Abstract
Herbivores are constitutive elements of most terrestrial ecosystems. Understanding effects of herbivory on ecosystem dynamics is thus a major, albeit challenging task in community ecology. Effects of mammals on plant communities are typically explored by comparing plant densities or diversity in exclosure experiments. This might over-estimate long-term herbivore effects at community levels as early life stage mortality is driven by a multitude of factors. Addressing these challenges, we established a set of 100 pairs of ungulate exclosures and unfenced control plots (25 m2) in mixed montane forests in the Alps in 1989 covering a forest area of 90 km2. Investigations ran until 2013. Analogous to the gap-maker–gap-filler approach, dynamically recording the height of the largest trees per tree species in paired plots with and without exclosures might allow for assessing herbivore impacts on those individuals with a high probability of attaining reproductive stages. We thus tested if recording maximum heights of regenerating trees would better reflect effects of ungulate herbivory on long-term dynamics of tree regeneration than recording of stem density, and if species dominance patterns would shift over time. For quantifying the effects of ungulate herbivory simultaneously at community and species level we used principle response curves (PRC). PRCs yielded traceable results both at community and species level. Trajectories of maximum heights yielded significant results contrary to trajectories of total stem density. Response patterns of tree species were not uniform over time: e.g., both Norway spruce and European larch switched in their response to fencing. Fencing explained about 3% of the variance of maximum tree heights after nine years but increased to about 10% after 24 years thus confirming the importance of long-term surveys. Maximum height dynamics of tree species, addressed in our study, can thus reflect local dominance of tree species via asymmetric plant competition. Such effects, both within and among forest patches, can accrue over time shaping forest structure and composition.
Similar content being viewed by others
Introduction
Herbivores are constitutive elements of most terrestrial ecosystems in which the co-evolved interactions between plants and herbivores can vary widely in their intensity and feedbacks1. Herbivory is a major driver of dynamics in many ecosystems (e.g.2,3,4) and a major selective pressure leading to numerous evolutionary adaptations5,6 like chemical or morphological defence strategies of plants or strong dependencies of herbivores on food availability. In turn, plants impact herbivore survivorship and fecundity in terms of quantity and quality of feeding resources. As a numerical response, enhanced food availability is typically linked with increased consumption rates. Vice versa, herbivory by ungulates can reduce or promote structural diversity and plant species diversity depending on the intensity and selectivity of feeding, the palatability and functional redundancy of plants, and other factors7,8,9. Top-down effects of herbivores depend on (individual) feeding strategies, which include adaptive trade-offs between nutrient intake and avoidance of predation risk10. In the past, a multitude of studies on herbivory focused on the fraction of aboveground net primary production consumed by herbivores thereby highlighting the global role of herbivory and tackling questions of top-down versus bottom-up control in ecosystems10,11,12,13,14,15. Bottom-up forces comprise abiotic factors that drive primary production (like soil fertility), whereas top-down forces address impacts of higher trophic levels on lower levels16. Such studies usually do not clarify lasting effects of herbivory on dynamics of plant communities1,17.
Effects of mammals on plant communities are typically explored by exclosure experiments18,19,20,21,22,23 impeding access of mammalian herbivores to study plots. However, year-round absence of native herbivores does not display natural terrestrial ecosystem conditions. Correspondingly, a conceptual framework of effects of defaunation on different trophic levels within forest communities has been provided in the past, showing long-term effects of defaunation on a pristine tropical forest24. Comparing exclosures and coupled open access plots, the consumption of organic matter by herbivores might be expressed as differences in net primary production25, in plant densities8,18,20, or, at community level, in structural diversity or species diversity8,26,27,28,29,30.
Quantifying long-term effects of herbivory on plant species composition and dynamics of ecosystems faces several challenges: plants, and particularly trees, show strong ontogenetic variation in herbivory. This is caused by higher ratios of nutrient-rich (cotyledons, leaves) versus nutrient-poor (stem) plant structures, by reduced defence of early developmental stages after stored carbohydrate reserves in seeds and cotyledons are allocated31,32,33 and by a higher number of herbivore guilds being able to consume small plants. Assessing herbivory thus has to capture these early life stages, especially if consumption rates differ with species. Trees in these life stages, however, are frequently under-dispersed34 and show high densities. This leads to density-dependent mortality and is typically reflected in exponentially decreasing stem density distributions, caused by stage specific mortality factors (like adverse microsite conditions35, pathogens36, competition, or herbivory37). Consequently, only a comparatively small number of progenies survive38 and only a small portion of seedlings finally grows into the forest canopy and can thus reproduce. Thus, initial herbivore effects at early developmental stages might be accompanied by a multitude of co-occurring or subsequent processes and might not be significant drivers of dynamics at later stages. They are only part of the compensatory mortality dynamics. Accordingly, short time surveys of herbivore effects in terms of reductions in stem numbers or net primary production might over-estimate herbivore effects in the long-term and at community levels, particularly for tree species composition. These problems are well known in studies of tree regeneration in forest gaps where species composition at early stages based on seedling densities can be a misleading proxy of future species composition39,40,41. This is overcome by assessing gap fillers or definite gap fillers (sensu39) as those individuals with a high probability for replacing a gap maker. Analogous to this approach, dynamic recording of the height of the highest tree individuals per species in paired plots with and without exclosures over time (thereby allowing for changes in dominance with time) may allow for assessing herbivore impacts on those individuals with a high probability of reaching the main canopy and getting into reproductive stages. Although tree height growth is a fundamental factor in juvenile survivorship (e.g.42) in turn determining the chance of reaching reproductive size41,43, tree heights are less frequently considered30 than stem numbers (per height class14) or species abundances (e.g.18), when exploring effects of herbivores on forest tree communities20,30. When tree heights are used to depict herbivore impacts, growth patterns and growth histories of tagged tree individuals are frequently taken as reference for the quantification of herbivory-mediated reductions in height growth15,24,44,45,46. This approach, however, requires either tagging of individuals in high densities (with all associated challenges of adequate numbers of replications of study sites), or holds the risk of selecting individuals that do not represent the dominant ones that will have a high chance of forming the future canopy.
To compare approaches assessing herbivory effects based on stem density or on heights of highest individuals per plot, we used a unique data set of deer exclosures gained in mixed forest communities (particularly spruce, beech, fir) for up to 24 years47. For each tree species, both densities and heights were recorded, which allows for testing the following hypotheses: (1) recording maximum heights of regenerating trees better reflects effects of ungulate herbivory on long-term dynamics of tree regeneration than recording of stem density and (2) species dominance patterns shift over time; thus, results from surveys with short and medium duration differ from long-term results, when individuals have reached later development stages (with terminal heights being no longer accessible for browsing by ungulates as is the case with thickets).
Methods
Study area
Regeneration dynamics were monitored on the forest area (90 km2) of the mountain massif “Höllengebirge” (highest elevation 1862 m), being part of the northern Limestone Alps in Upper Austria, Central Europe (47°49′ N, 13°30′ E). The forest is located around a central plateau that lays above the forest line. Geological substrates are dominated by limestone and dolomite, typical soils are Rendzinas and relictic loams. The climate of the region is submaritime, with long winter periods and cool, wet and short summer periods. Mean annual temperature of the area is about 6–8 °C (depending on altitude) and mean annual precipitation reaches approx. 1800 mm. Annual precipitation is characterized by a bimodal temporal pattern, with one maximum occurring at July and another one between November and January. The area is typically covered by snow on approx. 170 days per year, with large inter-annual variation48. Typical forest communities are montane mixed forests comprising of Norway spruce (Picea abies), silver fir (Abies alba), European beech (Fagus sylvatica), ash (Fraxinus excelsior), and sycamore maple (Acer pseudoplatanus). They are classified as Helleboro nigri-Fagetum and Adenostylo glabrea-Fagetum49. The silvicultural system is permanent forest (shelterwood and strip-selection cutting) with natural forest regeneration and additionally strip clear-cuts with supplementary afforestation. During the investigation period no serious climatic events (e.g. drought, wind throw, snow break) or insect calamities occurred.
The mean ungulate density on the forested area in spring (without newborn calves, fawns and kids) is in total about 20 animals per km2, consisting of approximately four red deer (Cervus elaphus, counted at winter-feeding stations), five to ten roe deer (Capreolus capreolus, estimated by local hunters), and six to ten chamois (Rupicapra rupicapra, estimated by local hunters) per km2; the hunting season runs from May to December. Hunting bags were 1 to 2 red deer, 1 to 4 roe deer, and 1 to 2 chamois per km2 and year, on a constant level over the observation period.
Sampling method
We established 100 pairs of ungulate exclosures (excluding the occurring red deer, roe deer and chamois) and unfenced control plots at altitudes between 500–1100 a.s.l. in 1989 and investigated them until 2013. Minimum distance between plot pairs is 200 m. Plots were installed at forest sites where tree regeneration was potentially possible (i.e. mature forests providing seed trees), and where initial stages of regeneration already existed in the majority of cases or were expected to occur in the near future50,51. In choosing sites we also considered that light conditions would enable further development of seedlings (maximum canopy density by seed trees about 80%) and that a subsequent dense canopy closure was unlikely. Smaller mammals like hares (Lepus europaeus) or herbivore voles and mice were able to enter the exclosures. The type of treatment (fencing vs. control) was randomly attributed with a coin toss. Exclosures were constructed with a 2.0 m tall, galvanized fence outlining an area of 6 × 6 m. Distances between exclosures and corresponding control plots ranged from 5 m up to max. 20 m with largest possible congruence of site and stand conditions between exclosures and control plots. In this alpine environment with very high spatial heterogeneity, larger plots would have inevitably implicated more divergence between fence and control plots even at the beginning of the survey, which would have biased our records. Within the 6 × 6 m area of each fence and control plot, we recorded plant data within the central 5 × 5 m square (i.e. survey area without “edge effects”), which was permanently marked with metal rods. Data were first recorded in the year of establishment of exclosure/control pairs and afterwards in a three-year cycle. Thereby, the height of the tallest tree of all species per sampling plot (25 m2, each fenced and unfenced) was recorded using the following height classes: ≤ 10 cm, 11–25 cm, 26–40 cm, 41–70 cm, 71–100 cm, 101–130 cm, 131–160 cm, 161–200 cm, 201–250 cm, 251–300 cm, … (continuing 50 cm classes). Up to the 18-year period, we further recorded stem numbers and height classes of all trees. From the original 100 control samples 82 plots still existed after nine years, 65 after 12 years, 43 after 18 years and 17 plots could be further investigated until a 24-year period. The other plots dropped out mainly due to rockfall or single windthrown trees damaging them.
No animal experiments were carried out. The ungulates were neither caught nor influenced by medication. They were only denied access to small patches within forests by means of a fence. The fencing was carried out in accordance with all relevant guidelines and regulations. The entire sampling design was optimized to capture effects of ungulate herbivory and to keep other driving factors as constant as possible.
Data analyses
Targeting at synchronous analyses of the effects of ungulate herbivory on recruitment dynamics of tree communities and of all recorded tree species, we had to apply a method, which could cope with a large number of potentially occurring tree species (and thus with numerous categories of one input variable). Traditional approaches exploring effects of herbivory on tree recruitment dynamics like LMs, GLMs or GLMMs usually address only a few tree species (in most cases considering the dominant species15), or the response of single species within communities52 or community specific indices (e.g. diversity indices, species numbers, total seedling density or seedling heights14,30,52). In our study, we recorded up to 19 tree species at the fenced and/or unfenced plots, which would not allow for a convergence of traditional LMs, GLMs or GLMMs when considering interaction terms. We thus used principle response curves (PRC22,53,54,55 with a scaling of two) for data analyses, as we aimed to quantify the effects of ungulate herbivory on recruitment, height dynamics and stem density at both community level and at the level of all occurring single tree species. We presumed that PRC is a valuable approach for analysing regeneration dynamics on exclosure-control plots, looking both at total stem density and at stem heights of the highest trees per tree species (and plot). As height growth is strongly correlated with time, we particularly focused on height growth variance originating from treatment differences across time. PRC is a derivative of redundancy analysis (RDA), which uses sampling dates as set of co-variables and the interaction between sampling dates and treatment as set of explanatory variables. In our case, time series of treatment sites (fenced plots) is compared with corresponding control sites (unfenced plots) and effects of a treatment are represented as deviations from a control (= reference). Performing a PRC means variance partitioning56, yielding a variance part which origins from differences in time and another part which origins from treatment differences in time. The PRC focuses on effects of treatments factoring out variations between replicate microcosms and differing dates54. The first principal component of variance, being explained by the interaction of treatment and time, is displayed on the y-axis, whereas sampling dates are depicted on the x-axis54. Additionally, species scores on a second y-axis reflect the affinity of single species to the community-based response curve. This might be interpreted as weight of a species for the response diagram. Species with the same algebraic sign as the overall response curve show a corresponding response to a treatment, species with scores about zero (matching to the reference line) do not respond to the treatment or show distinctly differing response patterns, whereas species with an opposite sign than the community response are negatively correlated to the overall response curve. We used PRC to compare the outcome of two recording methods, i.e. dynamic maximum heights and total density of individuals.
In contrast to previous studies using PRC (e.g.22,55), we did not address changes in community composition (expressed in species numbers or coverage), but trajectories of maximum heights of all occurring tree species. As tree heights had been recorded in terms of height classes, we used the mid-point height per height class interval as representative (e.g. 85 cm for the 71–100 cm height class). To address both recruitment and height dynamics, we set the maximum tree heights of all potentially occurring tree species to zero when tree species were absent on single plots or points of time. This allowed us to adequately represent the following circumstances: (1) Tree species might be present on a fenced plot, while being absent on the control plot and vice versa. Without a zero maximum height value, we would have lost the information, that a given tree species is absent and related comparisons of fenced vs. control plots would have been biased. (2) Tree species might not be present during the first records, but might emerge and disappear at later points of time. Again, only zero maximum height values for all absent tree species could adequately represent such patterns. (3) Zero maximum height values allowed us to represent not only effects of herbivory on growth patterns of all potentially occurring tree species, but also on recruitment, which draws a more comprehensive picture of community and species responses to ungulate herbivory than it would have been the case otherwise. Due to the linear character of subsequent height classes, we did not log-transform height data, already matching the assumption of linearity for the PRC57. To address hypothesis (1), we also ran PRCs with total tree density. To depict regeneration, emerging after the installation of the fence/control-plots, we both considered stem numbers per hectare including height class 1 (i.e. plantlets < 10 cm) and stem numbers excluding height class 1. We tested the significance of the PRC with Monte Carlo permutations of the microcosms with permutations (n = 1000) of entire time series in partial RDAs. We further ran Monte Carlo permutation tests (n = 1000) for each sampling date, looking at the significance of the treatment per sampling date. As constrained proportions of variance increased with longer lasting survey periods (particularly relevant for maximum heights), we ran two analyses for testing the sensitivity of PRCs to sample size (i.e. the number of plot pairs): We first calculated PRCs with randomly reduced data sets without resampling and we used random sampling with replacement in a second step to draw subsets for the first two surveys (82 and 65 pairs of sampling plots respectively) consisting of 82 (for the first survey only), 65, 43 and 17 pairs of sampling plots with 500 iterations each. We then computed PRC analyses for each of the 3500 resulting subsets and extracted the constrained and conditional proportion of variance for comparison. To contrast results of PRCs roughly against a more traditional approach, we additionally ran LMs (for maximum height values) per tree species for the 18-year records, considering the treatment (fence vs. control), the point of time and their interaction as fixed factors. We used R 3.6.358 and vegan 2.5–459 for our analyses. For convenience of the workflow we further used RStudio 1.1.46360 and the following R-packages: dplyr 0.8.0.161, forcats 0.3.062, ggplot2 3.1.063, purrr 0.3.164, readr 1.3.165, stringr 1.4.066, tibble 2.0.167, tidyr 0.8.368, skimr 1.0.569, rstudioapi 0.770, rmarkdown 1.1071, knitr 1.2072 and hues 0.173.
Results
Dynamics of stem densities and maximum heights over time
Except for one tree species (i.e. rowan (Sorbus aucuparia) on the fenced plots) total stem numbers per hectare showed a typical overall decreasing trend in the course of the 18-year survey period (Fig. 1). At the end of the 18-year survey, ash and Norway spruce reached higher stem density on the control plots than on the fenced plots. Some rare species like Scots pine (Pinus sylvestris), Norway maple (Acer platanoides) or English yew (Taxus baccata) showed distinct responses to the exclosures in terms of lower maximum heights on the control plots. Within the frequently occurring tree species, European larch on average reached higher maximum heights on the control plots and Norway spruce did not respond distinctly to fencing in terms of maximum heights (within the 18-year study period; Fig. 1).
Trajectories (mean values ± std error) of (a) total stem density (n/ha), (b) stem density (n/ha) without height class one (< 10 cm) and (c) maximum heights per tree species for an 18-year time span, separated for the fenced plots (F) and the unfenced control plots (U); trajectories are separated for (A) the main tree species, also indicated with an + , and (B) co-occurring species. Species are ordered by their overall mean value (for total stem density, stem density without height class one and for maximums heights, respectively). Fraction numbers above the trajectories depict the number of main or co-occurring tree species on the fenced or unfenced plots, which show higher mean values (in terms of stem numbers, heights) than the remaining fraction. 3/5 at the first year of records of mean heights in panel A on the unfenced plots means, that three out of five main tree species are on average higher than the remaining two tree species. Aal Abies alba, Apl Acer platanoides, Aps Acer pseudoplatanus, Bpe Betula pendula, Fex Fraxinus excelsior, Fsy Fagus sylvatica, Lde Larix decidua, Msy Malus sylvaticus, Pab Picea abies, Pav Prunus avium, Psy Pinus sylvestris, Qsp Quercus sp., Sar Sorbus aria, Sau Sorbus aucuparia, Sca Salix caprea, Tba Taxus baccata, Ugl Ulmus glabra. Please note the different, scaled y-axes in the diagrams.
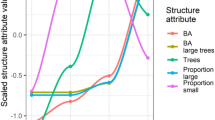
These patterns were conformingly reflected by the overall PRCs with a higher (and significant) response of trees to fencing in terms of maximum heights than of stem density (Fig. 2, Table 1). Differences in stem density or maximum heights between control plots and fenced plots, expressed in species scores (Fig. 2, Table 2), corresponded to the basic observed patterns (Fig. 1) as well, e.g. with higher stem numbers of Norway spruce on the control plots (Fig. 2) or larger maximum heights of European larch on the control plots during the 18-year survey period (Fig. 2).
Principle response curves showing trajectories of (a) total stem density, (b) stem density without height class one (< 10 cm), (c) maximum heights of trees along the first RDA axis over an 18-year period and (d) maximum heights over a 24-year period, contrasting fenced plots (-·-·-) to open control plots ( , depicted as reference line); the second y-axis represents single species scores on the first RDA axis. Species with the same algebraic sign as the overall response curve show a corresponding response to a treatment; species with scores about zero (matching to the reference line) do not respond to the treatment or show distinctly differing response patterns; species with a opposite sign than the overall community response (-·-·-) are negatively correlated to the overall response curve. Stem number PRCs (a,b) correspond to basic stem number patterns, but fencing is non-significant in terms of permutation testing. Aal Abies alba, Aps Acer pseudoplatanus, Bpe Betula pendula, Fex Fraxinus excelsior, Fsy Fagus sylvatica, Lde Larix decidua, Msy Malus sylvaticus, Pab Picea abies, Pav Prunus avium, Psy Pinus sylvestris, Qsp Quercus sp., Sar Sorbus aria, Sau Sorbus aucuparia, Sca Salix caprea, Tba Taxus baccata, Ugl Ulmus glabra. Please note the different, scaled y-axes in the diagrams.
, depicted as reference line); the second y-axis represents single species scores on the first RDA axis. Species with the same algebraic sign as the overall response curve show a corresponding response to a treatment; species with scores about zero (matching to the reference line) do not respond to the treatment or show distinctly differing response patterns; species with a opposite sign than the overall community response (-·-·-) are negatively correlated to the overall response curve. Stem number PRCs (a,b) correspond to basic stem number patterns, but fencing is non-significant in terms of permutation testing. Aal Abies alba, Aps Acer pseudoplatanus, Bpe Betula pendula, Fex Fraxinus excelsior, Fsy Fagus sylvatica, Lde Larix decidua, Msy Malus sylvaticus, Pab Picea abies, Pav Prunus avium, Psy Pinus sylvestris, Qsp Quercus sp., Sar Sorbus aria, Sau Sorbus aucuparia, Sca Salix caprea, Tba Taxus baccata, Ugl Ulmus glabra. Please note the different, scaled y-axes in the diagrams.
As highlighted in the summary of PRCs (Table 1), permutation tests of PRC axes did not yield significance for stem density in two thirds of tested cases. Significance for stem density was exclusively observed for stem density without height class 1 and never for total stem density. Contrary, all PRCs of maximum heights yielded significant values in permutation testing. Proportions of variance, captured by the treatment × time interaction, only reached low levels for the stem density. In contrast, all permutation tests of PRCs of maximum heights were significant and the constrained proportion of variance, reflecting treatment effects, was higher for all referring PRCs compared to stem density (Table 1). The constrained proportion of variance increased with longer duration of the observation period with highest values after 24 years. Permutation testing of the point of time, when the treatment becomes significant, only yielded results for maximum heights. Maximum heights responded to the fencing after 3–6 years. Significant deviations of the treatment plots from the control plots (= reference) were not detected at the first record (i.e. starting survey, Table 1, Fig. 2), indicating an adequate selection of comparable control and treatment plots.
Effects of ungulates on regeneration dynamics only reached comparably low levels: For the maximum heights, we observed the highest proportions of variance, captured by the treatment × time interaction (Table 1). This proportion was increasing with time. However, fencing only explained about 10% of variance of maximum heights after a survey period of 24 years. After nine years, only 3% of variance of maximum heights could be assigned to treatment × time interactions (Table 1). Contrary, time captured 12% of variance of maximum heights for the 24-year survey and 4% for the 9-year period. For stem density, all referring (significant) values were much lower.
Sensitivity of PRC to sample size
To test for the sensitivity of our PRCs to sample size, we also ran PRCs with randomly reduced data sets (Tables 3 and 4). Thereby, we used the number of exclosure/control pairs, which were available for the longer lasting survey periods (i.e. 65, 43, or 17 plot pairs). We further performed a random sampling with replacement building subsets for the first two surveys (82 and 65 pairs of sampling plots respectively) consisting of 82 (for the first survey only), 65, 43 and 17 pairs of sampling plots with 500 iterations each.
Except for one case, proportions of variance captured by the treatment × time interaction were not distinctly higher for the reduced data sets (Tables 3 and 4).
Tree species response
In total, we observed 19 tree species on the survey plots (Table 5). Of these, some species appeared after the first survey, some species like Scots pine (Pinus sylvestris) or wild cherry (Prunus avium) disappeared in the course of the experiment and some species could only be recorded in low numbers on single plots (Tilia sp., A. campestris, Quercus sp.).
For the 24-year period, sycamore maple showed the most distinct positive response to fencing in terms of maximum heights, followed by ash and rowan (Sorbus aucuparia) (Fig. 2, Table 5). Furthermore, European beech, common whitebeam (Sorbus aria) and goat willow (Salix caprea) showed high affinity to the first axis of the principle response curves. Maximum heights of silver fir followed a similar pattern. Several species like English yew, wych elm (Ulmus glabra), European crab apple (Malus sylvestris), Norway maple (Acer platanoides) and silver birch (Betula pendula) only reached species scores around zero, indicating a weak affinity to the first axis of the principle response curves. In contrast, Norway spruce was the only tree species following an opposite pattern of heights of highest trees, with higher trees on the control plots at the end of the 24-year survey period. However, the low proportion of variance explained by the treatment (10%) should be considered.
Traditional approach (LM) vs. PRC—contrasts at tree species level
The LMs of the maximum height values per tree species as response variables and the treatment, the point of time and their interaction as fixed factors yielded adjusted R2 values between zero (for silver birch, European larch, European crab apple, wild cherry, Scots pine) and a maximum of 24% (for common whitebeam) (see Table 6). Seven out of 15 species-specific maximum height models comprised significant interactions of the treatment and the point of time. No single species-specific LM yielded a significant coefficient of the main factor treatment, whereas three models (i.e. for silver birch, European larch and Norway spruce) yielded a significant effect of point of time without comprising significant interactions between the treatment and the point of time. Contrasting the species-specific maximum height models to the partial species-specific results of the PRC showed, that most models go conform with the PRC in terms of significance and algebraic sign of the effect coefficients of the LMs (Table 6).
Discussion
Analysing herbivore effects on the height of largest trees versus total stem density
As only a comparatively small number of progenies of forest trees survive till maturity74 and only a small portion of seedlings finally grows into the forest canopy38, we hypothesised that dynamic recording of heights of the highest tree seedlings, saplings and young trees per tree species (and plot) to be more meaningful than taking total stem density (see30) to depict net effects of herbivory on forest dynamics at relevant developmental stages (i.e. terminal heights being no longer accessible for browsing by ungulates). This hypothesis was confirmed through trajectories of maximum heights per tree species that yielded significant results of PRCs contrary to trajectories of total stem density. More significant responses of tree communities to browsing in terms of height growth than in total stem numbers have already been documented in previous studies30. Since the highest trees have a higher likelihood of canopy recruitment as compared to average individuals42,43, an analysis of average responses only leads to a misinterpretation of herbivore effects41,75,76. A dynamic recording of highest trees that allows for shifting dominance focuses on net results of regeneration processes and includes compensation of losses in height growth caused by browsing by other, non-browsed tree individuals at community level41,74. For monitoring of ungulate impacts in practical forestry and wildlife management, the focus on highest trees also has advantages in terms of lower recording efforts in the field.
We assumed that trajectories of height growth of all occurring trees of each species might be less relevant than height dynamics of the respective highest trees of each species, as herbivory-mediated suppression of one tree might favour height growth of a neighbouring tree (even of the same species) and this spatial impact might change with time.
This is in line with results of other studies77, predicting selective effects of browsing in terms of reduced abundance of small trees, which in turn results in a lower recruitment into higher height classes. Modelling juvenile survivorship of tree species, mortality probabilities of saplings might be predicted as functions of recent growth histories42. Height class distributions of plant taxa or functional groups are typical response variables when addressing herbivore effects on plant communities (see77). However, for tree species composition of mature forests the number and mixture of trees in the reproductive upper layer is important. In contrast to the high numbers of trees in forest regenerations with initial seedling numbers up to some 100,000 trees per hectare, in mature forests, the tree density naturally decreases to some hundred trees per hectare. For this decrease, many causes are possible (compensatory mortality). Within this forest dynamic, the number of surviving trees and the heights of the species’ dominant trees in each stage of forest development are decisive whereas total seedling densities and their height classes are less important for the further forest development (cf.41,43,51,78). In agreement with other authors79, we see deer browsing on woody seedlings as a function of species preference, height structure, growth, and seasonal phenology of seedlings. Maximum height dynamics, addressed in our study, reflect local dominance of tree species via asymmetric plant competition, and such effects, both within and among forest patches, can accrue over time shaping forest structure and composition. As in other studies, high variance in growth rates might be attributed to differences in phenotypes, resulting from various impacts like canopy shading, variation in soil nutrient and moisture conditions, herbivory, or intraspecific competition76,80. Accordingly, growth rates can vary widely even under controlled experimental conditions81.
Community approach versus single-species focus
In previous studies, most species-oriented approaches either focused on single (often rare or dominant) target species or were based on community-related values (e.g. diversity indices; see82,83,84). As regeneration dynamics and survivorship of tree species unfold as a result of neighbourhood processes of individuals and their species-specific life-history traits in connection with environmental filters29,35,85,86,87,88, a synchronous analysis of both the entire regenerating tree community and all tree species (vs. subjectively focusing on certain species only) seems to be essential. However, many standard statistical approaches like GL(M)Ms are not applicable in case of largely multiple response variables. Such approaches are typically applied to total stem numbers, to regeneration cover or to responses of single key species (e.g.89). In contrast, PRCs proved to yield traceable results at community and species level. For species with weak affinity to the first PRC axis, pictures of the second and following axis might be relevant57. Thereby, proportions of variance explained by the treatment × time interaction should be considered.
Long term versus short term studies
The proportion of variance in maximum heights, explained by the treatment × time interaction (i.e. the fencing) was 3% after nine years but increased to about 10% after 24 years of monitoring. This highlights the importance of long-term surveys, when exploring herbivore effects on forest regeneration (see also30) and confirms our second hypothesis. In half of our analyses, PRCs became significant at the 6th year of monitoring and at subsequent phases. We thus infer, that short-time surveys might not catch relevant developmental stages (i.e. heights of terminal shoots being no longer accessible for browsing ungulates) and might thus not adequately represent net effects of browsing at community level. Even at tree species level, response patterns to herbivory might not be uniform over time: For example, both Norway spruce and European larch changed in their response to fencing. Norway spruce showed no distinct response in terms of maximum heights over 18 years and negatively responded to fencing afterwards. Contrary, European larch switched from positive response to neutral and negative response to fencing and ended after 24 years with positive response in terms of maximum heights. Specific responses of tree species to ungulate browsing might derive from different feeding preferences of ungulates, selecting for the most palatable species and from tree species´ tolerance to browsing52,90,91. Most likely, the observed patterns for Norway spruce and European larch reflect their low preference by browsing ungulates92 due to low palatability and effects of competition with broadleaf trees on the fenced plots. Furthermore, European larch typically germinates successfully on exposed mineral soil, which might be promoted by ungulate trampling (see93). Windows of opportunity for successful recruitment of European larch might thus positively correlate with periods of higher ungulate presence.
The percentages of variance explained by the PRCs appear to be rather low. It is thus evident that, despite considerable population densities of ungulates, other factors than browsing distinctly drove the dynamics of the largest individuals per species (and plot) in our study such as responses to (micro-)sites and light conditions, intra- and interspecific competition and pathogens29,35,85,86,87,88. We attribute this to the following causes: (1) In the montane forests of the study region, the natural species composition has a high ratio of Norway spruce49, and the ratio has been further increased by forestry since the mid of the nineteenth century94,95. The response of Norway spruce to the fencing was weak and, at the end of the survey period, contrary to all other tree species. The low percentages of variance explained may thus carry a strong signal of this species; (2) The study sites represent mesic conditions on average and did not show strong disturbances during the observation periods. On such sites, herbivory-caused mortality or retarding of individual growth through herbivory may be compensated at community or individual levels. This may not be the case on e.g. drier sites after large disturbances in comparable regions, where intense ungulate herbivory had strong effects on post-disturbance species composition and lead to higher soil CO2-effluxes caused by higher soil temperatures96. Moderate intensities of herbivory, however, did not show differences in soil CO2-effluxes96. It may thus be that thresholds exist in terms of impacts of ungulate herbivory on ecosystem parameters that were not crossed on the sites in our study area.
In the given study, we focused on effects of ungulate herbivory at different developmental stages of tree species, targeting their chance of reaching reproductive size. Future analyses might as well address bottom-up forces (abiotic factors) together with top–down effects as related data have been recorded on the study plots. Other factors, additionally affecting forest regeneration structures and partly interacting with ungulate impacts, could be included in further studies testing different constellations for long-term effects on forests, e.g. changing light conditions in the understorey, other abiotic site factors, frequency of seed trees per species, insects, rodents, tree diseases, competitive vegetation such as shrubs, grasses, and herbs.
General considerations for analyses and interpretations of exclosure experiments
For analyses and interpretations of exclosure experiments—as done in this study—several factors have to be considered: (1) tree regeneration in control plots and fenced plots should not differ at the beginning of records. In other words, the status of already present regeneration, site conditions and the presumed development of the crown canopy should be the same for both plot types. Thus, the choice of treatment–control pairs is a crucial point in sampling design. In our case, we did not find any significant deviations of the treatment (fenced) plots from the control plots (= reference) for the starting survey. We thus conclude, that we adequately selected comparable control and treatment plots. (2) Due to the sampling design, control-fence plots are usually located in areas with beginning/already existing forest regeneration. In case that the surroundings of small regeneration spots are comparatively poor in forest regeneration (and ground vegetation) cover (depending on site and silvicultural system), regeneration at such small spots might be more intensively used by ungulates and herbivory effects might be boosted there, compared to regeneration on extended areas. In our study, the high number of control-fence pairs should compensate for such possibly occurring effects. Furthermore, PRCs proved to be comparatively non-sensitive to changes in sample size. Potential compensation effects or amplification effects of deer impacts on forests trees might occur at later stages (older thickets, pole stand). Such effects could not be investigated in the given study up to now (needing longer periods than 24 years), but the exclosures hold the potential for referring future analyses.
Conclusion
Trees show a strong ontogenetic variation in terms of consumption rates by herbivores. Effects of herbivory, occurring at early developmental stages, might be accompanied or followed by a cascade of other processes, that drive plant community dynamics. Using principle response curves (PRC) we could show, that early stage herbivory might thus not be a significant driver of dynamics at later stages and it might not necessarily determine the chance of reaching reproductive size in forest trees. Repeated records of the heights of the highest individuals per tree species captures changes in dominance with time, focusing on individuals with a high probability of reaching the main canopy and getting into reproductive stages. Recording maximum heights of all regenerating tree species in paired plots with and without exclosures thus better reflects effects of ungulate herbivory on long-term dynamics of tree regeneration than recording stem densities. Results from surveys with short and medium duration differ from long-term results, when trees have reached later development stages (thickets, pole stands). Regeneration dynamics and survivorship of tree species are the result of neighbourhood processes of individuals and species-specific life-history traits together with environmental filters. Thus, synchronous analyses of both the entire regenerating tree community and all occurring tree species (vs. subjectively focusing on certain target species) are ecologically informative.
Data availability
Datasets of the enclosure-control pairs are available at https://doi.org/10.6084/m9.figshare.13160159.v1.
Code availability
Program source codes are maintained by U. Nopp-Mayr.
References
Crawley, M. Herbivory: The Dynamics of Animal–Plant Interactions (Blackwell Scientific, Oxford, 1983).
Putman, R. Grazing in Temperate Ecosystems: Large Herbivores and the Ecology of the New Forest (Springer, Berlin, 1986).
Huntly, N. Herbivores and the dynamics of communities and ecosystems. Annu. Rev. Ecol. Syst. 1, 477–503 (1991).
Skarpe, C. Impact of grazing in savanna ecosystems. Ambio 20, 351–356 (1991).
Agrawal, A. A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 22, 103–109 (2007).
Bruce, T. C. Interplay between insects and plants–dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 2, 391 (2014).
Mason, N. W. H., Peltzer, D. A., Richardson, S. J., Bellingham, P. J. & Allen, R. B. Stand development moderates effects of ungulate exclusion on foliar traits in the forests of New Zealand: Ungulate impacts on foliar traits. J. Ecol. 98, 1422–1433 (2010).
Faison, E. K., DeStefano, S., Foster, D. R., Motzkin, G. & Rapp, J. M. Ungulate browsers promote herbaceous layer diversity in logged temperate forests. Ecol. Evol. 6, 4591–4602 (2016).
Simončič, T., Bončina, A., Jarni, K. & Klopčič, M. Assessment of the long-term impact of deer on understory vegetation in mixed temperate forests. J. Veg. Sci. 30, 108–120 (2019).
Schmitz, O. J. Herbivory from individuals to ecosystems. Annu. Rev. Ecol. Evol. Syst. 39, 133–152 (2008).
Riginos, C. & Grace, J. B. Savanna tree density, herbivores, and the herbaceous community: Bottom-up vs. top-down effects. Ecology 89, 2228–2238 (2008).
Turkington, R. Top-down and bottom-up forces in mammalian herbivore–vegetation systems: An essay review. Botany 87, 723–739 (2009).
Kos, M. et al. Relative importance of plant-mediated bottom-up and top-down forces on herbivore abundance on Brassica oleracea: Bottom-up and top-down effects on herbivores. Funct. Ecol. 25, 1113–1124 (2011).
Kuijper, D. P. J. et al. Bottom-up versus top-down control of tree regeneration in the Białowieża Primeval Forest, Poland: Abiotic and biotic control of tree regeneration. J. Ecol. 98, 888–899 (2010).
Churski, M., Bubnicki, J. W., Jędrzejewska, B., Kuijper, D. P. J. & Cromsigt, J. P. G. M. Brown world forests: Increased ungulate browsing keeps temperate trees in recruitment bottlenecks in resource hotspots. New Phytol. 214, 158–168 (2017).
Fretwell, S. D. Food chain dynamics: The central theory of ecology?. Oikos 50, 291–301 (1987).
Reimoser, F. & Putman, R. Impacts of wild ungulates on vegetation: Costs and benefits. In Ungulate Management in Europe—Problems and Practices (eds Putman, R. et al.) 144–191 (Cambridge University Press, Cambridge, 2011).
Bellingham, P. J. & Allan, C. N. Forest regeneration and the influences of white-tailed deer (Odocoileus virginianus) in cool temperate New Zealand rain forests. For. Ecol. Manag. 175, 71–86 (2003).
Russell, F. L. & Fowler, N. L. Effects of white-tailed deer on the population dynamics of acorns, seedlings and small saplings of Quercus buckleyi. Plant Ecol. 173, 59–72 (2004).
Casabon, C. & Pothier, D. Browsing of tree regeneration by white-tailed deer in large clearcuts on Anticosti Island, Quebec. For. Ecol. Manag. 253, 112–119 (2007).
Pellerin, M. et al. Impact of deer on temperate forest vegetation and woody debris as protection of forest regeneration against browsing. For. Ecol. Manag. 260, 429–437 (2010).
Tschöpe, O., Wallschläger, D., Burkart, M. & Tielbörger, K. Managing open habitats by wild ungulate browsing and grazing: A case-study in North-Eastern Germany: Managing open habitats by wild ungulate browsing and grazing. Appl. Veg. Sci. 14, 200–209 (2011).
Millett, J. & Edmondson, S. The impact of 36 years of grazing management on vegetation dynamics in dune slacks. J. Appl. Ecol. 50, 1367–1376 (2013).
Beck, H., Snodgrass, J. W. & Thebpanya, P. Long-term exclosure of large terrestrial vertebrates: Implications of defaunation for seedling demographics in the Amazon rainforest. Biol. Conserv. 163, 115–121 (2013).
Charles, G. K., Porensky, L. M., Riginos, C., Veblen, K. E. & Young, T. P. Herbivore effects on productivity vary by guild: Cattle increase mean productivity while wildlife reduce variability. Ecol. Appl. 27, 143–155 (2017).
Castleberry, S. B., Ford, W. M., Miller, K. V. & Smith, W. P. Influences of herbivory and canopy opening size on forest regeneration in a southern bottomland hardwood forest. For. Ecol. Manag. 131, 57–64 (2000).
Filazzola, A., Tanentzap, A. J. & Bazely, D. R. Estimating the impacts of browsers on forest understories using a modified index of community composition. For. Ecol. Manag. 313, 10–16 (2014).
Nishizawa, K., Tatsumi, S., Kitagawa, R. & Mori, A. S. Deer herbivory affects the functional diversity of forest floor plants via changes in competition-mediated assembly rules. Ecol. Res. 31, 569–578 (2016).
Boulanger, V. et al. Ungulates increase forest plant species richness to the benefit of non-forest specialists. Glob. Change Biol. 24, e485–e495 (2018).
McGarvey, J. C., Bourg, N. A., Thompson, J. R., McShea, W. J. & Shen, X. Effects of twenty years of deer exclusion on woody vegetation at three life-history stages in a mid-atlantic temperate deciduous forest. Northeast. Nat. 20, 451–468 (2013).
Kabeya, D. & Sakai, S. The role of roots and cotyledons as storage organs in early stages of establishment in Quercus crispula: a quantitative analysis of the nonstructural carbohydrate in cotyledons and roots. Ann. Bot. 92, 537–545 (2003).
Boege, K. & Marquis, R. J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 20, 441–448 (2005).
Hanley, M. E., Lamont, B. B., Fairbanks, M. M. & Rafferty, C. M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 8, 157–178 (2007).
Diggle, P. J. Statistical Analysis of Spatial Point Patterns. (Arnold, 2003).
Gratzer, G. & Waagepetersen, R. Seed dispersal, microsites or competition—What drives gap regeneration in an old-growth forest? An application of spatial point process modelling. Forests 9, 230 (2018).
Szwagrzyk, J., Gratzer, G., Stępniewska, H., Szewczyk, J. & Veselinovic, B. High reproductive effort and low recruitment rates of European beech: Is there a limit for the superior competitor?. Pol. J. Ecol. 63, 198–212 (2015).
Nopp-Mayr, U., Kempter, I., Muralt, G. & Gratzer, G. Herbivory on young tree seedlings in old-growth and managed mountain forests. Ecol. Res. 30, 479–491 (2015).
Shugart, H. H. A theory of forest dynamics. (Springer, 1984).
Lertzman, K. B. Patterns of gap-phase replacement in a subalpine, old-growth forest. Ecology 73, 657–669 (1992).
Kneeshaw, D. D. & Bergeron, Y. Canopy gap characteristics and tree replacement in the Southeastern Boreal forest. Ecology 79, 783–794 (1998).
Wakeling, J. L., Staver, A. C. & Bond, W. J. Simply the best: The transition of savanna saplings to trees. Oikos 120, 1448–1451 (2011).
Kobe, R. K., Pacala, S. W., Silander, J. A. Jr. & Canham, C. D. Juvenile tree survivorship as a component of shade tolerance. Ecol. Appl. 5, 517–532 (1995).
Zuidema, P. A., Brienen, R. J. W., During, H. J. & Güneralp, B. Do persistently fast-growing juveniles contribute disproportionately to population growth? A new analysis tool for matrix models and its application to rainforest trees. Am. Nat. 174, 709–719 (2009).
Tremblay, J.-P., Huot, J. & Potvin, F. Density-related effects of deer browsing on the regeneration dynamics of boreal forests. J. Appl. Ecol. 44, 552–562 (2007).
Speed, J. D. M., Austrheim, G., Hester, A. J., Solberg, E. J. & Tremblay, J.-P. Regional-scale alteration of clear-cut forest regeneration caused by moose browsing. For. Ecol. Manag. 289, 289–299 (2013).
Shelton, A. L., Henning, J. A., Schultz, P. & Clay, K. Effects of abundant white-tailed deer on vegetation, animals, mycorrhizal fungi, and soils. For. Ecol. Manag. 320, 39–49 (2014).
Reimoser, F. & Reimoser, S. Ergebnisse aus dem Vergleichsflächenverfahren ('Wildschaden-Kontrollzäune’) – ein Beitrag zur Objektivierung der Wildschadensbeurteilung. In Ist die natürliche Verjüngung des Bergwaldes gesichert? (ed. Müller, F.) 151–159 (Austrian Research Centre for Forests, Vienna, 2003).
ZAMG. Klimadaten von Österreich 1971–2000. (2013).
Mucina, L., Grabherr, G. & Wallnöfer, S. Die Pflanzengesellschaften Österreichs. Teil III - Wälder und Gebüsche (Gustav Fischer Verlag, Stuttgart, 1993).
Reimoser, F., Schodterer, H. & Reimoser, S. Beurteilung des Schalenwildeinflusses auf die Waldverjüngung – Vergleich verschiedener Methoden des Wildeinfluss-Monitorings („WEM - Methodenvergleich”) (Austrian Research Centre for Forests, Vienna, 2014).
Reimoser, F., Armstrong, H. & Suchant, R. Measuring forest damage of ungulates: What should be considered. For. Ecol. Manag. 120, 47–58 (1999).
Long, Z. T., Pendergast, T. H. & Carson, W. P. The impact of deer on relationships between tree growth and mortality in an old-growth beech-maple forest. For. Ecol. Manag. 252, 230–238 (2007).
Van den Brink, P. J. & Ter Braak, C. J. Principal response curves: Analysis of time-dependent multivariate responses of biological community to stress. Environ. Toxicol. Chem. 18, 138–148 (1999).
van den Brink, P. J., den Besten, P. J., de Bij, V. A. & ter Braak, C. J. F. Principal response curves technique for the analysis of multivariate biomonitoring time series. Environ. Monit. Assess. 152, 271–281 (2009).
Poulin, M., Andersen, R. & Rochefort, L. A new approach for tracking vegetation change after restoration: A case study with peatlands. Restor. Ecol. 21, 363–371 (2013).
Borcard, D., Gillet, F. & Legendre, P. Numerical Ecology with R (Springer International Publishing, Berlin, 2018).
Van den Brink, P. J., Van den Brink, N. W. & Ter Braak, C. J. Multivariate analysis of ecotoxicological data using ordination: demonstrations of utility on the basis of various examples. Austr. J. Ecotoxicol. 9, 141–156 (2003).
R Development Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2020).
Oksanen, J. et al. vegan: Community Ecology Package. (2019).
RStudio Team. RStudio: Integrated Development Environment for R. (RStudio, Inc., 2016).
Wickham, H., François, R., Henry, L. & Müller, K. dplyr: A Grammar of Data Manipulation. (2019).
Wickham, H. forcats: Tools for Working with Categorical Variables (Factors). (2018).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, Berlin, 2016).
Henry, L. & Wickham, H. purrr: Functional Programming Tools. (2019).
Wickham, H., Hester, J. & Francois, R. readr: Read Rectangular Text Data. (2018).
Wickham, H. stringr: Simple, Consistent Wrappers for Common String Operations. (2019).
Müller, K. & Wickham, H. tibble: Simple Data Frames. (2019).
Wickham, H. & Henry, L. tidyr: Easily Tidy Data with ‘spread()’ and ‘gather()’ Functions. (2019).
McNamara, A., Rubia, E. A. de la, Zhu, H., Ellis, S. & Quinn, M. skimr: Compact and Flexible Summaries of Data. (2019).
Allaire, J. J., Wickham, H., Ushey, K. & Ritchie, G. rstudioapi: Safely Access the RStudio API. (2017).
Allaire, J. J. et al. rmarkdown: Dynamic Documents for R. (2018).
Xie, Y. knitr: A General-Purpose Package for Dynamic Report Generation in R. (2018).
Baumgartner, J. hues: Distinct Colours Palettes Based on ‘iwanthue’. (2017).
Jones, O. R. et al. Diversity of ageing across the tree of life. Nature 505, 169–173 (2014).
Pacala, S. W. et al. Forest models defined by field measurements: Estimation error analysis and dynamics. Ecol. Monogr. 66, 1–43 (1996).
Beckage, B. & Clark, J. S. Seedling survival and growth of three forest tree species: The role of spatial heterogeneity. Ecology 84, 1849–1861 (2003).
Peltzer, D. A. et al. Disentangling drivers of tree population size distributions. For. Ecol. Manag. 331, 165–179 (2014).
Reimoser, F., Odermatt, O., Roth, R. & Suchant, R. Die Beurteilung von Wildverbiss durch SOLL-IST-Vergleich. Allg Forst Jagdztg 168, 214–227 (1997).
Pépin, D. et al. Relative impact of browsing by red deer on mixed coniferous and broad-leaved seedlings—An enclosure-based experiment. For. Ecol. Manag. 222, 302–313 (2006).
Jurena, P. N. & Archer, S. Woody plant establishment and spatial heterogeneity in grasslands. Ecology 84, 907–919 (2003).
Cramer, M. D., Chimphango, S. B. M., Cauter, A. V., Waldram, M. S. & Bond, W. J. Grass competition induces N2 fixation in some species of African Acacia. J. Ecol. 95, 1123–1133 (2007).
Hegland, S. J., Lilleeng, M. S. & Moe, S. R. Old-growth forest floor richness increases with red deer herbivory intensity. For. Ecol. Manag. 310, 267–274 (2013).
Lilleeng, M. S., Hegland, S. J., Rydgren, K. & Moe, S. R. Red deer mediate spatial and temporal plant heterogeneity in boreal forests. Ecol. Res. 31, 777–784 (2016).
Laurent, L., Mårell, A., Balandier, P., Holveck, H. & Saïd, S. Understory vegetation dynamics and tree regeneration as affected by deer herbivory in temperate hardwood forests. IForest - Biogeosciences For. 10, 837–844 (2017).
Holladay, C.-A., Kwit, C. & Collins, B. Woody regeneration in and around aging southern bottomland hardwood forest gaps: Effects of herbivory and gap size. For. Ecol. Manag. 223, 218–225 (2006).
Smit, C., Gusberti, M. & Müller-Schärer, H. Safe for saplings; safe for seeds?. For. Ecol. Manag. 237, 471–477 (2006).
Pröll, G., Darabant, A., Gratzer, G. & Katzensteiner, K. Unfavourable microsites, competing vegetation and browsing restrict post-disturbance tree regeneration on extreme sites in the Northern Calcareous Alps. Eur. J. For. Res. 134, 293–308 (2015).
Stephan, J. G. et al. Long-term deer exclosure alters soil properties, plant traits, understorey plant community and insect herbivory, but not the functional relationships among them. Oecologia 184, 685–699 (2017).
Hidding, B., Tremblay, J.-P. & Côté, S. D. A large herbivore triggers alternative successional trajectories in the boreal forest. Ecology 94, 2852–2860 (2013).
Augustine, D. J. & McNaughton, S. J. Ungulate effects on the functional species composition of plant communities: Herbivore selectivity and plant tolerance. J. Wildl. Manag. 62, 1165–1183 (1998).
Owen-Smith, N. R. Adaptive Herbivore Ecology. From Resources to Populations in Variable Environments (Cambridge University Press, Cambridge, 2002).
Reimoser, F. & Reimoser, S. Richtiges Erkennen von Wildschäden am Wald (Zentralstelle Österr, Landesjagdverbände, 2017).
Ramirez, J. I. et al. Above- and below-ground cascading effects of wild ungulates in temperate forests. Ecosystems https://doi.org/10.1007/s10021-020-00509-4 (2020).
Kral, F. Spät- und postglaziale Waldgeschichte der Alpen aufgrund der bisherigen Pollenanalysen (Österreichischer Agrarverlag, Vienna, 1979).
Mayer, H. & Ott, E. Gebirgswaldbau, Schutzwaldpflege: ein waldbaulicher Beitrag zur Landschaftsökologie und zum Umweltschutz (G. Fischer, Mumbai, 1991).
Mayer, M., Keßler, D. & Katzensteiner, K. Herbivory modulates soil CO2 fluxes after windthrow: A case study in temperate mountain forests. Eur. J. For. Res. 139, 383–391 (2020).
Acknowledgements
We thank the Austrian Federal Forests (ÖBf AG), who made this long-term investigation possible.
Author information
Authors and Affiliations
Contributions
S.R. and F.R. designed and conducted the field work and provided all data. U.N.-M. and G.G. conceptualised the study. U.N.-M. selected and conducted the statistical analyses, assisted by F.S. and L.O. S.R., F.R. and G.G. supervised the analyses. F.S. prepared the figures. U.N.-M. and G.G. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nopp-Mayr, U., Reimoser, S., Reimoser, F. et al. Analyzing long-term impacts of ungulate herbivory on forest-recruitment dynamics at community and species level contrasting tree densities versus maximum heights. Sci Rep 10, 20274 (2020). https://doi.org/10.1038/s41598-020-76843-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76843-3
This article is cited by
-
Plant–plant interaction by Aster leiophyllus affects herbivory by Sika deer, Cervus nippon
The Science of Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.