Abstract
The purpose of our study was to investigate the relationship between plasma growth differentiation factor-15 (GDF-15) concentrations and diabetic retinopathy in patients with type 2 diabetes mellitus (DM). We evaluated 235 patients with type 2 DM in a cross-sectional study. Significantly increased levels of the plasma GDF-15 were found in individuals with diabetic retinopathy versus those without. According to the degree of diabetic retinopathy, there was a significant difference in the average plasma GDF-15 levels (no diabetic retinopathy, 1114 ng/L; nonproliferative diabetic retinopathy, 1327 ng/L; proliferative diabetic retinopathy, 1445 ng/L; p for trend = 0.035) after adjustments for confounders. Logistic regression analyses indicated that plasma GDF-15 concentrations were significantly associated with diabetic retinopathy (odds ratio per 1 standard deviation increment in the log-transformed value, 1.78; 95% confidence interval, 1.05–3.03, p = 0.032). Our study showed a significant positive relationship between plasma GDF-15 concentrations and diabetic retinopathy in type 2 DM patients.
Similar content being viewed by others
Introduction
Diabetic retinopathy is a common manifestation of diabetes-related microvascular damage in the retina, which can result in blindness in patients with diabetes mellitus (DM)1. Apart from the deleterious effect on vision, many studies have shown that diabetic retinopathy is linked to increased risks of systemic vascular diseases and mortality in type 2 DM patients1. In addition, diabetic retinopathy may result in a significant reduction in functional status and a decline in the quality of life2. Despite many studies demonstrating that diabetic retinopathy is primarily driven by hyperglycemia, it is unlikely that the risk of diabetic retinopathy can be accounted for by a single factor, indicating that its pathogenesis is multifactorial1.
Growth differentiation factor-15 (GDF-15) belongs to the transforming growth factor-β family and is also known as macrophage inhibitory cytokine-1. Its expression is enhanced in response to tissue ischemia3. Many previous studies have demonstrated that elevated GDF-15 concentrations were positively related with cardiovascular injury and mortality in humans4,5, suggesting its role as a novel biomarker for cardiovascular diseases6. Previous investigations have also shown a positive association between GDF-15 concentrations and diabetes risk6,7. Moreover, GDF-15 is reported to be implicated in the retinal inflammatory response8. However, the association GDF-15 levels and diabetic retinopathy in type 2 DM patients is not clear. Therefore, investigating whether GDF15 is implicated in the risk of diabetic retinopathy in type 2 DM patients is warranted.
The purpose of our study was to examine the association between plasma GDF-15 concentrations and diabetic retinopathy in individuals with type 2 DM.
Results
Table 1 presents the characteristics of the participants. The patients with diabetic retinopathy had a higher prevalence of hypertension, lower BMIs, higher HbA1c levels, a longer duration of DM, lower eGFRs, and higher AERs than those without. The patients with diabetic retinopathy more frequently received insulin treatment compared with those without. Among those with diabetic retinopathy, the prevalence of hypertension was greater and the plasma GDF-15 concentrations were significantly higher (Table 1).
Table 2 displays the average (95% confidence interval [CI]) plasma GDF-15 concentrations based on the degree of diabetic retinopathy. After adjusting for sex, age, hyperlipidemia, hypertension, hs-CRP, BMI, HbA1c, DM duration, eGFR, AER, and the use of insulin and OHAs, there was a significant difference in the average GDF-15 plasma levels according to the degree of diabetic retinopathy (no diabetic retinopathy, 1114 ng/L, 95% CI: 1033–1199; NPDR, 1327 ng/L, 95% CI: 1143–1538; PDR, 1445 ng/L, 95% CI: 1161–1803; p for trend = 0.035).
As shown in Table 3, we conducted logistic regression analysis to assess the effects of GDF-15 concentrations on diabetic retinopathy. After adjustment for sex, age, hyperlipidemia, hypertension, hs-CRP, BMI, HbA1c, DM duration, eGFR, AER, and the use of insulin and OHAs, a statistically significant association was persistent between plasma GDF-15 concentrations and diabetic retinopathy (OR per 1 SD increment in the log-transformed value, 1.78; 95% CI: 1.05–3.03, p = 0.032).
Analysis of the ROC curve of GDF-15 is displayed in Fig. 1. The AUC for GDF-15 was 0.701 (95% CI: 0.629–0.774; p < 0.001), and the optimal cut-off value was 1336 ng/L with 70.2% specificity and 64.2% sensitivity in predicting diabetic retinopathy.
ROC analysis of GDF-15 levels in predicting diabetic retinopathy in individuals with type 2 diabetes. AUC = 0.701 (p < 0.001), 95% CI: 0.629–0.774. Identified GDF-15 cutoff value = 1336 ng/L; sensitivity: 64.2%; specificity: 70.2%. ROC, receiver operating characteristic; AUC, area under the curve, GDF-15, growth differentiation factor-15.
Discussion
Our study found that plasma GDF-15 concentrations were independently and positively associated with diabetic retinopathy in individuals with type 2 DM. Our data revealed that the severity of diabetic retinopathy might be related with plasma GDF-15 concentrations. In addition, our findings suggest that GDF-15 may be a valuable biomarker for discriminating diabetic retinopathy in patients with type 2 DM.
GDF-15 has been increasingly recognized as a stress response cytokine3, and suggested to play a role in cell growth, differentiation, and inflammation3. GDF-15 is secreted from various cells, including endothelial cells and macrophages after tissue injury6, and increased GDF-15 levels may indicate tissue damage9. Previous studies emphasizing the association between plasma GDF-15 concentrations and cardiovascular disease have suggested GDF-15 as a potential biomarker for cardiovascular diseases. This was verified by numerous investigations demonstrating that high circulating levels of GDF-15 were related to the increased risks of heart failure, stroke, coronary heart disease, and cardiovascular mortality4,10,11.
Close associations between GDF-15 and increased risk of diabetes have been recently reported 6,9,12. Kempf et al.12 showed a positive correlation between plasma GDF-15 levels and insulin resistance. In addition, previous clinical investigations showed that GDF-15 levels were higher in individuals with type 2 DM compared with those without type 2 DM13,14. In a study with obese women, Dostálová et al.7 found that serum GDF-15 concentrations were increased in women with type 2 DM compared with control participants. Recently, Bao et al.15 reported that higher levels of GDF-15 were associated with increased incidence of diabetes. In addition, GDF-15 might be implicated in retinal inflammation and injury8,16. In response to optic nerve injury, the GDF-15 levels in the retina might be upregulated, as shown in previous research16. Ilhan et al.8 reported that increased levels of GDF-15 in the vitreous were found in inflammatory vitreoretinal disorders. However, the relationship between plasma GDF-15 concentrations and diabetic retinopathy in type 2 DM patients is not clear. Our data indicate a close association between GDF-15 levels and diabetic retinopathy in type 2 DM patients. We observed that there were significant differences in DM duration and HbA1c levels between patients with diabetic retinopathy and those without. Diabetic retinopathy was also related to impaired renal function and hypertension, consistent with previous reports1,17. Moreover, previous investigations have shown that GDF-15 levels were related to HbA1c levels4,6, and have reported that increased levels of GDF-15 were associated with renal impairment and increased prevalence of hypertension4,6. Hence, it is presumed that these might be partially responsible for the observed relationship between GDF-15 and retinopathy. However, our multivariable analysis showed that the statistically significant relationship between GDF-15 and diabetic retinopathy persisted despite adjustment for confounders including hypertension, HbA1c, DM duration, eGFR, and AER (Table 3). Consequently, the findings indicate that those variables did not exert significant influences on the relationship between them.
Even though the mechanisms by which GDF-15 might be implicated in the pathogenesis of diabetic retinopathy is not clear, possible explanations have been suggested. GDF-15 is involved in oxidative stress and endothelial function6,18. GDF-15 is also implicated in inflammatory and immune processes19,20. These are key pathogenic pathways of diabetic retinopathy1. Consequently, the close relationship between GDF-15 and diabetic retinopathy may be explained by the common pathways involved, while the causal inferences could not be drawn in our study. Further research is required to investigate the exact underlying mechanisms.
In the present study, we also found a positive association between plasma GDF-15 levels and the severity of diabetic retinopathy. Even though so far there are no available clinical data on the relationship between GDF-15 and pathogenesis of diabetic retinopathy in patients with type 2 DM, a possible implication of GDF-15 in progression of diabetic microvascular injury has been suggested. Hellemons et al.9 reported that increased circulating levels of GDF-15 were related with transition from normo- to micro- and from micro- to macroalbuminuria in patients with type 2 DM. Thus, large longitudinal studies are necessary to verify the usefulness of GDF-15 in prediction of retinal disease progression.
CRP is a well-recognized marker of systemic inflammation21. Despite extensive evidence indicating a link between inflammation and diabetic retinopathy1,22, the data in the literature in regard to the association between CRP and retinopathy are controversial23,24. In addition, in our study, multivariable analysis showed that the statistically significant relationship between GDF-15 levels and diabetic retinopathy persisted despite adjustment for confounders including hs-CRP levels. As a consequence, our findings suggest that GDF-15 might be linked to diabetic retinopathy through mechanisms independent of CRP. However, further research is necessary as other factors besides inflammatory stimuli can influence CRP levels25.
This study has some limitations. Due to a cross-sectional nature of the study, the causal relationship could not be established. Because of the relatively small sample size in this study, we were unable to perform further analysis on the differences between individual grades of NPDR. In addition, the number of patients with diabetic macular edema observed in our study was small (< 10%); hence, we could not conduct an analysis on diabetic macula edema. In spite of these limitations, our data might provide a valuable information on associations between GDF-15 levels and diabetic retinopathy in patients with type 2 DM.
In conclusion, plasma GDF-15 concentrations were positively associated with diabetic retinopathy in patients with type 2 DM. It is necessary to explore the underlying mechanisms between GDF-15 and retinopathy in individuals with type 2 DM in the future research.
Methods
Participants
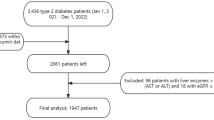
In the current cross-sectional study, we studied consecutively enrolled 235 patients with type 2 DM attending the diabetes clinic of our hospital. According to the Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus26, type 2 DM was diagnosed. Patients using glucocorticoids, those with renal impairment (serum creatinine ≥ 177 µmol/L), infections or inflammatory disorders, chronic liver disease, occlusive peripheral artery disease, stroke, cardiac disease, or malignancies were excluded from the study. Clinical information regarding past medical history and diabetes duration was obtained through a standardized inquiry. Hypertension was defined as taking anti-hypertensive medications or blood pressure ≥ 140/90 mmHg. Hyperlipidemia was considered as the use of lipid-lowering medications, total serum cholesterol concentrations ≥ 6.5 mmol/L, or triglyceride concentrations ≥ 2.3 mmol/L. The protocol was approved by the ethics committee of Chonnam National University Hospital, and all participants provided informed consent. The study was performed according to the Helsinki Declaration-based ethical principles for medical research involving human subjects.
Measurements
After the patients fasted overnight, venous blood samples were collected. Ion-exchange liquid chromatography (Tosoh, Tokyo, Japan) was used to assay glycated hemoglobin (HbA1c) levels. We measured high-sensitivity C-reactive protein (hs-CRP) levels by an immunonephelometric assay (Dade Behring, Marburg, Germany). The plasma GDF-15 concentrations were measured using a Human GDF-15 Quantikine enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). The intra-assay and inter-assay variabilities were < 5.0% and < 8.0%, respectively. Using the equation from the Chronic Kidney Disease Epidemiology Collaboration27, we assessed the estimated glomerular filtration rate (eGFR). We determined the urinary albumin excretion rate (AER) using the urinary albumin-to-creatinine ratio in random urine samples. An ophthalmologist carried out a dilated fundus examination to evaluate diabetic retinopathy. The patients were classified into three groups: no diabetic retinopathy, nonproliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR). Diabetic retinopathy referred to NPDR or PDR in the current study. Intra-grader reliability was assessed for 50 randomly chosen cases by re-classifying the retinal photographs. The intra-grader reliability kappa value was 0.91, indicating good reliability.
Statistical analyses
Sample size was determined to detect a medium effect size (d) of 0.5 with an alpha of 0.05 and 90% power. By using G*Power 3.1.9.228, this sample size was calculated for a 1:2 ratio of retinopathy/no retinopathy using two-tailed test. The estimated sample size was 192.
Statistical analysis was done using the Statistical Package for the Social Sciences software version 20.0 (SPSS, Chicago, IL, USA). The means ± standard deviation (SD) and frequencies (percentages) for the variables are presented unless otherwise described. Using the Kolmogorov–Smirnov test, we assessed the normal distribution of each variable. Differences in categorical and continuous variables for individuals with and without diabetic retinopathy were compared using chi-squared test, the Student’s t-test, or Mann–Whitney U test when appropriate. Differences among three groups were tested using analysis of variance or Kruskal–Wallis test. In order to compare the average GDF-15 concentrations according to the degree of diabetic retinopathy, we performed an analysis of covariance after adjustments for other covariates. For variables with skewed distributions, we performed logarithmic transformation before the analyses. To investigate the association between the GDF-15 concentrations and diabetic retinopathy, we carried out multiple logistic regression analyses using independent variables and factors with independent associations. Since the GDF-15 levels were not normally distributed, the odds ratios (ORs) per 1 SD increase in the log-transformed value of GDF-15 were calculated. A dummy variable was used to code the use of insulin and oral hypoglycemic agents (OHAs). In the fully adjusted regression model, age, sex, body mass index (BMI), hs-CRP, hyperlipidemia, hypertension, HbA1c, DM duration, AER, eGFR, and use of insulin and OHAs were included as covariates. A receiver operating characteristic (ROC) curve was generated to evaluate the specificity, sensitivity, and area under the curve (AUC) for predicting diabetic retinopathy. To determine the optimal cut-off values, the Youden index was estimated. A p-value of < 0.05 indicated statistical significance.
References
Cheung, N., Mitchell, P. & Wong, T. Y. Diabetic retinopathy. Lancet 376, 124–136. https://doi.org/10.1016/S0140-6736(09)62124-3 (2010).
Brown, M. M., Brown, G. C., Sharma, S. & Shah, G. Utility values and diabetic retinopathy. Am. J. Ophthalmol. 128, 324–330. https://doi.org/10.1016/s0002-9394(99)00146-4 (1999).
Fairlie, W. D. et al. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 65, 2–5. https://doi.org/10.1002/jlb.65.1.2 (1999).
Wollert, K. C., Kempf, T. & Wallentin, L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin. Chem. 63, 140–151. https://doi.org/10.1373/clinchem.2016.255174 (2017).
Lajer, M., Jorsal, A., Tarnow, L., Parving, H. H. & Rossing, P. Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care 33, 1567–1572. https://doi.org/10.2337/dc09-2174 (2010).
Adela, R. & Banerjee, S. K. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J. Diabetes Res. 2015, 490842. https://doi.org/10.1155/2015/490842 (2015).
Dostalova, I. et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur. J. Endocrinol. 161, 397–404. https://doi.org/10.1530/EJE-09-0417 (2009).
Ilhan, H. D., Bilgin, A. B., Toylu, A., Dogan, M. E. & Apaydin, K. C. The expression of GDF-15 in the human vitreous in the presence of retinal pathologies with an inflammatory component. Ocul. Immunol. Inflamm. 24, 178–183. https://doi.org/10.3109/09273948.2014.981549 (2016).
Hellemons, M. E. et al. Growth-differentiation factor 15 predicts worsening of albuminuria in patients with type 2 diabetes. Diabetes Care 35, 2340–2346. https://doi.org/10.2337/dc12-0180 (2012).
Brown, D. A. et al. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet 359, 2159–2163. https://doi.org/10.1016/S0140-6736(02)09093-1 (2002).
Schopfer, D. W., Ku, I. A., Regan, M. & Whooley, M. A. Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (The Heart and Soul Study). Am. Heart J. 167, 186–192. https://doi.org/10.1016/j.ahj.2013.09.013 (2014).
Kempf, T. et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur. J. Endocrinol. 167, 671–678. https://doi.org/10.1530/EJE-12-0466 (2012).
Vila, G. et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin. Chem. 57, 309–316. https://doi.org/10.1373/clinchem.2010.153726 (2011).
Shin, M. Y. et al. Association between Growth Differentiation Factor 15 (GDF15) and cardiovascular risk in patients with newly diagnosed type 2 diabetes mellitus. J. Korean Med. Sci. 31, 1413–1418. https://doi.org/10.3346/jkms.2016.31.9.1413 (2016).
Bao, X. et al. Growth differentiation factor 15 is positively associated with incidence of diabetes mellitus: the Malmo Diet and Cancer-Cardiovascular Cohort. Diabetologia 62, 78–86. https://doi.org/10.1007/s00125-018-4751-7 (2019).
Charalambous, P., Wang, X., Thanos, S., Schober, A. & Unsicker, K. Regulation and effects of GDF-15 in the retina following optic nerve crush. Cell Tissue Res. 353, 1–8. https://doi.org/10.1007/s00441-013-1634-6 (2013).
Ola, M. S., Nawaz, M. I., Siddiquei, M. M., Al-Amro, S. & Abu El-Asrar, A. M. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diab. Compl. 26, 56–64. https://doi.org/10.1016/j.jdiacomp.2011.11.004 (2012).
Kempf, T. et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 17, 581–588. https://doi.org/10.1038/nm.2354 (2011).
Bootcov, M. R. et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 94, 11514–11519. https://doi.org/10.1073/pnas.94.21.11514 (1997).
Schlittenhardt, D. et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 318, 325–333. https://doi.org/10.1007/s00441-004-0986-3 (2004).
Pepys, M. B. & Hirschfield, G. M. C-reactive protein: a critical update. J. Clin. Investig. 111, 1805–1812. https://doi.org/10.1172/JCI18921 (2003).
Tang, J. & Kern, T. S. Inflammation in diabetic retinopathy. Prog. Retin Eye Res. 30, 343–358. https://doi.org/10.1016/j.preteyeres.2011.05.002 (2011).
Spijkerman, A. M. et al. Endothelial dysfunction and low-grade inflammation and the progression of retinopathy in Type 2 diabetes. Diabet. Med. 24, 969–976. https://doi.org/10.1111/j.1464-5491.2007.02217.x (2007).
Muni, R. H. et al. Prospective study of inflammatory biomarkers and risk of diabetic retinopathy in the diabetes control and complications trial. JAMA Ophthalmol. 131, 514–521. https://doi.org/10.1001/jamaophthalmol.2013.2299 (2013).
Kang, E. S. et al. Relationship of serum high sensitivity C-reactive protein to metabolic syndrome and microvascular complications in type 2 diabetes. Diabetes Res. Clin. Pract. 69, 151–159. https://doi.org/10.1016/j.diabres.2004.11.014 (2005).
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26, S5–S20. https://doi.org/10.2337/diacare.26.2007.s5 (2003).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 (2009).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. https://doi.org/10.3758/bf03193146 (2007).
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1C1B5045647), Chonnam National University (2018-3551) and Chonnam National University Hospital Biomedical Research Institute (BCRI20035).
Author information
Authors and Affiliations
Contributions
J.O.C., S.Y.P. and M.Y.C. designed the study, drafted the manuscript, and approved its final version. S.Y.P. and J.O.C. contributed to the statistical analyses and interpretation of data. J.O.C., D.H.C., and D.J.C. acquired data. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chung, J.O., Park, SY., Cho, D.H. et al. Relationship between plasma growth differentiation factor-15 levels and diabetic retinopathy in individuals with type 2 diabetes. Sci Rep 10, 20568 (2020). https://doi.org/10.1038/s41598-020-77584-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77584-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.