Abstract
Previous studies of serum 25-hydroxyvitamin D (25(OH)D) in relation to melanoma have shown conflicting results. We conducted a nested case–control study of 708 cases and 708 controls, using prediagnostically collected serum, to study 25(OH)D and melanoma risk in the population-based Janus Serum Bank Cohort. Stratified Cox regression was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) adjusted for ultraviolet radiation (UVR) indicators and stratified by ambient UVB of residence and body mass index (BMI). Non-linear associations were studied by restricted cubic splines. Missing data were handled with multiple imputation by chained equations. We found an HR of melanoma risk of 1.01 (95% CI: 0.99, 1.04) and an HRimputed of 1.02 (95% CI: 1.00, 1.04) per 5-nmol/L increase. The spline model showed exposure-risk curves with significantly reduced melanoma risk between 60 and 85 nmol/L 25(OH)D (reference 50 nmol/L). Non-significant J-shaped curves were found in sub-analyses of subjects with high ambient UVB of residence and of subjects with BMI < 25 kg/m2. Our data did not yield persuasive evidence for an association between 25(OH)D and melanoma risk overall. Serum levels within the medium range might be associated with reduced risk, an association possibly mediated by BMI.
Similar content being viewed by others
Introduction
Incidence and mortality rates of cutaneous melanoma (hereafter melanoma) are increasing in fair-skinned populations worldwide1. Melanoma is currently the third and fifth most frequent cancer in Europe and USA, respectively1,2. Ultraviolet radiation (UVR) exposure is the primary environmental cause of melanoma3, and has been estimated to account for 80–90% of all cases4,5,6.
UVR exposure is also the main source of vitamin D7. Since the 1980s, vitamin D has been hypothesized to reduce both cancer incidence and mortality8. Laboratory studies have demonstrated anticancer properties of the hormonal form of vitamin D, 1,25-dihydroxycholecalciferol (1,25(OH)2D) in melanoma cell lines9,10,11. At UVR wavelengths of 290–320 nm, 7-dehydrocholesterol in the keratinocytes is converted to previtamin D3 (cholecalciferol). Previtamin D3 and D2 (ergocalciferol) are then hydroxylated in the liver to 25-hydroxyvitamin D (25(OH)D), representing the circulating form of vitamin D. The hormonal form, 1,25(OH)2D, is primarily formed in the kidney through endocrine synthesis (classical synthesis), but may also be formed in the skin and in non-renal cells by conversion of 25(OH)D to 1,25(OH)2D in an autocrine manner (non-classical synthesis), which has given support also for a role of 25(OH)D in cancer prevention12,13,14,15,16. Low 25(OH)D levels have been associated with obesity due to the fat-soluble properties of 25(OH)D,17,18,19 suggesting that body mass index (BMI) should be included as a factor when examining the association between 25(OH)D and melanoma association.
Although laboratory studies provide a biological plausibility that an adequate level of 25(OH)D may inhibit cancer development, some recent studies have not found evidence of an association20,21, while others have reported an inverse association between 25(OH)D and cancer risk22,23. The three most recent meta-analyses of serum 25(OH)D and melanoma risk concluded that there is no association24,25,26. However, the two earlier meta-analyses did not discriminate between pre- and postdiagnostic sampling of sera24,26, while the most recent only included prospective studies25. Studies with postdiagnostic serum 25(OH)D have generally shown lower 25(OH)D concentrations in melanoma patients than in controls27,28,29,30, but these findings might be due to reverse causation by the carcinogenic process itself31,32,33. Prospective studies have reported increased melanoma risk with increasing prediagnostic 25(OH)D serum levels34,35,36,37,38, most likely due to confounding by UVR exposure25,35,38. However, the prospective studies also vary in sample size, adjustment for UVR exposure, whether both sexes were studied, previous cancer history, and whether the melanoma cases were invasive or in situ. To our knowledge, no study has prospectively examined the 25(OH)D-melanoma association by ambient UVB of residence, BMI, anatomical site and histological subtype.
In the present study, we used stored sera from the population-based and prospective Janus Serum Bank Cohort (hereafter Janus Cohort) to examine the association between prediagnostic 25(OH)D and melanoma risk with adjustment for UVR indicators. Further, we aimed to assess the 25(OH)D-melanoma association with non-linear models, and by stratification on ambient UVB of residence, BMI, anatomical site and histological subtype.
Methods
Study population
We performed a case–control study nested in the Janus Cohort, a population-based biobank for prospective cancer studies containing serum samples from 318,628 Norwegians collected 1972–2003. Anthropometric measurements and questionnaire data are available for 292,851 cohort members who also participated in at least one of five regional health surveys. Detailed descriptions of the cohort establishment and data have been published elsewhere39,40.
The present study was designed as a nested case–control study within the prospective Janus Cohort to reduce laboratory costs. No samples were analyzed for 25(OH)D immediately after blood draw, but stored at − 25 °C and thawed in 2016–2017 for the cases and controls selected for this study according to a pre-defined study protocol33. The design and analysis-time of this study is therefore prospective since all study participants were cancer free at blood-draw. The study research file was created by linkage of the Janus Cohort to Statistics Norway, the Norwegian National Population Register and the Cancer Registry of Norway (CRN) by the use of the 11-digit personal identification number assigned to all Norwegian citizens. Additionally it contains group level information on ambient ultraviolet-B (UVB) of residence and sun tanning behavior from the Norwegian Women and Cancer (NOWAC) cohort study. A study protocol with details about the linkage and data sources for the current study has been published33.
Legal and ethical approval was obtained from the Regional Committee for Medical Research Ethics.
Identification of cancer cases
The linkage to the Norwegian National Registry (Population Register) provided information on vital status, year of death or year of emigration. The CRN linkage provided a complete cancer history (1953–2009) for all individuals with a melanoma diagnosis. Reporting of incident cancers to the CRN is compulsory by law, and data from several sources ensure high quality data with 99.5% being morphologically verified41,42. Cases were required to (1) be histologically verified melanomas, (2) have no cancer before their melanoma diagnosis (except basal cell carcinoma, which is not registered in the CRN), (3) be diagnosed after recruitment into the Janus Cohort, (4) be aged < 75 years at diagnosis, (5) be diagnosed before 2009 and (6) have at least 2 years between time from blood draw and subsequent diagnosis33. Information on tumour localization was based on a local CRN modified version of the International Classification of Diseases, 7th revision (ICD-7 codes 1900–1909), converted into ICD-10 codes (head and neck = C43.0–4; trunk = C43.5; upper limbs = C43.6; lower limbs = C43.7; other and not otherwise specified = C43.8–9). Histological subtypes of melanoma were defined by codes from ICD-Oncology, 3rd edition (superficial spreading melanoma = 8743; nodular melanoma = 8721; other = 8000, 8723, 8730, 8742, 8744, 8745, 8770, 8772; not otherwise specified = 8720).
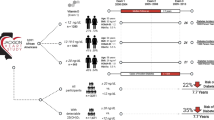
Follow-up was defined as from the year recruited into the Janus Cohort (baseline, ranging 1972–2003) through 31 December 2009. During follow-up, a total of 1810 incident melanoma cases were identified (using the abovementioned case criteria). The number of included cases was limited to a random selection of 710 of the identified melanomas, based on power calculations to reduce laboratory costs33. A flow chart of the study design and exclusions is shown in Fig. 1. Two cases were excluded (together with their controls) post-selection as they were not histologically verified (Fig. 1).
Identification of controls
Controls were drawn at random with replacement from a pool of available controls according to an incidence density sampling scheme43, and required to be alive, resident in Norway and without a cancer history before the melanoma diagnosis of the case. One control was matched to each case on sex, date of blood draw (+ /− 2 years), year of birth (+ /− 1 year), season of blood draw to account for seasonal variation in 25(OH)D (December–February, March–May, June–August, September–November)44, and county of blood draw.
Assessment of exposures
Laboratory analyses
Concentrations of 25(OH)D were measured during 2016–2017 using an in-house developed liquid chromatography/tandem mass spectrometry method at the Hormone Laboratory, Oslo University Hospital45. The laboratory participates in the Vitamin D External Quality Assessment Scheme for total 25(OH)D. Further, the laboratory is approved by Norwegian Accreditation and complies with the requirements of the NS-EN ISO/IEC 17025 standards. Laboratory results of 25(OH)D were provided in nmol/L on a continuous scale. We categorized 25(OH)D into quantiles based on the 25(OH)D distribution among controls (quintile 1: < 49.2, quintile 2: 49.2–61.8, quintile 3: 61.9–74.7, quintile 4: 74.8–89.2, quintile 5: > 89.2; and tertiles in sub-analyses tertile 1: < 58.5 tertile 2: 58.5–79.8, tertile 3: > 79.8 nmol/L), and according to clinical cut points (11–29, 30–49, 50–74, 75–212 nmol/L).
Health survey and registry data
Baseline measurements of height (to the nearest 1 cm) and weight (to the nearest 0.5 kg) were obtained by trained staff according to a standardized protocol. BMI was calculated (weight/height2). Body surface area (BSA, m2) was calculated using the DuBois and DuBois’ equation (weight0.4253 × height0.7253 × 0.007184)46.
The variable ‘Ambient UVB of residence’ at baseline was constructed by categorizing county of residence into north, mid, southwest, southeast inland, and southeast coast (in an ascending order from lower to higher UVB doses). The variable ‘Lifetime ambient UVB’ was constructed by linking county-specific UVB doses to county of residence at baseline, and then by cumulating yearly UVB doses from birth to diagnosis for each case–control set. UVB data were derived from UV measurement stations and from modelled values as described by Medhaug et al.47.
The Janus Cohort lacked individual data on UVR behavior variables. Therefore, group level data on annual mean number of sunburns, sunbathing vacations, and solarium sessions from members of the NOWAC study were linked to the Janus Cohort members, based on combinations of age, county and time period33,48. Continuous variables for lifetime number of sunburns, sunbathing vacations, and solarium sessions were constructed by cumulating annual mean numbers from birth to diagnosis for each case–control set. The rationale for conducting this group-level data linkage between the NOWAC (women only) and the Janus Cohort (men and women) was based on a survey conducted by the Norwegian Cancer Society49, showing only small gender differences for sunburns and sunbathing vacations. However, as solarium use was found to be more frequently used by women, group-level data on solarium use was only applied to women49.
The linkage to Statistics Norway provided information on occupation and highest attained educational level (none, compulsory, upper secondary, college/university, unknown) at baseline. Occupational UVR exposure at baseline (indoor, mixed, outdoor, unknown) was constructed by categorizing occupational codes according to Alfonso et al.50 who used these categories as proxies for occupational sun exposure.
The questions about physical activity and smoking were worded differently in each survey and have been harmonized: physical activity: inactive, low, medium, high, unknown; and smoking status: never, former, current, unknown40.
Data analysis
Continuous variables are presented as means (with standard deviation or range) or medians (with interquartile range) and categorical variables as frequencies (%). Stratified Cox regression was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the association between 25(OH)D and melanoma risk43. Restricted cubic splines with 5 knots (3 knots in stratified analyses) were incorporated into the Cox models to assess the shape of the 25(OH)D-melanoma association using the R package rms51. A likelihood ratio test was used to compare the fit of the linear and spline models. We adjusted for BMI, BSA, lifetime ambient UVB, lifetime sunburns, lifetime sunbathing vacations, occupational UVR, education, physical activity, and smoking status in all models. Additional adjustment for solarium use (women only) did not change the results.
Likelihood ratio tests were used to test the statistical significance of interaction between continuous 25(OH)D and ambient UVB of residence (dichotomized into low: north, mid, southwest and high: southeast inland, southeast coast) and BMI (dichotomized into normal weight: BMI < 25 kg/m2 and overweight: BMI ≥ 25 kg/m2). For ambient UVB of residence, HRs and 95% CI were estimated from stratified analyses, whereas for BMI HRs and 95% CI were computed as linear combinations of the estimated regression coefficients to not loose case–control pairs in the opposite BMI strata. Levels of 25(OH)D in persons with BMI < 25 and ≥ 25 kg/m2 were compared by a two sample t-test.
We performed analyses stratified by anatomical site and histological subtype and tested whether 25(OH)D-melanoma associations differed between anatomical sites and between histological subtypes by a contrast test (test for heterogeneity)52.
To assess the influence of extreme values we performed sensitivity analyses excluding persons with 25(OH)D below the 2.5 percentile or above the 97.5 percentile (Supplemental Material). To assess the influence of missing values we used multiple imputation with chained equations, assuming missing at random53. The imputation model included the outcome and all exposures and adjustment variables. We imputed 30 datasets, and the estimates and standard errors were combined using Rubin's rules (Supplemental Material)54.
Tests for significance were two-sided, and the statistical significance level set to < 0.05. Analyses were performed using Stata version 16.1 (StataCorp, College Station, TX, USA) and R version 3.6.1 (https://cran.r-project.org). The R package mice, version 3.6.0, was used for multiple imputation55.
Ethical approval
The study has approval from the Regional Committee for Medical and Health Research Ethics (no. 2014/185).
Results
Characteristics of the 708 cases and 708 controls are presented in Table 1. Mean 25(OH)D level at baseline was significantly higher among melanoma cases than controls (73.9 vs 70.8 nmol/L, respectively; P = 0.03). BMI, BSA, lifetime ambient UVB, sunburns, sunbathing vacations, solarium use, occupational UVR and physical activity were comparable between cases and controls. More cases (22%) held a college/university degree than controls (19%), while a larger proportion of controls (42%) than cases (32%) were current smokers (Table 1). In total, 607 case–control sets had complete information on all covariates (Fig. 1) and their characteristics (Table S1) were largely similar to the characteristics of the whole sample (Table 1), but differed by having comparable proportions of current smokers (cases 32% and controls 33%).
Table 2 shows associations between 25(OH)D and risk of melanoma for both complete-case analyses and imputed analyses. Little difference was seen between the effect estimates of the complete-case analyses and those of the imputed analyses. The complete-case and the imputed analyses showed HRs of 1.01 (95% CI: 0.99, 1.04) and 1.02 (95% CI: 1.00, 1.04) per 5 nmol/L increase, respectively. No significant effect estimates were found for quintiles or clinical cut points.
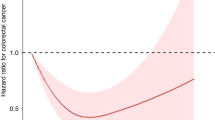
In Fig. 2, we modelled the 25(OH)D-melanoma association with restricted cubic splines (complete case). The exposure-risk curve dipped to HRs below 1.00 for 25(OH)D levels between 60 and 85 nmol/L (CI upper bound < 1.00), but the spline model did not show a better fit than the linear model (likelihood ratio test P = 0.15). Sensitivity analyses excluding the lowest and highest 25(OH)D values, did not change the shape of the curve materially (Figure S1).
Restricted cubic splines displaying hazard ratios of melanoma with 95% confidence intervals according to prediagnostic serum 25-hydroxyvitamin D in the Janus Serum Bank Cohort, Norway, 1972–2009. Complete case sample: 607 cases and 607 controls. Reference set to 50 nmol/L. Knots located at 36.35, 55.82, 69.83, 85.9, 121.82 nmol/L (first and last at 5 and 95 percentile, the remaining equally spaced), P value for non-linearity 0.15. Adjusted for body mass index, body surface area, lifetime ambient ultraviolet (UV)-B, lifetime sunburns, lifetime sunbathing vacations, occupational UV radiation exposure, education, physical activity, smoking status.
Figure 3 shows splines for 25(OH)D and melanoma risk stratified by ambient UVB of residence (complete case). The exposure-risk curve was slightly U-shaped for those residing in the low UVB exposure regions (panel A: north, mid, southwest) but with large uncertainty, whereas a more J-shaped curve was seen among residents with the highest UVB exposure (panel B: southeast). Linear modelling (Table S2, complete case) showed no significant associations and no significant interaction was found (Pinteraction 0.237). Multiple imputation analyses yielded similar results (Table S2). The non-linear models did not show a better fit than the linear models (P values 0.46 and 0.16 for lowest and highest ambient UVB of residence, respectively). Sensitivity analyses excluding the lowest and highest 25(OH)D values, made the curve in panel A point slightly more downwards, while the curve in panel B did not change materially (Figure S2).
Restricted cubic splines displaying hazard ratios (HRs) of melanoma with 95% confidence intervals according to prediagnostic serum 25-hydroxyvitamin D by ambient UVB of residence in the Janus Serum Bank Cohort, Norway, 1972–2009. (A) Splined HRs for north, mid and southwest with knots located at 41.14, 67.48, 106 nmol/L, P value for non-linearity 0.456. (B) Splined HRs for southeast inland and coast with knots located at 41.43, 70.78, 109.96 nmol/L, P value for non-linearity 0.16. (A,B) Complete case sample: 607 cases and 607 controls. Reference set to 50 nmol/L. Adjusted for body mass index, body surface area, lifetime ambient ultraviolet (UV)-B, lifetime sunburns, lifetime sunbathing vacations, occupational UV radiation exposure, education, physical activity, smoking status.
Figure 4 shows splines for 25(OH)D and melanoma risk among the normal weight (panel A: BMI < 25 kg/m2) and the overweight (panel B: BMI ≥ 25 kg/m2). Among the normal weight, the curve indicated a J-shape with reduced risk for 25(OH)D between 60–80 nmol/L (CI upper bound ≈1.00), while among overweight a non-significant curve with a flatter shape was seen. Significant interaction was found in the spline model (Pinteraction = 0.04 and 0.01 for linear and non-linear associations, respectively). Linear models (Table S3) showed no significant associations and no significant interaction (Pinteraction = 0.73), and the spline model showed a better fit than the linear model (P = 0.01). Sensitivity analyses excluding the lowest and highest 25(OH)D values, did not change the shape of the curve in panel A materially, while the curve in panel B pointed slightly more upwards (Figure S3). Mean 25(OH)D was significantly lower in overweight than in normal weight (69.6 vs 75.2 nmol/L, respectively; P < 0.001).
Restricted cubic splines displaying hazard ratios (HRs) of melanoma with 95% confidence intervals according to prediagnostic serum 25-hydroxyvitamin D by body mass index (BMI) in the Janus Serum Bank Cohort, Norway, 1972–2009. The curves are estimated based on linear combinations of the main effect term and the interaction term. Complete case sample: 607 cases and 607 controls. Reference set to 50 nmol/L. Knots located at 41.22, 69.83, 108.43 nmol/L (10, 50 and 90 percentile, the remaining equally spaced). Pinteraction 0.04 and 0.01 for the first (knot 1–2) and second (knot 2–3) curve-segments, respectively. P value for non-linearity 0.011. Adjusted for BMI (continuous), body surface area, lifetime ambient ultraviolet (UV)-B, lifetime sunburns, lifetime sunbathing vacations, occupational UV radiation exposure, education, physical activity, smoking status.
We also examined the 25(OH)D-melanoma association by anatomic site (Tables S4a and S4b) and found no clear pattern and no significant difference between the sites (Pheterogeneity = 0.24). When examined by histological subtype (Table S5), no clear pattern was seen and no significant difference was found between the subtypes (Pheterogeneity = 0.37).
Discussion
In this prospective analysis, we estimated an overall non-significant positive association between prediagnostic serum 25(OH)D and melanoma risk using linear models. However, when we explored the shape of the association by employing non-linear models, we found an exposure-risk curve suggesting reduced risk of melanoma for 25(OH)D levels between 60 and 85 nmol/L. Indications of exposure-risk curves with a J-shape were seen in sub-analyses of persons with high ambient UVB of residence and BMI < 25 kg/m2, although not statistically significant.
Studying the 25(OH)D–melanoma association is challenging due to the possible reverse causation from the melanoma diagnosis itself31,32,33, and due to potentially strong confounding from UVR exposure35,38, which is an important source of 25(OH)D and the primary risk factor of melanoma. Our finding of a non-significant positive association is largely in line with other studies examining the 25(OH)D-melanoma association using prediagnostic samples and linear models34,36,37,38. This positive association indicates confounding by UVR as high levels of 25(OH)D are unlikely to cause melanoma56.
Our finding of reduced risk for levels between 60 and 85 nmol/L, might be explained by that persons within this range pursue a more healthy lifestyle with moderate UVR exposure and a diet rich on vitamin D57. For people living in northern and western Norway, with lower ambient UVB of residence and higher dietary intake of vitamin D57, the exposure-risk curve did not rise at higher 25(OH)D levels. The increase in risk seen from 100 nmol/L onwards among persons living in southeastern Norway with the highest ambient UVB exposure of residence, might, however, reflect increased sun exposure. These findings are in line with the results from a comparable Danish cohort that concluded that increasing 25(OH)D levels were associated with increased melanoma risk, and that 25(OH)D is a surrogate marker of UVB exposure34. However, high, non-UVB induced, levels of 25(OH)D above 100 nmol/L may also indicate vitamin D treatment due to deficiency among patients58.
We found indications of effect modification by BMI in the spline model; the exposure-risk curve among normal weight persons suggested a similar dip (Fig. 4A; 60–80 nmol/L) as in the overall analysis (Fig. 2; 60–85 nmol/L), lending support to the explanation that this might reflect a healthy lifestyle. A flatter shape of the exposure-risk curve was seen among overweight persons, although with large uncertainty for 25(OH)D levels of 100 nmol/L onwards. This might indicate that overweight persons sunbathe less than normal weight persons59, and hence that the 25(OH)D-melanoma association is less prone to confounding by UVR in overweight. Our finding that mean 25(OH)D was significantly lower in overweight compared with normal weight, accords with that from another Norwegian cohort18, and might be a marker of less UVB exposure34 or be due to decreased bioavailability of 25(OH)D among overweight19. Importantly, being overweight is per se a possible melanoma risk factor but in comparison with UVR exposure, only a small fraction of the melanoma cases may be attributed to overweight or body size60.
In line with previous studies of 25(OH)D and melanoma risk34,35,36,37,38, a recent Mendelian randomization study did not find evidence for causal link between genetic determinants of 25(OH)D levels and melanoma risk61. Also, we recognize that our spline regressions yielded wide CIs and only showed a significantly better fit to the data for the model including the interaction term with BMI. Further, a J-shaped curve may also reflect underlying co-morbidity resulting in higher risk for lower concentrations of 25(OH)D, or it may be a mathematical side effect of combining a spline model and a Cox model62. Although radiation over the whole UV-spectrum (100–400 nm) is considered to cause melanoma in humans63, the mechanistic role of UVA and UVB in the melanomagenic process differs. Melanoma induction by UVA is dependent on melanin pigment, while UVB initiates the melanomagenesis independent of melanin pigment64,65.
We are therefore precautious about overstating a possible preventive role of 25(OH)D on melanoma risk, and interpret this finding as possible. If, however, 25(OH)D is inversely associated with melanoma risk, it is likely through activation of the 1,25(OH)2D receptor12, which has been shown to have various anti-proliferative and anti-inflammatory effects11. Further, 1,25(OH)2 may inhibit growth of neoplasms through cell-cycle arrest66, and reduce inflammation and serum cytokine levels through reduction of stress-activated kinase signaling67,68.
Our study is the largest prospective study to evaluate the association between 25(OH)D and melanoma risk that includes only invasive and histologically verified incident melanomas from a complete and nationwide cancer registry. To our knowledge, it is also the first study to explore the shape of the 25(OH)D-melanoma association and to conduct sub-analyses by ambient UVB of residence and BMI in a high-latitude population. An important strength is that we only sampled cases and controls without a cancer history, which together with the use of prediagnostic serum samples limit the possibility of reverse causation. Also, our study was nested in the population-based Janus Cohort and linked to the National Population Register securing complete control of loss to follow-up. A major limitation was that our sub-analyses were not adequately powered to exclude or confirm possible medium range associations. Further, we did not have individual information on pigmentary traits and only group level data on sunburns, sunbathing and solarium, which hampered adjustment of these factors and likely resulted in confounding by UVR exposure. Difference in skin color could potentially bias our results, but since the fraction of non-whites in Norway was less than 1% during 1970–1991 (when 97.5% of the Janus Cohort members were recruited), we consider the risk of such bias as small33. Serum 25(OH)D levels have been shown to be stable after storage at − 25 °C69. A possible time-dependent degradation was compensated for by matching cases and controls on date of blood draw. Difference in time since blood draw might have affected our results, although a recent study from the Janus Cohort, using repeated 25(OH)D measurements in relation to cancer survival, showed that 25(OH)D was stable over time70. Quality control samples (results not shown) showed that measurement precision for 25(OH)D was variable, but since matched case–control pairs were placed next to each other on the same batch, intra-batch variability was reduced.
In this large prospective case–control study nested in a population-based cohort, we did not find overall persuasive evidence for an association between 25(OH)D and melanoma risk, although spline curves suggested a possible reduced risk for 25(OH)D serum levels within the normal range (60–85 nmol/L) overall, and in sub-analyses of individuals with BMI < 25 kg/m2. The fact that UVR exposure is a common causal factor for both 25(OH)D production and melanoma, may lead to confounding, as was suggested in our results by risk estimates increasing at higher 25(OH)D values in the most sunny parts of the country.
Data availability
Requests for data sharing/case pooling may be directed to the corresponding author. This project uses third-party data derived from State government registries, which are ultimately governed by their ethics committees and data custodians. Thus, any requests to share these data will be subject to formal approval from each data source used in this project.
References
Karimkhani, C. et al. The global burden of melanoma: Results from the Global Burden of Disease Study 2015. Br. J. Dermatol. 177, 134–140 (2017).
2Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30, https://doi.org/10.3322/caac.21387 (2017).
3Berwick, M. et al. Melanoma 17–49 (Springer, 2016).
Arnold, M. et al. Cutaneous melanoma in France in 2015 attributable to solar ultraviolet radiation and the use of sunbeds. J. Eur. Acad. Dermatol. Venereol. 32, 1681–1686 (2018).
Parkin, D. M., Boyd, L. & Walker, L. C. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br. J. Cancer 105 Suppl 2, S77–S81, https://doi.org/10.1038/bjc.2011.489 (2011).
Whiteman, D. C. & Wilson, L. F. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiol. 44, 203–221. https://doi.org/10.1016/j.canep.2016.06.013 (2016).
Bikle, D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 94, 26–34. https://doi.org/10.1210/jc.2008-1454 (2009).
Garland, C. F. & Garland, F. C. Do sunlight and vitamin D reduce the likelihood of colon cancer?. Int. J. Epidemiol. 9, 227–231 (1980).
Reichrath, J. et al. In vitro comparison of the vitamin D endocrine system in 1, 25 (OH) 2D3-responsive and- resistant melanoma cells. Cancer Biol. Ther. 6, 48–55 (2007).
Seifert, M., Rech, M., Meineke, V., Tilgen, W. & Reichrath, J. Differential biological effects of 1, 25-dihydroxy vitamin D3 on melanoma cell lines in vitro. J. Steroid Biochem. Mol. Biol. 89, 375–379 (2004).
Krishnan, A. V. & Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 51, 311–336. https://doi.org/10.1146/annurev-pharmtox-010510-100611 (2011).
Barreto, A. M., Schwartz, G. G., Woodruff, R. & Cramer, S. D. 25-Hydroxyvitamin D3, the prohormone of 1, 25-dihydroxyvitamin D3, inhibits the proliferation of primary prostatic epithelial cells. Cancer Epidemiol. Prevent. Biomark. 9, 265–270 (2000).
Bikle, D. & Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 16, 234–252. https://doi.org/10.1038/s41574-019-0312-5 (2020).
Schwartz, G. G., Whitlatch, L. W., Chen, T. C., Lokeshwar, B. L. & Holick, M. F. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol. Biomark. Prevent. 7, 391–395 (1998).
Swami, S. et al. Dietary vitamin D3 and 1, 25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 153, 2576–2587 (2012).
Verone-Boyle, A. R. et al. Diet-derived 25-hydroxyvitamin D3 activates vitamin D receptor target gene expression and suppresses EGFR mutant non-small cell lung cancer growth in vitro and in vivo. Oncotarget 7, 995 (2016).
Holick, M. F. Vitamin D deficiency. N. Engl. J. Med. 357, 266–281. https://doi.org/10.1056/NEJMra070553 (2007).
Mai, X. M., Chen, Y., Camargo, C. A. Jr. & Langhammer, A. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: The HUNT study. Am. J. Epidemiol. 175, 1029–1036. https://doi.org/10.1093/aje/kwr456 (2012).
Wortsman, J., Matsuoka, L. Y., Chen, T. C., Lu, Z. & Holick, M. F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 72, 690–693. https://doi.org/10.1093/ajcn/72.3.690 (2000).
Keum, N., Lee, D. H., Greenwood, D. C., Manson, J. E. & Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 30, 733–743. https://doi.org/10.1093/annonc/mdz059 (2019).
Manson, J. E., Bassuk, S. S. & Buring, J. E. Vitamin D, calcium, and cancer: Approaching daylight?. JAMA 317, 1217–1218. https://doi.org/10.1001/jama.2017.2155 (2017).
Budhathoki, S. et al. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site specific cancers in Japanese population: Large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ (Clin. Res. Ed.) 360, k671 (2018).
McCullough, M. L. et al. Circulating vitamin D and colorectal cancer risk: An international pooling project of 17 cohorts. J. Natl Cancer Inst. 111, 158–169. https://doi.org/10.1093/jnci/djy087 (2019).
Caini, S. et al. Vitamin D and melanoma and non-melanoma skin cancer risk and prognosis: A comprehensive review and meta-analysis. Eur. J. Cancer 50, 2649–2658. https://doi.org/10.1016/j.ejca.2014.06.024 (2014).
Mahamat-Saleh, Y., Aune, D. & Schlesinger, S. 25-Hydroxyvitamin D status, vitamin D intake, and skin cancer risk: A systematic review and dose–response meta-analysis of prospective studies. Sci. Rep. 10, 13151. https://doi.org/10.1038/s41598-020-70078-y (2020).
Tsai, T. Y., Kuo, C. Y. & Huang, Y. C. The association between serum vitamin D level and risk and prognosis of melanoma: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. (JEADV) https://doi.org/10.1111/jdv.16189 (2020).
Bade, B. et al. Low serum 25-hydroxyvitamin D concentrations are associated with increased risk for melanoma and unfavourable prognosis. PLoS ONE 9, e112863 (2014).
Navarrete-Dechent, C. et al. Circulating vitamin D-binding protein and free 25-hydroxyvitamin D concentrations in patients with melanoma: A case-control study. J. Am. Acad. Dermatol. 77, 575–577 (2017).
Cattaruzza, M. S. et al. 25-Hydroxyvitamin D serum levels and melanoma risk: A case–control study and evidence synthesis of clinical epidemiological studies. Eur. J. Cancer Prevent. 28, 203–211 (2019).
Befon, A. et al. Serum total 25-hydroxyvitamin D levels in patients with cutaneous malignant melanoma: A case-control study in a low-risk southern European population. Dermatol. Pract. Concept. 10, e2020010. https://doi.org/10.5826/dpc.1001a10 (2020).
Berwick, M. & Erdei, E. O. Vitamin D and melanoma incidence and mortality. Pigment Cell Melanoma Res. 26, 9–15. https://doi.org/10.1111/pcmr.12015 (2013).
Robsahm, T., Schwartz, G. & Tretli, S. The inverse relationship between 25-hydroxyvitamin D and cancer survival: Discussion of causation. Cancers 5, 1439–1455 (2013).
Stenehjem, J. S. et al. A protocol for prospective studies of 25-hydroxyvitamin D, leptin and body mass index in relation to cutaneous melanoma incidence and survival. BMJ Open 7, e014829 (2017).
Afzal, S., Nordestgaard, B. G. & Bojesen, S. E. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: A prospective cohort study. J. Invest. Dermatol. 133, 629–636. https://doi.org/10.1038/jid.2012.395 (2013).
Kwon, G. P. et al. Association of 25-hydroxyvitamin D levels and cutaneous melanoma: A nested case-control study of the Women’s Health Initiative Observation Study. J. Am. Acad. Dermatol. 79, 145–147. https://doi.org/10.1016/j.jaad.2017.05.037 (2018).
Major, J. M. et al. Pre-diagnostic circulating vitamin D and risk of melanoma in men. PLoS ONE 7, e35112. https://doi.org/10.1371/journal.pone.0035112 (2012).
Skaaby, T. et al. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancer. Cancer Epidemiol. Prevent. Biomark. 23, 1220–1229 (2014).
van der Pols, J. C. et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J. Invest. Dermatol. 133, 637–641, https://doi.org/10.1038/jid.2012.346 (2013).
Langseth, H., Gislefoss, R. E., Martinsen, J. I., Dillner, J. & Ursin, G. Cohort profile: The Janus Serum Bank Cohort in Norway. Int. J. Epidemiol. https://doi.org/10.1093/ije/dyw027 (2016).
Hjerkind, K. V. G. R., Tretli, S., Nystad, W., Bjørge, T., Engeland, A., Meyer, H.E., Holvik, K., Ursin, G., Langseth, H. Cohort profile update: The Janus Serum Bank Cohort in Norway. Int. J. Epidemiol., https://doi.org/10.1093/ije/dyw302 (2016).
Larsen, I. K. et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur. J. Cancer 45, 1218–1231. https://doi.org/10.1016/j.ejca.2008.10.037 (2009).
Robsahm, T. E., Bergva, G., Hestvik, U. E. & Moller, B. Sex differences in rising trends of cutaneous malignant melanoma in Norway, 1954–2008. Melanoma Res. 23, 70–78. https://doi.org/10.1097/CMR.0b013e32835c7e48 (2013).
Pearce, N. What does the odds ratio estimate in a case-control study?. Int. J. Epidemiol. 22, 1189–1192 (1993).
Aalerud, T. N. & Johnsen, B. The Norwegian UV Monitoring Network. 1995/96-2004. (Statens Straalevern, 2006).
Frigstad, S. O. et al. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 1–7, https://doi.org/10.1080/00365521.2016.1233577 (2016).
Wentworth, J. (Elsevier, 1982).
47Medhaug, I., Olseth, J. A. & Reuder, J. UV radiation and skin cancer in Norway. J. Photochem. Photobiol. B Biol. 96, 232–241, https://doi.org/10.1016/j.jphotobiol.2009.06.011 (2009).
Lund, E. et al. Cohort profile: The Norwegian Women and Cancer Study-NOWAC-Kvinner og kreft. Int. J. Epidemiol. 37, 36–41. https://doi.org/10.1093/ije/dym137 (2008).
Norwegian-Cancer-Society. Solvaner i den norske befolkningen (Sunhabits in the Norwegian population). in Report in Norwegian (2012).
Alfonso, J. H. et al. Occupation and relative risk of cutaneous squamous cell carcinoma (cSCC): A 45-year follow-up study in 4 Nordic countries. J. Am. Acad. Dermatol. 75, 548–555. https://doi.org/10.1016/j.jaad.2016.03.033 (2016).
Harrell Jr, F. E. rms: Regression Modeling Strategies. R Package Version 3.6-3. (2013).
Wang, M. et al. Statistical methods for studying disease subtype heterogeneity. Stat. Med. 35, 782–800 (2016).
Bartlett, J. W., Seaman, S. R., White, I. R., Carpenter, J. R. & Initiative*, A. S. D. N. Multiple imputation of covariates by fully conditional specification: accommodating the substantive model. Stat. Methods Med. Res. 24, 462–487 (2015).
Rubin, D. B. Multiple Imputation for Nonresponse in Surveys. Vol. 81 (Wiley, 2004).
Buuren, S. V. & Groothuis-Oudshoorn, K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 1–68 (2010).
Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 88, 582S-586S (2008).
Brustad, M., Alsaker, E., Engelsen, O., Aksnes, L. & Lund, E. Vitamin D status of middle-aged women at 65–71 N in relation to dietary intake and exposure to ultraviolet radiation. Public Health Nutr. 7, 327–335 (2004).
Kroll, M. H. et al. Temporal relationship between vitamin D status and parathyroid hormone in the United States. PLoS ONE 10, e0118108. https://doi.org/10.1371/journal.pone.0118108 (2015).
Sergentanis, T. N. et al. Obesity and risk of malignant melanoma: A meta-analysis of cohort and case–control studies. Eur. J. Cancer 49, 642–657 (2013).
Stenehjem, J. S. et al. Anthropometric factors and cutaneous melanoma: Prospective data from the population-based Janus Cohort. Int. J. Cancer 142, 681–690 (2018).
Liyanage, U. E., Law, M. H., Barrett, J. H., Iles, M. M. & MacGregor, S. Is there a causal relationship between vitamin D and melanoma risk? A Mendelian randomization study. Br. J. Dermatol. 182, 97–103. https://doi.org/10.1111/bjd.18238 (2020).
Long, D. Cox proportional hazard regression and splines: A cautionary tale for exposure-response assessment. in Lecture Notes (held May 3, 2019). Department of Biostatistics, University of Alabama at Birmingham, AL, USA. https://www.sra.org/sites/default/files/u35/Long_Cox-Regression-n-Spline-Caution_03-05-19.pdf. Accessed 15 Jan 2020.
El Ghissassi, F. et al. A review of human carcinogens—part D: Radiation. Lancet Oncol. 10, 751–752 (2009).
Moan, J., Baturaite, Z., Porojnicu, A. C., Dahlback, A. & Juzeniene, A. UVA, UVB and incidence of cutaneous malignant melanoma in Norway and Sweden. Photochem. Photobiol. Sci. 11, 191–198. https://doi.org/10.1039/c1pp05215b (2012).
Noonan, F. P. et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 3, 884. https://doi.org/10.1038/ncomms1893 (2012).
Deeb, K. K., Trump, D. L. & Johnson, C. S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 7, 684 (2007).
Kim, S. J. et al. Vitamin D deficiency is associated with inferior survival of patients with extranodal natural killer/T-cell lymphoma. Cancer Sci. 109, 3971 (2018).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436 (2008).
Agborsangaya, C. et al. The effects of storage time and sampling season on the stability of serum 25-hydroxy vitamin D and androstenedione. Nutr. Cancer 62, 51–57. https://doi.org/10.1080/01635580903191460 (2010).
Robsahm, T. E., Tretli, S., Torjesen, P. A., Babigumira, R. & Schwartz, G. G. Serum 25-hydroxyvitamin D levels predict cancer survival: A prospective cohort with measurements prior to and at the time of cancer diagnosis. Clin. Epidemiol. 11, 695–705. https://doi.org/10.2147/CLEP.S207230 (2019).
Funding
Funding has been granted by the Norwegian Cancer Society (no. 5829980–2014), the Cancer Registry of Norway Research Fund and UiO:Life Science Internationalization Support.
Author information
Authors and Affiliations
Contributions
T.E.R. conceived the study. J.S.S., T.K.G., J.R.R., R.B., M.B.V. and T.E.R. contributed to the project design. N.C.S. and J.S.S. performed the data analyses. R.B. and J.S.S. performed the data management. R.G. and M.B.V. prepared and provided group level data on sunburns, sunbathing vacations, and solarium sessions. L.T.N. and B.J. prepared and provided ambient UVB data from UV measurement stations. P.M.T. was responsible for the laboratory analyses. J.S.S. drafted the manuscript and all authors reviewed and revised it critically for important intellectual content and approved the final version for submission. J.S.S. and T.E.R. are the guarantors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stenehjem, J.S., Støer, N.C., Ghiasvand, R. et al. Prediagnostic serum 25-hydroxyvitamin D and melanoma risk. Sci Rep 10, 20129 (2020). https://doi.org/10.1038/s41598-020-77155-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77155-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.