Abstract
To investigate the safety and efficacy of Self-Assembling Peptide P11-4 (SAP P11-4) compared to placebo or fluoride varnish (FV), a randomized, controlled, blinded, split-mouth study with sequential design was conducted. Subjects presenting two teeth with White-Spot-Lesions (WSLs) were included and teeth were randomly assigned to test or control. Control received placebo at baseline (D0) and test SAP P11-4, all received FV at Day 90 (D90). Standardized photographs were taken at each visit, and WSL size changes were morphometrically assessed. Hierarchical Linear Modelling, considering paired and sequential design, was used to test four hypotheses. SAP P11-4 lesions (test, D90–D0) showed significant WSL size reduction compared to placebo (control, D90–D0; p = 0.008) or FV (control, D180–D90; p = 0.001). Combination of SAP P11-4 and delayed FV after 90 days (test, D180–D0), showed a significant difference compared to FV alone (control D270–D90; p = 0.003). No significant difference on FV efficacy was found when SAP P11-4 was applied 3 months before FV (test D270–D90; control D270–D90, p = 0.70). SAP P11-4 treatment resulted in superior caries regression compared to either placebo or FV, and FV efficacy seems not to be affected by SAP P11-4. SAP P11-4 was found to be a safe and effective WSL treatment.
Similar content being viewed by others
Introduction
Modern dentistry has witnessed a paradigm shift in dental caries management. The medical model, which focuses on caries prevention, reduction and reversal of incipient lesions, has gradually replaced the traditional interventional model known as the “drill and fill model”1. Over the past decades, fluoride, in various application forms, has been the predominant tool for caries prevention, with sufficient supporting evidence for its efficacy and safety2,3. Its widespread use is generally considered as the main cause for dental caries reduction4. However, shortcomings of the present fluoride therapies have been reported, including limited efficacy in some subjects, a caries reduction plateau at the population level5, and ineffectiveness in caries already manifested as white spots6. Subsequently, new remineralization therapies have been called for7 and are being developed to close this treatment gap by either adding or enhancing the effect of fluoride or replacing them, also for subjects reluctant to use fluoride4.
Breakthroughs in biomimetic remineralization have enabled the oral remineralization process, driven by saliva, beyond its normal level by providing additional nucleation sites for the crystallization of calcium phosphate8. Recently, a rationally designed monomeric self-assembling peptide P11-4 (SAP P11-4) solution9, which is able to diffuse into the subsurface carious lesions and self-assembles into a 3D biomimetic scaffold within, can guide the remineralization process10,11. SAP P11-4 has been shown to diffuse into the body of the carious lesion, and supporting de novo hydroxyapatite formation below the porous mineralized surface11,12,13,14. A first in man clinical trial has shown that SAP P11-4 is safe for human use and can reverse caries progression, significantly reducing the size of buccal white spot lesions as assessed by a visual analogue scale15. Another early clinical trial has shown that, when SAP P11-4 is applied on proximal carious surfaces, it leads to an arrest or even regression of caries in 85% of cases as judged by standardized clinical radiographs16. Recent randomized gold-standard controlled clinical trials have shown SAP P11-4 combined with fluoride varnish or a Self-Assembling Peptide Matrix Gel to be superior for the treatment of early occlusal caries compared to fluoride varnish alone17,18. The combination treatment inactivated 80–100% of the carious lesions and caused regression into a lower ICDAS-II class in up to 30% of cases. In contrast, in the control groups solely receiving fluoride varnish, only 35% of the carious lesions were inactivated, and 6% regressed to smaller ICDAS-II class17 and 20% needed restorative treatment during the 12 months study period18.
The aims of the present randomized and quadruple-blinded clinical split-mouth trial with the unique sequential study design allowing in a single trial to investigate the following objectives: (1) the efficacy of SAP P11-4 in the treatment of incipient buccal carious lesions in a comparative study, (2) compare, for the first time, the efficacy of SAP P11-4 with a placebo and with a fluoride varnish treatment alone, (3) assess the additional benefit of SAP P11-4 in the combined treatment with fluoride varnish in comparison to fluoride varnish alone, and (4) evaluate the influence of SAP P11-4 on the efficacy of fluoride varnish.
Results
The study protocol was approved by the Commission d’Ethique of the Hospitaux Universitaires de Geneve (12-275, approval date: 22/05/2013) and registered in clinicaltrials.gov (NCT02020681, registration date: 19/12/2013) prior to study start.
Subjects
Overall, 44 subjects (26 female, average 27.1 years) were enrolled from the daily practice and the student body of the University of Geneva between November 2013 (first patient’s first visit) and January 2016 (last patient’s last visit). All subjects signed informed consent prior to any study-related procedures. Subject’s as well as study lesion’s baseline characteristics are presented in Table 1. Initial characteristics of the study lesions were recorded by two clinicians and were comparable for the test and control arms regarding the Clinical Index White Spot Lesion19, lesion’s appearance and texture, and lesion activity. Lesions of subjects who had an orthodontic treatment and finished it more than one year before study inclusion were not classified as lesions originating from orthodontic treatment. Notably, white spot lesions originating from orthodontic treatment, known to have a partial remineralization potential without any treatment20,21 were absent in the present study. In total, 44 tooth-pairs (one per subject) were randomly assigned to either test or control treatment arm and analysed. One subject was lost to follow-up after the Day 30 (D30) visit due to relocation, and 3 subjects were lost to follow-up after Day 90 (D90) due to unknown reasons (Consort chart Supplementary Fig. S1).
Morphometry
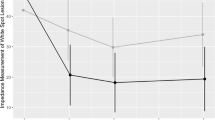
Both test and control lesions had a comparative size at baseline (test arm: 53′023 ± 70′022 pixel; control arm: 50′787 ± 69′584 pixel).
The relative size of the control arm lesions decreased on average by:
-
− 0.07 ± 0.24 between D0 and D90 (placebo, 11/43 decreasing by ≥ − 0.2; 5/43 increasing by ≥ + 0.2),
-
− 0.03 ± 0.13 between D90 and D180 (effect of fluoride varnish for 90 days, whereby 4/40 decreased by ≥ − 0.2 and 2/40 increased by ≥ + 0.2),
-
− 0.04 ± 0.19 between D90 and D270 (effect of fluoride varnish for 180 days, whereby 7/39 decreased by ≥ − 0.2 and 2/39 increased by ≥ + 0.2).
The relative size of the test arm lesions decreased on average by:
-
− 0.19 ± 0.25 between D0 and D90 (SAP P11-4, with 17/41 decreasing by ≥ − 0.2; 0/41 increasing by ≥ + 0.2),
-
− 0.20 ± 0.28 between D0 and D180 (SAP P11-4 followed by a 3 months delayed fluoride varnish, whereby 20/39 decreased by ≥ − 0.2 and 1/39 increased by ≥ + 0.2),
-
− 0.06 ± 0.18 between D90 and D270 (fluoride varnish with prior SAP P11-4, whereby 6/38 decreased by ≥ − 0.2 and 2/38 increased by ≥ + 0.2).
(Table 2, Box plots see Supplementary Fig. S2, Supplementary Table 1).
Group comparisons
The difference in WSL size for the four group comparisons of interest (Hypotheses H1–H4) was investigated using Hierarchical Linear Modelling (HLM) controlling for confounding factors (Table 2, Supplementary Tables 2–5).
Hypothesis 1 (H1): A statistically significant difference was observed for the change of WSL size treated with SAP P11-4 showing a size reduction of − 0.14 compared to WSL treated with placebo (95% CI − 0.04 to − 0.24, p = 0.008).
Hypothesis 2 (H2): A statistically significant difference was observed for the change of WSL size treated with SAP P11-4 showing a size reduction of − 0.18 compared to WSL treated with fluoride varnish (95% CI − 0.08 to − 0.27, p < 0.001).
Hypothesis 3 (H3): A statistically significant difference was observed for the change of WSL size treated with SAP P11-4 followed by a 3-month delayed fluoride varnish treatment, showing a size reduction of − 0.17 compared to WSL treated fluoride varnish alone (95% CI − 0.06 to − 0.28, p = 0.003), indicating an additional benefit of SAP P11-4 in a treatment period of 180 days compared to fluoride varnish alone.
Hypothesis 4 (H4): No statistically significant difference was observed for change of WSL size treated with fluoride varnish after receiving SAP P11-4 3 months earlier (estimate of size reduction − 0.02), compared to lesions treated with fluoride varnish that had not received SAP P11-4 previously (95% CI − 0.10 to 0.06, p = 0.70).
The results computed with HLM proved to be robust in the sensitivity analysis (Supplementary Tables 6–12).
VAS progression scores
The result of the evaluation of the progression/regression of the WSL by means of VAS with respect to D0 was as follows:
-
A.
Placebo (control arm: D90–D0) showed an average of − 4.8 ± 13.9; a median of − 2.0 and a minimum–maximum range of − 30 to + 31, indicating overall values just below "0" corresponding to a slight regression;
-
B.
SAP P11-4 (test arm: D90–D0) showed an average of − 8.5 ± 12.9, a median of − 5.0 and range of − 36 to + 17 indicating an overall regression of the lesions with individual exceptions;
-
C.
SAP P11-4 with delayed fluoride varnish (test arm: D180-D0) showed an average of − 8.8 ± 12.5, median of − 7.0 and range of − 38 to + 17 indicating overall regression after SAP P11-4 application followed by a stabilization after fluoride varnish application.
Global impression of change
The results of the GIOCQ for the meaningful groups were as follows:
-
A.
Placebo (control arm: D90): very much worse: 0; worse: 3; a little worse: 5; unchanged: 14; a little better 12; better: 9; very much better: 0; missing: 1.
-
B.
SAP P11-4 (test arm: D90–D0): very much worse: 0; worse: 0; a little worse: 5; unchanged: 14; a little better 13; better: 6; very much better: 3; missing: 3.
-
C.
SAP P11-4 with delayed fluoride varnish (test arm: D180): very much worse: 0; worse: 1; a little worse: 2 unchanged: 14; a little better 14; better: 7; very much better: 1; missing: 5.
Caries diagnostics
Laser Fluorescence values at D0 for test arm lesions were 6.7 ± 5.3 and 6.5 ± 4.9 for the control arm lesions. According to the manufacturer’s instructions, values < 10 are regarded as healthy enamel. At D0, 4/44 test lesions and 5/44 control lesions indicated carious enamel. On D90, 4/43 of the test lesions were assessed to be carious, whereas in the control lesions 3/43 indicated carious enamel. On D180, the number of carious findings increased to 5/40 in the test lesions and remained at 3/40 in the control lesions. On D270, 5/40 of the test lesions and 3/40 control lesions indicated carious enamel (Supplementary Table 13).
Nyvad caries activity criteria
The results of the Nyvad Caries Activity Criteria for the meaningful groups were as follows (Supplementary Table 14):
-
A.
Placebo (control arm: D90–D0): becoming inactive: 10.
-
B.
SAP P11-4 (test arm: D90–D0): becoming inactive: 8.
-
C.
SAP P11-4 with delayed fluoride varnish (test arm: D180–D0): becoming inactive: 10.
Plaque index and oral hygiene data are presented in Supplementary Table 15 and 16.
Safety
No trial nor intervention-related safety concerns were reported.
Discussion
SAP P11-4 has previously been shown to be a successful treatment for early carious lesions (i.e. White Spot Lesions—WSL) that in combination with fluoride varnish or Self-Assembling Peptide Matrix Gel led to regression and inactivation of occlusal caries17,18 as well as to regression of proximal lesions, the later assessed on standardised clinical radiographs22. Furthermore, it has been shown to positively influence the appearance of buccal white spot lesions15. In a recently reported split-mouth study, investigating SAP P11-4 in early buccal caries within a private practice setting, morphometry was also used as primary outcome measure similar to the present study23. In Broeseler et al. 2020 the fluoride varnish control group showed a stabilisation of the lesion’s size over a duration of a year, whereas the SAP P11-4 test group resulted in a size reduction.
The current clinical trial supplied additional information relating to the performance of SAP P11-4 to standard clinical care, such as fluoride varnish or wait-and-see (i.e., placebo), as measured by the WSL size. SAP P11-4 induced regression of caries and has shown to be both superior to the placebo control (including cleaning and etching of the lesion) and the gold-standard fluoride varnish. SAP P11-4 facilitates de novo hydroxyapatite formation throughout the subsurface carious lesion, clinically visible as a decrease in lesion size10. In contrast, fluoride ions promote remineralisation only within the top 20 μm of the enamel surface24 resulting in stabilisation of the carious lesion as observed morphometrically in the present clinical trial.
The sequential design of the present study allowed for multiple hypothesis to be tested, and it was shown that treatment with SAP P11-4 does not impede on the efficacy of fluoride varnish, when SAP P11-4 is applied three months before fluoride varnish. The present study cannot rule out an effect of SAP P11-4 on fluoride varnish’s efficacy as there maybe absence of evidence which does not proof an absence of effect. However, the present results add evidence to two previously reported SAP P11-4 studies on occlusal caries, but as these studies did not include a treatment group solely receiving SAP P11-4, inhibitory effects of SAP P11-4 on fluoride varnish could not be excluded. It should be noted that the WSLs treated with SAP P11-4 in the present clinical trial did not disappear completely, in agreement with previous reports15,21,25 and consistent with the fact that the formed hydroxyapatite crystals formed on the SAP P11-4 scaffold surface show a fan-type structure not a prismatic arrangement10 and thus do not result in the same translucency as natural prismatic enamel. The changes in the white spot lesion should be and were in the present study regarded as a surrogate parameter and not a direct measure of caries or a clinical success parameter. An estimation of clinical success might be the number of WSLs decreasing by > 20% in size and are therefore clearly regressing by visual inspection or the number of buccal lesions progressing in size > 20%, in analogy clearly progressing. For those criteria, SAP P11-4 led to 42% of the lesions regressing, while none progressed in size, the placebo group led to 26% regressing and 12% progressing, and the fluoride varnish group led to 10% regressing and 5% progressing. These findings correspond to the assumption that SAP P11-4 remineralizes the subsurface body of active carious lesions and has little effect on inactive carious lesions, fluoride varnish closes the mineralized surface and thus inactivates the lesion, and placebo-treated lesions are more dynamic and progress or regress depending on subject behaviour.
The results of the present trial are consistent with previous clinical studies on SAP P11-415,17,18,21,22, indicating that SAP P11-4 could be a suitable clinical option for the non-invasive treatment of early carious lesions, allowing a more comprehensive and in-depth regression of decayed enamel7,26.
As spontaneous enamel regression is generally not observed27, except in WSLs originating from orthodontic treatment after debonding28, the treatment with SAP P11-4 can ultimately facilitate the preservation of the tooth structure and delay or avoid entry into the deadly spiral of restorations29,30 allowing potentially a longer lifetime of the tooth.
In the present study, the appearance and progression/regression of WSLs was chosen as the primary parameter, as it resembles the clinical indicator on which clinicians base their treatment decisions31,32. This is apparent from the primarily used classification system of caries (ICDAS-II) and clinical radiographs, which rely on visual or radiographic appearance33.
Although WSLs seldomly receive dedicated treatment, as they do not pose an imminent clinical problem, the appearance and WSL size is closely followed by clinicians, triggering invasive treatment when significant progression is observed34. However, other factors, such as opaqueness as an indicator for activity, add to the treatment decision35.
The limitations of the present study are that WSL size is an indirect surrogate measurement of caries, which cannot be directly and quantitatively related to clinical success, and in order to obtain high quality standardized photographs, buccal carious lesions were chosen, of which many were inactive at the study start as evident from the Nyvad Caries Activity Criteria and Laser Fluorescence readings. This led to an effectively smaller sample size with regard to lesion activity, resulting in low laser fluorescence baseline values and preventing meaningful quantitative assessment. In a future study carious lesion activity should be an inclusion criteria as defined by Nyvad Caries Activity Criteria and/or Laser Fluorescence threshold values.
The used sequential study design could also be regarded as a limitation. Yet to avoid biases in a corresponding study with six arms, it would have needed significantly more subjects to be enrolled, resulting in greater human and financial resources as well a longer recruitment time and multiple investigators/sites, which would have required extensive calibration among them. With the HLM analysis, precautions were taken to counteract bias by controlling for confounders. The present design was chosen to obtain as much information as possible with as little downsides as possible.
Lastly, due to the split-mouth study design and ethical implications of giving oral hygiene instructions and fluoride toothpaste at every study visit, the placebo group was not without treatment.
Conclusion
Treatment of early buccal carious lesions with SAP P11-4 led to superior regression of caries decay compared to either placebo or fluoride varnish. Furthermore, it was demonstrated that SAP P11-4 did not impede the effectiveness of fluoride varnish, and treatment with SAP P11-4 with delayed fluoride varnish was superior to treatment with fluoride varnish alone. Based on current and previous clinical studies, SAP P11-4 may change the daily management of initial carious lesions from a wait-and-see approach to early non-invasive interventions with the intent to regenerating the enamel tissue. Since SAP P11-4 supports the formation of de novo hydroxyapatite crystals deep within and throughout the carious lesion body, it offers the clinician a new, effective, non-aerosol generating and non-invasive treatment option.
Materials and methods
Subjects
All clinical procedures were performed at the University of Geneva, University Clinics of Dental Medicine according to the approved study protocol and current versions of ISO 14155, Declaration of Helsinki and Swiss regulations on clinical trials. Subjects presenting with at least two Class V lesions not in need of invasive treatment and not on adjacent teeth were eligible for the study. The main selection criteria were: lesions must be fully visible, assessable and accessible; > 20 teeth and permanent dentition; < 65 years of age; written informed consent before study participation; negative pregnancy test; willing to observe good oral hygiene throughout the study; no adjacent restorations on the study tooth surface; fluoride varnish application ≥ 6 months prior to study; no evidence of tooth erosion; and no concurrent participation in another trial.
Study design and randomization and treatments
This randomized, placebo and gold-standard controlled, blinded split-mouth clinical trial with sequential design was designed to investigate the clinical effect of SAP P11-4 for the treatment of early buccal carious lesions compared to a sham/placebo treatment, and fluoride varnish, as well as to assess the additional benefit of SAP P11-4 in a combination treatment with fluoride varnish in comparison to fluoride varnish alone and to evaluate the influence of SAP P11-4 on the effectiveness of fluoride varnish (Fig. 1). The trial was quadruple-blinded (subject, investigator, assessor, statistician). A blinded randomization sequence (defined via higher/lower tooth number) was generated by a third party and kept in numbered, closed envelopes at the investigator site. The computer-generated randomisation sequence of the test and control tooth allocation was kept under lock at third party (sealed envelopes were available at trial site in the case of an emergency) and was provided for unblinding only after morphometric assessment and statistical analysis was performed. Bags with the same numbering as on the envelopes containing the study material were provided to the study centre. Each numbered bag contained one vial of verum and one of placebo (indistinguishable from verum vial). The vials were labelled with "higher tooth number" or "lower tooth number" according to the computer-generated tooth assignment. After subjects met the selection criteria, a sequential envelope was drawn providing the tooth allocation for the treatments of the two teeth per study participant to either the test or control arm (simple randomization; 1:1). At baseline (D0), test lesions received SAP P11-4 (50 µL Curodont Repair, credentis ag), and control lesions received placebo (50 µL). All study teeth received fluoride varnish according to manufacturer’s instructions (~ 25 µL/tooth; 22,600 ppm fluoride, Duraphat, Colgate Palmolive Co.) at D90 (Fig. 1).
Clinical trial design depicting the recalls and interventions for test and control lesions. Time intervals within the sequential design corresponding to groups in the comparisons are depicted as follows: Placebo (Sham) (blue); fluoride varnish (control arm) (red); SAP P11-4 (green); fluoride varnish (test arm) (black).
Procedures
To ensure blinding and reduce methodological bias, both subject’s study teeth received pre-treatment as recommended for SAP P11-4: dental cleaning with pumice stone (incl. 2% NaOCl, Districhemie SA, Ecublens, Switzerland) and etching (Etching Gel, Ultra-etch, Ultradent, UT, USA 35% H3PO4, 20 s). Treatment was provided on D0, and all study teeth received fluoride varnish at D90. Additional study visits were D30, D180 and D270. Standardized clinical photographs including a grey-scale in every picture for calibration were taken15,36,37,38,39, oral hygiene, plaque index (according to Silness and Loe 196440), Nyvad Caries Activity Criteria35 and laser fluorescence reading (Diagnodent Pen; KaVo) recorded as well as oral hygiene instructions provided at every study visit.
Outcomes
The primary outcome was the regression/progression of white spot lesions as judged by the change in WSL size assessed by morphometry15,20,21,23,41 on standardized, calibrated clinical photographs as described in Broeseler et al.23 (Fig. 2). Morphometric assessment was performed by a blinded assessor (Image J, Version 1.45, NIH, Bethesda, MA, USA) and measurements were reviewed by another blinded person for plausibility and for consistency of morphometric measurements over time (March 2016–October 2018).
Clinical standardized photographs of one subject, showing the control lesion (A–E) and the test lesion (F–J) at each interval: Day 0 (A, F); Day 30 (B, G); Day 90 (C, H); Day 180 (D, I); Day 270 (E, I). The treatments and their time periods within the clinical design are indicated at the bottom of the photographs.
The secondary parameters were: lesion’s activity assessed by Nyvad Caries Activity Criteria, Global Impression of Change Questionnaire (GIOCQ), Laser Fluorescence value readings and Visual Analogue Scale (VAS) for the progression/regression of each lesion with respect to baseline as judged by the clinician at each visit. Due to the non-linearity of VAS, only meaningful study groups starting at D0 were assessed (same argument is valid also for GIOCQ and Nyvad Caries Activity Criteria). The VAS scale ranged from − 50 to + 50, with negative values indicating regression and “0” arrested and positive values progression. Safety of treatments was assessed by recording adverse events per Directive 2001/20/EC, ISO 14155 and MDD93/42.
Statistical analysis
The changes in WSL size between the test and control arm were evaluated using Hierarchical Linear Modelling (HLM, two-sided), which allowed to integrate important variables into the model, to account for confounding factors and to evaluate the effect of treatment, taking into account correlations resulting from the paired measurements. The dependent variable of the HLM models was the morphometric change in WSL size due to the treatments over time from D0, whilst the independent variables were: gender (dummy variable with “female” as a baseline), race (converted in a dummy variable with “Caucasian” as a baseline), age, DMFT, WSL clinical index, oral hygiene and plaque index, with all explanatory variables adjusted for their respective baseline values. The effect of the treatment(s) was evaluated using a dummy variable with the control group as baseline. The following four group comparisons (corresponding to the four Hypothesis H1–H4) were of interest in the present study:
-
(H1) Placebo vs SAP P11-4 (D90–D0 of the control arm vs. D90–D0 of the test arm).
-
(H2) Fluoride varnish (FV) vs SAP P11-4 (D180–D90 of the control arm vs. D90–D0 of the test arm).
-
(H3) FV vs SAP P11-4 + delayed FV (D270–D90 of the control arm vs. D180–D0 of the test arm) to compare FV with a combined treatment of SAP P11-4 and FV in a period of 180 days.
-
(H4) FV vs FV with prior SAP P11-4 application (D270–D90 of the control arm vs. D270–D90 of the test arm) to investigate whether prior application of SAP P11-4 had an influence on FV.
The null hypothesis for each of the four comparisons of interest was that there was no difference in the morphometric change between each comparison, while the alternative hypothesis was that there was a difference. One HLM fixed effects model for each of the four hypotheses above was estimated for treatment and control variables and the associated statistics presented. The assumptions for the HLM models (normality and homoscedasticity) have been tested and hold for the four hypotheses. The effect of treatment was considered statistically significant if the p-value of the treatment dummy variable was smaller than 0.01. All analyses were run in IBM SPSS Statistics Version 24 (January 2019–June 2019). Intention-to-treat analysis was performed.
Sensitivity analysis of HLM was performed varying the inclusion of parameters in the model as well as computed parameter values themselves (average oral hygiene or plaque index value of the two investigated time points for each group during a comparison and their respective value at the start time of the comparison interval in both arms) in order to investigate the robustness of the model or the result.
At study start, the available literature did not yield suitable data for a sample size calculation. Therefore, the estimation for the primary outcome based on successful alike studies42. A post hoc calculation of the sample size for a 0.8 power level and 0.01 significance level for the three comparisons (H1–H3) ranged between 42 and 51 pairs of observations. Sample size calculations were computed using STATA 15.1 by StataCorp (College Station, TX, USA) "Power and Sample size" module.
All secondary parameters were analysed descriptively, since the estimation of the sample size was based only on the primary parameter.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on request.
References
Featherstone, J. D. B. & Chaffee, B. W. The evidence for caries management by risk assessment (CAMBRA(R)). Adv. Dent. Res. 29, 9–14. https://doi.org/10.1177/0022034517736500 (2018).
Amaechi, B. T. & van Loveren, C. Fluorides and non-fluoride remineralization systems. Monogr. Oral Sci. 23, 15–26. https://doi.org/10.1159/000350458 (2013).
Fontana, M. Enhancing fluoride: Clinical human studies of alternatives or boosters for caries management. Caries Res. 50(Suppl 1), 22–37. https://doi.org/10.1159/000439059 (2016).
Gonzalez-Cabezas, C. & Fernandez, C. E. Recent advances in remineralization therapies for caries lesions. Adv. Dent. Res. 29, 55–59. https://doi.org/10.1177/0022034517740124 (2018).
Haugejorden, O. & Magne Birkeland, J. Ecological time-trend analysis of caries experience at 12 years of age and caries incidence from age 12 to 18 years: Norway 1985–2004. Acta Odontol. Scand. 64, 368–375. https://doi.org/10.1080/00016350600856083 (2006).
Oliveira, B. H. et al. Biannual fluoride varnish applications and caries incidence in preschoolers: A 24-month follow-up randomized placebo-controlled clinical trial. Caries Res. 48, 228–236. https://doi.org/10.1159/000356863 (2014).
Fisher, J. et al. FDI Global Caries Initiative; implementing a paradigm shift in dental practice and the global policy context. Int. Dent. J. 62, 169–174. https://doi.org/10.1111/j.1875-595X.2012.00128.x (2012).
Philip, N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 53, 284–295. https://doi.org/10.1159/000493031 (2018).
Alkilzy, M., Santamaria, R. M., Schmoeckel, J. & Splieth, C. H. Treatment of carious lesions using self-assembling peptides. Adv. Dent. Res. 29, 42–47. https://doi.org/10.1177/0022034517737025 (2018).
Kirkham, J. et al. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 86, 426–430 (2007).
Kind, L. et al. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 96, 790–797. https://doi.org/10.1177/0022034517698419 (2017).
Deyhle, H. et al. Mineralization of early stage carious lesions in vitro—A quantitative approach. Dent. J. 3, 111–122 (2015).
Schmidlin, P., Zobrist, K., Attin, T. & Wegehaupt, F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J. Appl. Oral Sci. 24, 31–36. https://doi.org/10.1590/1678-775720150352 (2016).
Kamal, D., Hassanein, H., Elkassas, D. & Hamza, H. Comparative evaluation of remineralizing efficacy of biomimetic self-assembling peptide on artificially induced enamel lesions: An in vitro study. J. Conserv. Dent. 21, 536–541. https://doi.org/10.4103/JCD.JCD_123_18 (2018).
Brunton, P. A. et al. Treatment of early caries lesions using biomimetic self-assembling peptides—A clinical safety trial. Br. Dent. J. 215, E6. https://doi.org/10.1038/sj.bdj.2013.741 (2013).
Schlee, M., Schad, T., Koch, J. H., Cattin, P. C. & Rathe, F. Clinical performance of self-assembling peptide P11–4 in the treatment of initial proximal carious lesions: A practice-based case series. J. Investig. Clin. Dent. 9, e12286. https://doi.org/10.1111/jicd.12286 (2018).
Alkilzy, M., Tarabaih, A., Santamaria, R. M. & Splieth, C. H. Self-assembling peptide P11–4 and fluoride for regenerating enamel. J. Dent. Res. 97, 148–154. https://doi.org/10.1177/0022034517730531 (2018).
Doberdoli, D. et al. Randomized clinical trial investigating self-assembling peptide P11–4 for treatment of early occlusal caries. Sci. Rep. 10, 4195. https://doi.org/10.1038/s41598-020-60815-8 (2020).
Gorelick, L., Geiger, A. M. & Gwinnett, A. J. Incidence of white spot formation after bonding and banding. Am. J. Orthod. 81, 93–98 (1982).
Willmot, D. R., Benson, P. E., Pender, N. & Brook, A. H. Reproducibility of quantitative measurement of white enamel demineralisation by image analysis. Caries Res. 34, 175–181 (2000).
Welk, A., Ratzmann, A., Reich, M., Krey, K. F. & Schwahn, C. Effect of self-assembling peptide P11–4 on orthodontic treatment-induced carious lesions. Sci. Rep. 10, 6819. https://doi.org/10.1038/s41598-020-63633-0 (2020).
Schlee, M., Schad, T., Koch, J. H., Cattin, P. C. & Rathe, F. Clinical performance of self-assembling peptide P11–4 in treatment of initial proximal carious lesions—A practice based case series. J. Investig. Clin. Dent. 9, e12286. https://doi.org/10.1111/jicd.12286 (2017).
Bröseler, F. et al. Randomised clinical trial investigating self-assembling peptide P11–4 in the treatment of early caries. Clin. Oral. Investig. 24, 123–132. https://doi.org/10.1007/s00784-019-02901-4 (2020).
Müller, F. et al. Elemental depth profiling of fluoridated hydroxyapatite: Saving your dentition by the skin of your teeth?. Langmuir 26, 18750–18759. https://doi.org/10.1021/la102325e (2010).
Wierichs, R. J., Kogel, J., Lausch, J., Esteves-Oliveira, M. & Meyer-Lueckel, H. Effects of self-assembling peptide P11–4, fluorides, and caries infiltration on artificial enamel caries lesions in vitro. Caries Res. 51, 451–459. https://doi.org/10.1159/000477215 (2017).
Featherstone, J. D. Remineralization, the natural caries repair process—The need for new approaches. Adv. Dent. Res. 21, 4–7 (2009).
Bergman, G. & Lind, P. O. A quantitative microradiographic study of incipient enamel caries. J. Dent. Res. 45, 1477–1484 (1966).
Höchli, D., Hersberger-Zurfluh, M., Papageorgiou, S. N. & Eliades, T. Interventions for orthodontically induced white spot lesions: A systematic review and meta-analysis. Eur. J. Orthod. 39, 122–133. https://doi.org/10.1093/ejo/cjw065 (2017).
Burke, F. J. T. & Lucarotti, P. S. K. The ultimate guide to restoration longevity in England and Wales. Part 2: Amalgam restorations—time to next intervention and to extraction of the restored tooth. Br. Dent. J. 224, 789–800. https://doi.org/10.1038/sj.bdj.2018.354 (2018).
Ramos-Gomez, F. J., Crystal, Y. O., Domejean, S. & Featherstone, J. D. Minimal intervention dentistry: Part 3 Paediatric dental care—prevention and management protocols using caries risk assessment for infants and young children. Br. Dent. J. 213, 501–508. https://doi.org/10.1038/sj.bdj.2012.1040 (2012).
Makhija, S. K. et al. Characteristics, detection methods and treatment of questionable occlusal carious lesions: Findings from the national dental practice-based research network. Caries Res. 48, 200–207. https://doi.org/10.1159/000354841 (2014).
Makhija, S. K. et al. Twenty-month follow-up of occlusal caries lesions deemed questionable at baseline: Findings from the National Dental Practice-Based Research Network. J. Am. Dent. Assoc. 145, 1112–1118. https://doi.org/10.14219/jada.2014.82 (2014).
Ismail, A. I. et al. The International Caries Detection and Assessment System (ICDAS): An integrated system for measuring dental caries. Community Dent. Oral. Epidemiol. 35, 170–178. https://doi.org/10.1111/j.1600-0528.2007.00347.x (2007).
Makhija, S. K. et al. The prevalence of questionable occlusal caries: Findings from the Dental Practice-Based Research Network. J. Am. Dent. Assoc. 143, 1343–1350 (2012).
Nyvad, B. & Baelum, V. Nyvad criteria for caries lesion activity and severity assessment: A validated approach for clinical management and research. Caries Res. 52, 397–405. https://doi.org/10.1159/000480522 (2018).
Bock, N. C. et al. Changes in white spot lesions following post-orthodontic weekly application of 1.25 per cent fluoride gel over 6 months-a randomized placebo-controlled clinical trial. Part I: photographic data evaluation. Eur. J. Orthod. 39, 134–143. https://doi.org/10.1093/ejo/cjw060 (2017).
Bock, N. C. et al. Changes in white spot lesions following post-orthodontic weekly application of 1.25 per cent fluoride gel over 6 months-a randomized placebo-controlled clinical trial. Part II: Clinical data evaluation. Eur. J. Orthod. 39, 144–152. https://doi.org/10.1093/ejo/cjw061 (2017).
Huang, G. J. et al. Effectiveness of MI Paste Plus and PreviDent fluoride varnish for treatment of white spot lesions: A randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 143, 31–41. https://doi.org/10.1016/j.ajodo.2012.09.007 (2013).
Akin, M. & Basciftci, F. A. Can white spot lesions be treated effectively?. Angle Orthod. 82, 770–775. https://doi.org/10.2319/090711.578.1 (2012).
Silness, J. & Loe, H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta Odontol. Scand. 22, 121–135. https://doi.org/10.3109/00016356408993968 (1964).
Benson, P. E., Shah, A. A. & Willmot, D. R. Measurement of white lesions surrounding orthodontic brackets: Captured slides vs digital camera images. Angle Orthod. 75, 226–230 (2005).
Paris, S., Hopfenmuller, W. & Meyer-Lueckel, H. Resin infiltration of caries lesions: An efficacy randomized trial. J. Dent. Res. 89, 823–826. https://doi.org/10.1177/0022034510369289 (2010).
Acknowledgements
The blinded test material and placebo was provided by credentis ag, Windisch, Switzerland. This study was kindly funded by InnoSuisse (# 14934.1 PFLS-LS).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: I.K., L.D., C.B. Investigators performing the study: P.S., A.M., M.A., L.D., I.K. Analyzed the data: E.B., C.B. Wrote and reviewed the manuscript: P.S., A.M., M.A., C.B.; E.B., L.D., I.K.
Corresponding author
Ethics declarations
Competing interests
C. Bommer is an employee of credentis. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sedlakova Kondelova, P., Mannaa, A., Bommer, C. et al. Efficacy of P11-4 for the treatment of initial buccal caries: a randomized clinical trial. Sci Rep 10, 20211 (2020). https://doi.org/10.1038/s41598-020-77057-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77057-3
This article is cited by
-
White spot lesions: diagnosis and treatment – a systematic review
BMC Oral Health (2024)
-
Comparison of therapies of white spot lesions: a systematic review and network meta-analysis
BMC Oral Health (2023)
-
Self-assembling peptides for managing white spot lesions: a systematic review and meta-analysis
European Archives of Paediatric Dentistry (2023)
-
Management of white spot lesions induced during orthodontic treatment with multibracket appliance: a national-based survey
Clinical Oral Investigations (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.