403–26409. https://doi.org/10.1016/j.ceramint.2020.02.210 (2020).
Wang, R. et al. Enhancement mechanisms of Tm3+-codoping on 2 μm emission in Ho3+ doped fluoroindate glasses under 888 nm laser excitation. Ceram. Int. 46, 6973–6977. https://doi.org/10.1016/j.ceramint.2019.11.108 (2020).
Wang, R. et al. 3.9 μm emission and energy transfer in ultra-low OH-, Ho3+/Nd3+ co-doped fluoroindate glasses. J. Lumin. 225, 117363. https://doi.org/10.1016/j.jlumin.2020.117363 (2020).
Liu, X., Chen, B. J., Pun, E. Y. B. & Lin, H. Ultra-broadband near-infrared emission in praseodymium doped germanium tellurite glasses for optical fiber amplifier operating at E-, S-, C-, and L-band. J. Appl. Phys. 111, 116101. https://doi.org/10.1063/1.4722997 (2012).
Coleman, D. J., Jackson, S. D., Golding, P. & King, T. A. Measurements of the spectroscopic and energy transfer parameters for Er3+-doped and Er3+, Pr3+-codoped PbO–Bi2O3–Ga2O3 glasses. J. Opt. Soc. Am. B 19, 2927–2937. https://doi.org/10.1364/JOSAB.19.002927 (2002).
Park, S. H., Lee, D. C., Heo, J. & Shin, D. W. Energy transfer between Er3+ and Pr3+ in chalcogenide glasses for dual-wavelength fiber-optic amplifiers. J. Appl. Phys. 91, 9072–9077. https://doi.org/10.1063/1.1476965 (2002).
Li, G. et al. Er3+ doped and Er3+/Pr3+ co-doped gallium antimony-sulphur chalcogenide glasses for infrared applications. Opt. Mater. Express 6, 3849–3856. https://doi.org/10.1364/OME.6.003849 (2016).
Zhou, B., Tao, L., Tsang, Y. H., Jin, W. & Pun, E. Y. B. Superbroadband near-infrared emission and energy transfer in Pr3+-Er3+ codoped fluorotellurite glasses. Opt. Express 20, 12205–12211. https://doi.org/10.1364/OE.20.012205 (2012).
Xu, Q. et al. Er3+ cross-section spectra of Er3+/Pr3+:Gd3Ga5O12 single-crystal: Pr3+-codoping effect. J. Am. Ceram. Soc. 102, 6407–6413. https://doi.org/10.1111/jace.16554 (2019).
Judd, B. R. Optical absorption intensities of rare-earth ions. Phys. Rev. 127, 750–761. https://doi.org/10.1103/PhysRev.127.750 (1962).
Ofelt, G. S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 37, 511–520. https://doi.org/10.1063/1.1701366 (1962).
Carnall, W. T., Fields, P. R. & Rajnak, K. Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 49, 4424–4442. https://doi.org/10.1063/1.1669893 (1968).
Zur, L., Janek, J., Sołtys, M., Pisarska, J. & Pisarski, W. A. Effect of BaF2 content on luminescence of rare-earth ions in borate and germanate glasses. J. Am. Ceram. Soc. 99, 2009–2016. https://doi.org/10.1111/jace.14223 (2016).
Zhang, L., Hu, H. & Lin, F. Emission properties of highly doped Er3+ fluoroaluminate glass. Mater. Lett. 47, 189–193. https://doi.org/10.1016/S0167-577X(00)00233-0 (2001).
Zhou, B., Tao, L., Tsang, Y. H., Jin, W. & Pun, E. Y. B. Superbroadband near-IR photoluminescence from Pr3+-doped fluorotellurite glasses. Opt. Express 20, 3803–3813. https://doi.org/10.1364/OE.20.003803 (2012).
Pisarska, J. et al. Influence of BaF2 and activator concentration on broadband near-infrared luminescence of Pr3+ ions in gallo-germanate glasses. Opt. Express 24, 2427–2435. https://doi.org/10.1364/OE.24.002427 (2016).
Zhang, F., Bi, Z., Huang, A. & Xiao, Z. Visible luminescence properties of Er3+–Pr3+ codoped fluorotellurite glasses. Opt. Mater. 41, 112–115. https://doi.org/10.1016/j.optmat.2014.10.029 (2015).
Zhou, M. et al. Broadband near-infrared luminescence at around 1.0 μm in Pr3+/Er3+ codoped tellurite glass. J. Lumin. 203, 689–695. https://doi.org/10.1016/j.jlumin.2018.07.021 (2018).
Choi, Y. G., Baik, J. H. & Heo, J. Spectroscopic properties of Pr3+: 1D2 → 1G4 transition in SiO2-based glasses. Chem. Phys. Lett. 406, 436–440. https://doi.org/10.1016/j.cplett.2005.03.028 (2005).
Chen, F. et al. Investigation of mid-infrared emission characteristics and energy transfer dynamics in Er3+ doped oxyfluoride tellurite glass. Sci. Rep. 5, 10676. https://doi.org/10.1038/srep10676 (2015).
Tian, Y., Xu, R., Hu, L. & Zhang, J. Enhanced 2.7 µm emission from Er3+/Tm3+/Pr3+ triply doped fluoride glass. J. Am. Ceram. Soc. 94, 2289–2291. https://doi.org/10.1111/j.1551-2916.2011.04645.x (2011).
Li, L., Zhou, Y., Qin, F., Zheng, Y. & Zhang, Z. On the Er3+ NIR photoluminescence at 800 nm. Opt. Express 28, 3995–4000. https://doi.org/10.1364/OE.386792 (2020).
Pelé, A. L. et al. Wavelength conversion in Er3+ doped chalcogenide fibers for optical gas sensors. Opt. Express 23, 4163–4172. https://doi.org/10.1364/OE.23.004163 (2015).
Golding, P. S., Jackson, S. D., King, T. A. & Pollnau, M. Energy transfer processes in Er3+-doped and Er3+, Pr3+-codoped ZBLAN glasses. Phys. Rev. B 62, 856–864. https://doi.org/10.1103/PhysRevB.62.856 (2000).
Cheng, P. et al. Pr3+/Er3+ co-doped tellurite glass with ultra-broadband near-infrared fluorescence emission. J. Lumin. 197, 31–37. https://doi.org/10.1016/j.jlumin.2018.01.005 (2018).
Shen, X. et al. Dual super-broadband NIR emissions in Pr3+-Er3+-Nd3+ tri-doped tellurite glass. Ceram. Int. 46, 14284–14286. https://doi.org/10.1016/j.ceramint.2020.02.196 (2020).
Li, G. S. et al. Broadband near-infrared emission in Pr3+-Er3+ codoped phosphate glasses for optical amplifiers. Ceram. Int. 42, 5558–5561. https://doi.org/10.1016/j.ceramint.2015.12.026 (2016).
Chu, Y. et al. Ce3+/Yb3+/Er3+ triply doped bismuth borosilicate glass: a potential fiber material for broadband near-infrared fiber amplifiers. Sci. Rep. 6, 33865. https://doi.org/10.1038/srep33865 (2016).
Huang, F. et al. Origin of near to middle infrared luminescence and energy transfer process of Er3+/Yb3+ co-doped fluorotellurite glasses under different excitations. Sci. Rep. 5, 8233. https://doi.org/10.1038/srep08233 (2015).
Huang, F., Liu, X., Hu, L. & Chen, D. Spectroscopic properties and energy transfer parameters of Er3+- doped fluorozirconate and oxyfluoroaluminate glasses. Sci. Rep. 4, 5053. https://doi.org/10.1038/srep05053 (2014).
Choi, Y. G., Kim, K. H., Park, B. J. & Heo, J. 1.6 μm emission from Pr3+:(3F3, 3F4) → 3H4 transition in Pr3+- and Pr3+/Er3+-doped selenide glasses. Appl. Phys. Lett. 78, 1249–1251. https://doi.org/10.1063/1.1350958 (2001).
Park, S. H., Lee, D. C., Heo, J. & Kim, H. S. Pr3+/Er3+ codoped Ge-As-Ga-S glasses as dual-wavelength fiber-optic amplifiers for 1.31 and 1.55 µm windows. J. Am. Ceram. Soc. 83, 1284–1286. https://doi.org/10.1111/j.1151-2916.2000.tb01370.x (2000).
Pisarski, W. A., Pisarska, J. & Ryba-Romanowski, W. Effect of erbium concentration on physical properties of fluoroindate glass. Chem. Phys. Lett. 380, 604–608. https://doi.org/10.1016/j.cplett.2003.08.055 (2003).
Gauthier, J.-C. et al. Mid-IR supercontinuum from 2.4 to 5.4 μm in a low-loss fluoroindate fiber. Opt. Lett. 41, 1756–1759. https://doi.org/10.1364/OL.41.001756 (2016).
Jia, S. et al. 2875 nm lasing from Ho3+-doped fluoroindate glass fibers. IEEE Photon. Technol. Lett. 30, 223–326. https://doi.org/10.1109/LPT.2017.2787119 (2018).
Majewski, M. R. et al. Emission beyond 4 μm and mid-infrared lasing in a dysprosium-doped indium fluoride (InF3) fiber. Opt. Lett. 43, 1926–1929. https://doi.org/10.1364/OL.43.001926 (2018).
Maes, F. et al. Room-temperature fiber laser at 392 μm. Optica 5, 761–764. https://doi.org/10.1364/OPTICA.5.000761 (2018).
Acknowledgements
The research project was funded by the National Science Centre (Poland) granted on the basis of the decision No. 2017/25/B/ST8/02530.
Author information
Authors and Affiliations
Contributions
W.A.P. and D.D. originated the research concept and interpreted results. M.K., M.K., J.Ż., P.M., A.B., M.L. carried out most of the experiments and data analysis. J.P., J.D., D.D. discussed the results and commented on the manuscript. W.A.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pisarski, W.A., Pisarska, J., Kuwik, M. et al. Fluoroindate glasses co-doped with Pr3+/Er3+ for near-infrared luminescence applications. Sci Rep 10, 21105 (2020). https://doi.org/10.1038/s41598-020-77943-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77943-w
This article is cited by
-
Impact of erbium oxide on structure, linear and nonlinear optical properties of bismo-borate glass systems for using in optical applications
Optical and Quantum Electronics (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.