Abstract
H6 subtype avian influenza viruses spread widely in birds and pose potential threats to poultry and mammals, even to human beings. In this study, the evolution and pathogenicity of H6 AIVs isolated in live poultry markets from 2011 to 2017 were investigated. These H6 isolates were reassortant with other subtypes of influenza virus with increasing genomic diversity. However, no predominant genotype was found during this period. All of the H6N2 and most of the H6N6 isolates replicated efficiently in lungs of inoculated mice without prior adaptation. All of the H6N2 and two H6N6 isolates replicated efficiently in nasal turbinates of inoculated mice, which suggested the H6N2 viruses were more adaptive to the upper respiratory tract of mice than the H6N6 viruses. One of H6N2 virus caused systemic infection in one out of three inoculated mice, which indicated that H6 avian influenza virus, especially the H6N2 viruses posed a potential threat to mammals. Five H6 strains selected from different genotypes caused no clinical signs to inoculated chickens, and their replication were limited in chickens since the viruses have been detected only from a few tissues or swabs at low titers. Our study strongly suggests that the H6 avian influenza virus isolated from live poultry markets pose potential threat to mammals.
Similar content being viewed by others
Introduction
Avian influenza virus (AIV), belonging to influenza A virus in the family of Orthomyxoviridae, contains eight negative stranded RNA segments. Influenza A viruses are subdivided into 18 different hemagglutinin (HA) and 11 neuraminidase (NA) subtypes based on the surface proteins1,2,3,4. Subtype H6 is one of the widespread AIV subtypes though it is easy to be neglected for its low pathogenicity in birds5,6,7.
The H6 AIV was isolated first from a turkey in the United States in 19658,9. Since then, H6 AIVs have been detected frequently from wild aquatic birds and domestic poultry throughout the world10,11,12. During 2000–2002, H6N2 AIVs were isolated from chickens from 12 different locations in California. The pathological changes observed in the early cases were primarily associated with mild respiratory infections, but yolk peritonitis had become the main feature of all the subsequent cases through 2001 and 200213. In 2009, H6N1 AIV was isolated once again from turkey exhibiting typical signs associated with AIV infection in Israel14.
Domestic ducks act as an important reservoir for influenza viruses and have also facilitated the establishment of multiple H6 influenza virus lineages15. Among those, the H6N2 and H6N6 were the main epidemiological subtypes recently circulated in Southern China6,7,15,16. Though clinical disease caused by H6 in chicken farms has not been reported in China, H6 AIVs were isolated continually from chickens in the live poultry markets (LPMs) and might pose a huge threat on the poultry industry. Previous studies have shown that the H6 AIVs have a broad host range, and the H6 viruses might have crossed the species barrier and infected mammals, including humans, without adaptation17,18. In 2010, an avian-origin H6N6 swine influenza virus was isolated from sick pigs in Southern China19. Three years later, an H6N1 virus was isolated from a woman with flu-like symptoms in Taiwan18. In addition, an H6N1 virus with molecular characteristics closely related to the human isolates, was found to cause infections in dogs20. H6 AIVs have the potential to cross the species barrier and infect mammals, including humans18,20. Around 34% of H6 viruses isolated from Southern China could bind to the human-like receptor17. Specially, a single amino acid change from glutamine to leucine at position 226 of hemagglutinin causes a switch in receptor-binding preference from avian-like to mammalian-like21. In addition, H6 AIVs provided internal genes for other subtype viruses and generated novel viruses frequently, such as H5N1, H9N2 and H5N6 AIVs which were detected in humans22,23,24.
Recently, a large amount of H6 AIVs were isolated in Southern China through routine surveillance and their phylogenetic characters were analyzed comprehensively25. However, the pathogenicity of those H6 AIVs on chickens and mammals are unclear. In this study, the evolution and pathogenicity of 7 H6N2 and 15 H6N6 viruses, isolated from LPMs in Southern China from 2011 to 2017, were evaluated in mice and chickens.
Materials and methods
Virus isolation
Oropharyngeal swabs were collected at different LPMs in Hunan, Jiangsu, Zhejiang, Guangdong and Fujian provinces from 2011 to 2017. Each swab was soaked in phosphate-buffered saline (PBS) and tested by reverse transcription and polymerase chain reaction (RT-PCR) using influenza-specific HA primers for H6 subtype as described previously26,27. To isolate the viruses, the H6-positive samples were filtered through 0.22 μm filters and inoculated into allantoic cavity of 9-day-old specific pathogen free (SPF) embryonated chicken eggs (Beijing Merial Vital Laboratory Animal Technology Co., Ltd.,Beijing, China). The viruses were purified and propagated by 3 rounds of limiting dilution in embryonated SPF chicken eggs28. The viral titers were determined in SPF embryonated chicken eggs and calculated using the Reed and Muench method29.
Genetic analysis and phylogenetic analysis
Viral RNAs were extracted from allantoic fluid containing H6 viruses with the QIAamp viral RNA mini kit (Qiagen, Gemany) according to the manufacturer’s instructions. The first-strand cDNAs were synthesized using reverse transcriptase M-MLV (Takara Bio Inc., Dalian, China) with universal primer for influenza A viruses (5`- AGC RAA AGC AGG-3`) following the manufacturer’s protocol. The eight gene segments of each virus were amplified by PCR with universal primers for influenza30. The PCR were carried out in an C1000 Thermal cycler PCR Instrument (Biorad, U.S.A.) using Premix Ex Taq version 2.0 (Takara Bio Inc., Da Lian, China) following the manufacturer's instructions. The PCR products were purified using DNA Gel Extraction Kits (Axygen, Hangzhou, China) and sequenced by GENEWIZ Biotechnology Company (Suzhou, China). The sequences were edited with Seqman module of the DNAStar package. Phylogenetic trees were generated by the distance-based neighbor-joining method using Clustal W. The reliability of the trees was assessed by bootstrap analysis with 1000 replications31. Reference sequences were cited from GenBank of NCBI and GISAID.
Studies with mice
To determine the pathogenicity of theH6 AIVs in mice, each of eight 4-week-old female BALB/c mice (Beijing Merial Vital Laboratory Animal Technology Co., Ltd., Beijing, China) were anesthetized and inoculated intranasally (i.n.) with 106.0 EID50 in 30.0 μl PBS of each virus of the 22 H6 isolates. Eight control mice were inoculated intranasally with 30.0 μl of PBS. At five days post-inoculation (dpi), three mice in each group were euthanized, and their lungs, nasal turbinates, hearts, livers, spleens and brains were collected for virus titration. Each organ was homogenized in chilled PBS and centrifuged at 2500g for 10 min. The viruses in the supernatants were titrated using the methods described previously29. The body weight and clinical signs of remaining five mice in each group were recorded until 14 dpi.
Studies with chickens
The pathogenicity of 5 H6 viruses from different genotypes, was evaluated in SFP chickens. Each of six 4-week-old SPF chickens were inoculated i.n. with 106.0EID50/bird of each virus in a volume of 100.0 μl, respectively. One day later, three naïve chickens were introduced into each of these isolates to evaluate the transmission of viruses among chickens. Six control birds were inoculated i.n. with the same amount of PBS. Three inoculated chickens in each group were euthanized, and their tracheas, lungs, kidneys, spleens and duodenums were collected for virus isolation at 3 dpi. Cloacal and oropharyngeal swabs were collected from the other 3 chickens at 2, 4 and 6 dpi. All of the tissues were homogenized in PBS (0.1 g per 1.0 ml), and the swabs in 1.0 ml PBS were stirred on vortex, and then all samples were centrifuged at 2500 g for 10 min. Each supernatant was collected, filtered through 0.22 μm filters, and inoculated into allantoic cavities of three 9-day-old embryonated chicken eggs. At 14dpi, blood samples were drawn from the remaining inoculated chickens and three contact chickens for detection of H6 subtype specific antibodies.
Ethics statement and statistical analysis
All animal studies in this study were conducted in accordance to the guidelines of the Animal Care and Use Committee of Shanghai Veterinary Research Institute, and all animal studies protocols are approved by Shanghai Veterinary Research Institute (Permit number: SHVRI-Po-0120). Good living environment with sufficient food and water were available for all the animals. Comparisons of the weight changes between two groups were determined by nonparametric t-tests using GraphPad (Vision 6.0, GraphPad Prism). The differences were considered statistically significant if P values < 0.05(*), P < 0.01(**), P < 0.001(***).
Results
Virus isolation and identification
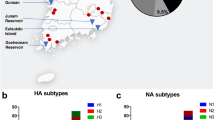
A total of 22 H6 AIVs were isolated from ducks and geese without any obvious clinical signs in LPMs during 2010–2017. All of viruses were propagated in SPF chicken embryonated eggs and their virus titers ranged from 106.3 to 109.5 EID50/100 μl. Full genomes of 7 H6N2 and 15 H6N6 viruses were sequenced and deposited to the NCBI database, the accession number and abbreviations of viruses used in this study have been listed in Table 1.
Molecular characterization
According to sequence analysis, all of the H6 isolates shared the same amino acids (PQIETR/GLF) with single basic amino acid at the cleavage site between HA1 and HA2, which displayed a low pathogenic feature32. The receptor-binding sites in HA protein possessed the amino acid residues Q226 and G228 (H3 HA numbering33, which is used throughout the manuscript), suggesting that all H6 isolates would have a higher affinity to α-2,3-linked sialic acid which is predominant in the upper respiratory tract of avian species34. Several mutations were found in the receptor-binding area in the HA proteins and listed in Table 2, including the amino acids from 169 to 171 which related to a potential glycosylation site, and the amino acid at 190 which was found to determine the binding capability of H9N2 viruses to lung epithelial cells of mouse and human35. The amino acid changes of A138S and P186T/I were appeared in ZJ/B1994/H6N6, FJ/D3480/H6N6and JS/F336/H6N6.
Those 22 viruses contained 119E, 274H and 294 N on the NA proteins (N2 numbering36), which suggested that all viruses are sensitive to oseltamivir37. There were 11 amino acids deletion in the stalk region of three H6N6 isolates (ZJ/B1994/H6N6, FJ/D3480/H6N6 and JS/F336/H6N6), which might be associated with increased virulence in mammals38. It is notable that S31N substitutions in the M2 protein, which are associated with amantadine resistance of influenza virus were observed in GD/E3503/H6N2 and GD/F3891/H6N2 viruses39. Amino acid residues were E and D at positions 627 and 701 of the PB2 protein in all those H6 isolates, which have been associated with the pathogenicity of avian influenza viruses in mammals40. Previous reports showed that the substitution D92E in the NS1 protein associated with reducing its phosphorylation and increasing the virus resistance to interferon41, but this substitution was not detected in any of the isolates. In addition, no amino acid changes associated with increased virulence in mammals were detected in the PA or PB1 proteins.
Phylogenetic analysis of HA and NA genes
The nucleotide and amino acid similarity of the HA from 22 isolates in this study were 82.0% to 99.9% and 84.0% to 99.8%, respectively, as shown in Table 3. The phylogenetic tree of HA gene (Fig. 1a) indicated that all of the H6 isolates were divided into two clades under Eurasian lineage. All the H6N2 isolates were clustered in A/duck/Shantou/339/2000(H6N2)-like (ST/339-like) virus clade except GD/F1473/H6N2. Together with all of the H6N6 viruses, GD/F1473/H6N2 was derived from A/wild duck/Shantou/2853/2003(H6N2)-like (ST/2853-like) virus clade. Additionally, 3 H6N6 strains (ZJ/B1994/H6N6, FJ/D3480/H6N6 and JS/F336/H6N6) belonged to A/swine/Guangdong/K6/2010(H6N6)-like (GD/K6-like) subclade.
Phylogenetic trees of H6 AIVs isolated from 2011 to 2017 in Southern China. The phylogenetic trees of the H6 HA (a), N2 NA (b) and N6 NA (c) genes were generated by using the neighbor-joining method using MEGA 6.0. The genomic sequences of the viruses listed in black were downloaded from available databases; the viruses listed in red were evaluated in this study. The scale bar represents the distance between sequence pairs, and horizontal distances are proportional to genetic distance.
The NA genes of 7 H6N2 isolates separated into two clades (ST/339-like and ST/2853-like clades). Only GD/F1473 clustered in the ST/2853-like clade, in which H3N2, H4N2, and H6N2 viruses were clustered. The other H6N2 viruses fell into ST/339-likeclade, in which the viruses were isolated from ducks, geese and chickens (Fig. 1b). The NA genes of 15 H6N6 isolates were divided into two clades as well. The majority of those viruses were clustered together with the clade of ST/192-like viruses. Only the JS/E1201/H6N6 strain clustered on the clade of A/Muscovy duck/Fujian/FZ01/2008(H5N6)-like (FJ/FZ01-like) virus (Fig. 1c).
Phylogenetic analysis of the internal genes
Phylogenetic analysis of the six internal genes showed that all those 22 H6 AIVs clustered in the Eurasian lineage. The PB1, PB2, PA and NS genes were divided into two clades, and the NP and M genes were fell into 3 clades (Fig. S1-6). Phylogenetic tree of PB1 genes showed that nine isolates were clustered in the ST339-like clade, in which the viruses were isolated from different birds including chicken, duck, goose and wild bird. The other thirteen isolates were closely related to A/duck/Guangdong/S3180/2010(H6N6) (GD/S3180) (Fig. S1). In the phylogenetic trees of the PB2 and NS genes, only GD/F1473/H6N2 located in a separated clade (Fig. S2 and S6). PB2 gene of GD/F1473/H6N2 was related closely to that of the early H5N1 AIV isolate A/goose/Guangdong/1/96 in the BJ/BJ/1/94-likeclade. The NS gene of GD/F1473/H6N2 was closely related to that of H3N2, H3N8, and H6N2 AIVs isolated from different waterfowls. As for the PA genes, all of the H6N6 viruses and one H6N2 virus GD/F1473/H6N2 were clustered in the ST339-like clade (Fig. S3). The other 5H6N2 viruses were located in the Gs/GD196-like clade. The NP gene of the virus JS/E1201/H6N6 was clustered in a separated A/duck/Hunan/573/2002(H6N2)-like (HN/573-like) clade, while other isolates were divided into ST339-likeand A/duck/Mongolia/54/2001(H5N2)-like (Mongolia/54-like) virus groups (Fig. S4). On the NP tree, most of the H6N6 and GD/F1473/H6N2were related to a H5N2 virus A/duck/Mongolia/54/2001. In the ST339-like clade, the NP genes of 6H6N2 isolates clustered into a A/chicken/Guangdong/C273/2011 related sub-group, while 3 H6N6 viruses clustered into the A/swine/Guangdong/K6/2010 related sub-group. In the phylogenetic tree of M gene, all the viruses were distributed in ST/339-like, China/G1291-like and ST/2853-like clades (Fig. S5). The M genes of 6 H6N2 viruses located in China/G1291-like, 3 H6N6 viruses located in ST/2853-like clade, and the most of H6N6 viruses clustered together with a H6N2 isolate GD/F1473/H6N2 on ST/339-like clade.
According to the phylogenetic analysis of the whole genomes of the AIVs isolated from 2011 to 2017, the genotypes of H6 viruses were classified. Based on the HA genes, the viruses were divided into A and B genotypes corresponding to ST/339-like and ST/2853-like clades. H6N2 and H6N6 viruses were divided into A2, B2, A6 and B6 accordingly. Based on the different clades of NA and other 6 internal genes, the genotypes were named according to the chronological order of virus isolation in this study (Fig. 2). The genetic reassortment of H6 AIVs occurred frequently from 2011 to 2017, and resulted in multiple genotypes. The genotype of B602 was detected in 2012, 2015 and 2017, suggesting that the viruses of this genotype have elevated adaptation and replication efficiency in their natural reservoirs.
Pathogenicity in mice
To evaluate the pathogenicity in mice, groups of white mice were inoculated i.n. with each H6 isolates (106.0 EID50/mice). All of the mice used for observation survived till 14 dpi. None of H6N2 viruses, except GD/1268/H6N2, caused significant body weight loss (P < 0.05) in mice compared with the control group (Fig. 3). As for H6N6 viruses, only HN/A729/H6N6 and ZJ/B1994/H6N6 caused significant growth retard in mice, while the clinical signs caused by other viruses were not obvious.
Bodyweight changes of BALB/c mice infected with the H6 AIVs. 4-week-old female BALB/c mice were inoculated i.n. with 106.0EID50 of virus in a volume of 30.0 μl. The body weight of 5 mice were measured daily until 14 dpi. Weight changes of the BALB/c mice infected with H6N2 (Fig. 3a) and H6N6 (Fig. 3b) were shown respectively. The data were graphed using the Prism 6.0 software (Vision 6.0, GraphPad Prism). Comparisons of the weight changes between two groups were determined by nonparametric t-tests using GraphPad. (*P < 0.05, **P < 0.01, ***P < 0.001).
All of the H6H2 isolates were able to replicate efficiently in mouse lung and nasal turbinate without prior adaptation. The mean virus titers were ranged from 103.4 to 106.0 EID50/ml in the lungs and from 101.8 to104.8 EID50/ml in the nasal turbinates (Table 4). Six out of seven H6N2 isolates were detectable in the heart of partial or all the three inoculated mice. Specially, two H6N2 viruses were found in the spleen of one out of three inoculated mice, and one of those viruses GD/E3503/H6N2 caused systemic infection in one inoculated mouse. In addition, the virus was recovered from liver and brain. The GD/1268/H6N2 and GD/1127/H6N2 viruses of A201 genotype were detectable in the lungs, nasal turbinates and hearts consistently, while the 4 isolates of A202 genotype showed diversity in the tissue tropisms (Table 4).
The H6N6 viruses, except ZJ/B2039/H6N6 and JS/G91/H6N6, replicated in the lungs of inoculated mice. ZJ/B2039/H6N6 has not been detected in any of the other tested tissues, and lower titers of JS/G91/H6N6 were detected only in the nasal turbinate of one out of three mice (Table 4), suggesting the limited replication of those 2 viruses in mice. In addition, the replication of GD/E3798/H6N6 was also limited in mice and lower virus titers were detected in the lungs and nasal turbinates from two out of three mice. Besides those 3 viruses, the other H6N6 isolates replicated well in the lungs and the virus titers ranged from 102.1 to 106.3 EID50/ml. Specially, the average virus titers of 5 isolates reached a high level of over 105.0 EID50/ml, which suggested those viruses replicated efficiently in the lungs of inoculated mice. In the nasal turbinates, only GD/E3415/H6N6 and FJ/D3480/H6N6 could be detected in all of the three mice, and the average virus titers were 101.1 and 102.5 EID50/ml, respectively. Except ZJ/B2039/H6N6 and ZJ/B2044/H6N6 which were not recovered from the nasal turbinates of all three mice, the remaining H6N6 viruses were detected in some inoculated mice. All of the H6N6 isolates were detectable in the hearts of some to all of the three inoculated mice, except ZJ/B2039/H6N6, GD/E3798/H6N6 and JS/G91/H6N6. Lower viral titers of some H6N6 viruses were detected in livers, spleens and brains in some inoculated mice, suggesting that those viruses replicate in mice (Table 4).
Pathogenicity and transmission in chickens
To evaluate the virulence of the H6 isolates in chickens, groups of six 4-week-old SPF chickens were inoculated intranasally with 106.0 EID50 of each H6 isolate. No clinical sign was observed in the inoculated chickens. We further examined the replication of the H6 viruses by detecting the viruses in different tissue and swabs of inoculated chickens. The GD/1127/H6N2 and GD/E3503/H6N2 viruses were detected in the trachea of one inoculated chicken, respectively. FJ/D3480/H6N6 was detected in the duodenum of one inoculated chicken, while HN/A729/H6N6 and ZJ/B2028/H6N6 were not detected in any tested tissues of 3 inoculated chickens at 3dpi. Only GD/E3503/H6N2 was detected in the oropharyngeal swab from one chicken at 4 dpi. GD/E3503/H6N2 and HN/A729/H6N6 were detected in the cloacal swab from one chicken at 2 dpi and 6 dpi, respectively. None of 3 chickens inoculated with HN/A729/H6N6 showed seropositive to specific H6 antibody, and only some chickens inoculated with the other four viruses were seroconverted. One contact chicken in the GD/E3503/H6N2 group was seroconverted at 14 dpi, while no specific H6 antibody was detected in other contact chickens (Table 5). The results suggested the replication and transmission of H6 viruses with different genotypes were limited in chickens.
Discussion
Since the late 1990s, H6 AIVs have been circulating in Southern China. The H6 viruses have been become more prevalent over time with an increasing isolation rate of H6N1, H6N2 and H6N6 subtypes6,15,42. In this study, we analyzed the molecular evolution and pathogenicity in mice and chickens of H6 AIVs isolated from the LPMs in Southern China from 2011 to 2017, and provided a glimpse of the genetic diversity and pathogenicity of H6 AIVs in mammals and chickens.
Multiple H6 virus genotypes emerged from 2011 to 2017 resulted from the internal gene reassortment between H6 and other subtype viruses. It has been noted that most of the H6N1/N2 viruses isolated from Southern China from 2000 to 2005 were clustered with the G1-like, W312-like or H9N2 Ck/Bei-like lineage based on the PB2 and PB1 genes, and the PA and NP genes of most H6 viruses clustered with H5 and H9 viruses, M and NS genes with G1-like or H9N2 Ck/Bei-like lineage viruses16,22. Previous studies showed that the H6 isolate from ducks replicated poorly in chicken trachea and that the viruses recovered were W312-like viruses43,44. However, in our study, none of the internal genes were related closely to G1-like or W312-like viruses. These results suggested that the H6 subtype viruses in Southern China were genetically diverse, which might increase the potential for H6 viruses to transmit from ducks to other animals.
Molecular analyses suggested that all of the 22 H6 isolates were low pathogenic AIVs. The receptor-binding sites in the viral HA proteins possess the residues Q226 and G228, similar to those H6 isolates reported previously, which preferentially bind to the α-2,3-linked sialic acid receptors predominant in avian species45,46. However, the E190V and N192D substitutions of H6 HA have been associated with interspecies transmission of AIVs from ducks to chickens47, but those amino acids were not found in the five H6 AIVs (GD/1127/H6N2, GD/E3503/H6N2, HN/A729/H6N6, ZJ/B2028/H6N6 and FJ/D3480/H6N6) in this study, which would be the most likely reason that the replication of the H6 isolates in chickens was surprisingly limited. The residues 228S, 137N, 186L, A13S, and A193N at HA associated with human receptor-binding preference45,47 were not found in H6 isolates in this study. Additionally, almost all H6N6 isolates have the substitution of HA at V187D, the binding affinity might be altered to adapt to mammalian receptors19.
None of E119V, H275Y, R293K and N295S substitution in the NA were found, which suggested those H6 isolates are sensitive to neuraminidase inhibitors such as oseltamivir. The deletion of 11 amino acids in the stalk region of the NA of FJ/D3480/H6N6, ZJ/B1994/H6N6and JS/F336/H6N6, which was also found in a swine H6N6 virus, might be associated with the infectivity of H6N6 viruses in mammals through affecting NA activity48 and the balance between HA and NA49. It is notable that the S31N substitution in the M2 protein, which is associated with amantadine resistance of influenza virus50, was found in GD/E3503/H6N2 and GD/F3891/H6N2.
Some of the H6 viruses replicated efficiently on MDCK and A549 cells and in the lungs of mice17,51. The direct contact transmission of H6 viruses in guinea pigs was confirmed previously, which suggested the H6 viruses pose a clear threat to mammals17. We found that all of the H6H2 isolates were able to replicate efficiently in lung and nasal turbinate without prior adaptation in mice, but the replication ability of H6N6 varies. Even with the same genotype, different H6N6 viruses showed distinct replication abilities in mice, suggesting some amino acid mutations affected the replication of the viruses. Lower titers of some H6 viruses were detected in livers, spleens and brains in a part of inoculated mice, which suggested those viruses posed a potential to adapt and caused a systemic infection in mammals.
Five H6 AIVs selected from different genotypes caused no clinical signs in any of the inoculated chickens. The replication and transmission of H6N2 and H6N6 viruses were limited in SFP chicken in this study, although the pathological changes caused by H6N2 in chickens were reported in California13. Previous study showed that H6N2 isolates caused seroconversion in infected chickens, but no virus was recovered from the tissue samples, which suggested that the H6N2 strains replicated poorly and were nonpathogenic to chickens52. Our findings showed that the five H6 strains selected from different genotypes caused no clinical signs in any of the inoculated chickens, but the serological test results suggested that chickens in four groups have been infected despite limited recovery of inoculated viruses from the tissue samples. The replication and transmission of H6N2 and H6N6 viruses were limited in SFP chicken in this study.
Overall, our study suggested that the H6 AIVs circulating in South China are genetically diverse and pose potential threat to mammals, and continual surveillance of H6 viruses is necessary in China.
References
Wu, H. et al. Isolation and characterization of novel reassortant H6N1 avian influenza viruses from chickens in Eastern China. Virol. J. 15, 164. https://doi.org/10.1186/s12985-018-1063-y (2018).
Cox, N. J. & Subbarao, K. Influenza. The Lancet 354, 1277–1282. https://doi.org/10.1016/s0140-6736(99)01241-6 (1999).
Tong, S. et al. New world bats harbor diverse influenza A viruses. PLoS Pathog 9, e1003657. https://doi.org/10.1371/journal.ppat.1003657 (2013).
Wu, Y., Wu, Y., Tefsen, B., Shi, Y. & Gao, G. F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 22, 183–191. https://doi.org/10.1016/j.tim.2014.01.010 (2014).
Jiao, P. et al. Complete genomic sequence of a novel natural recombinant H6N2 influenza virus from chickens in Guangdong, Southern China. J. Virol. 86, 7717–7718. https://doi.org/10.1128/jvi.00963-12 (2012).
Peng, Y. et al. Epidemiological surveillance of low pathogenic avian influenza virus (LPAIV) from poultry in Guangxi Province, Southern China. PLoS ONE 8, e77132. https://doi.org/10.1371/journal.pone.0077132 (2013).
Luo, S. et al. Surveillance of live poultry markets for low pathogenic avian influenza viruses in Guangxi Province, Southern China, from 2012–2015. Sci. Rep. 7, 17577. https://doi.org/10.1038/s41598-017-17740-0 (2017).
Olesiuk, O., Snoeyenbos, G. & Roberts, D. J. A. D. An influenza A virus isolated from turkeys. Avian Dis. 11, 203–208 (1967).
Pereira, H. G., Rinaldi, A. & Nardelli, L. Antigenic variation among avian influenza A viruses. Bull. World Health Organ. 37, 553–558 (1967).
Zhao, G. et al. Molecular evolution of the H6 subtype influenza A viruses from poultry in eastern China from 2002 to 2010. Virol. J. 8, 470. https://doi.org/10.1186/1743-422x-8-470 (2011).
Wu, H., Peng, X., Peng, X., Cheng, L. & Wu, N. Molecular characterization of novel reassortant H6N2 subtype avian influenza viruses isolated from poultry in Eastern China, in 2014. Infect. Genet. Evol. 36, 41–45. https://doi.org/10.1016/j.meegid.2015.08.043 (2015).
Everest, H. et al. The evolution, spread and global threat of H6Nx avian influenza viruses. Viruses https://doi.org/10.3390/v12060673 (2020).
Woolcock, P. R., Suarez, D. L. & Kuney, D. Low-pathogenicity avian influenza virus (H6N2) in chickens in California, 2000–02. Avian Dis. 47, 872–881. https://doi.org/10.1637/0005-2086-47.s3.872 (2003).
Shkoda, I. et al. Characterization of a low pathogenic avian influenza virus (H6N1) isolated from Turkeys. Influenza Res. Treat. 2011, 285218. https://doi.org/10.1155/2011/285218 (2011).
Huang, K. et al. Establishment and lineage replacement of H6 influenza viruses in domestic ducks in southern China. J. Virol. 86, 6075–6083. https://doi.org/10.1128/jvi.06389-11 (2012).
Huang, K. et al. Establishment of an H6N2 influenza virus lineage in domestic ducks in southern China. J. Virol. 84, 6978–6986. https://doi.org/10.1128/jvi.00256-10 (2010).
Wang, G. et al. H6 influenza viruses pose a potential threat to human health. J. Virol. 88, 3953–3964. https://doi.org/10.1128/jvi.03292-13 (2014).
Yuan, J. et al. Origin and molecular characteristics of a novel 2013 avian influenza A(H6N1) virus causing human infection in Taiwan. Clin. Infect. Dis. 57, 1367–1368. https://doi.org/10.1093/cid/cit479 (2013).
Zhang, G. et al. Identification of an H6N6 swine influenza virus in southern China. Infect. Genet. Evol. 11, 1174–1177. https://doi.org/10.1016/j.meegid.2011.02.023 (2011).
Lin, H. T., Wang, C. H., Chueh, L. L., Su, B. L. & Wang, L. C. Influenza A(H6N1) Virus in Dogs, Taiwan. Emerg. Infect. Dis. 21, 2154–2157. https://doi.org/10.3201/eid2112.141229 (2015).
Qu, Z. et al. Identification of a key amino acid in hemagglutinin that increases human-type receptor binding and transmission of an H6N2 avian influenza virus. Microbes Infect. 19, 655–660. https://doi.org/10.1016/j.micinf.2017.09.008 (2017).
Chin, P. S. et al. Molecular evolution of H6 influenza viruses from poultry in Southeastern China: prevalence of H6N1 influenza viruses possessing seven A/Hong Kong/156/97 (H5N1)-like genes in poultry. J. Virol. 76, 507–516 (2002).
Hotta, K. et al. Isolation and characterization of H6N1 and H9N2 avian influenza viruses from Ducks in Hanoi, Vietnam. Virus Res. 163, 448–453. https://doi.org/10.1016/j.virusres.2011.11.004 (2012).
Jiao, P. et al. New Reassortant H5N6 highly pathogenic avian influenza viruses in Southern China, 2014. Front. Microbiol. 7, 754. https://doi.org/10.3389/fmicb.2016.00754 (2016).
Li, J. et al. Continued reassortment of avian H6 influenza viruses from Southern China, 2014–2016. Transbound Emerg. Dis. 66, 592–598. https://doi.org/10.1111/tbed.13037 (2019).
Lee, M. S., Chang, P. C., Shien, J. H., Cheng, M. C. & Shieh, H. K. Identification and subtyping of avian influenza viruses by reverse transcription-PCR. J. Virol. Methods 97, 13–22 (2001).
Pedersen, J. C. Hemagglutination-inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. Methods Mol. Biol. 436, 53–66. https://doi.org/10.1007/978-1-59745-279-3_8 (2008).
Yuan, R. et al. Pathogenicity and transmission of H5N1 avian influenza viruses in different birds. Vet. Microbiol. 168, 50–59. https://doi.org/10.1016/j.vetmic.2013.10.013 (2014).
Stanic, M. A simplification of the estimation of the 50 percent endpoints according to the Reed and Muench method. Pathol. Microbiol. (Basel) 26, 298–302 (1963).
Hoffmann, E., Stech, J., Guan, Y., Webster, R. G. & Perez, D. R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146, 2275–2289 (2001).
Ge, F. F. et al. Genetic evolution of H9 subtype influenza viruses from live poultry markets in Shanghai, China. J. Clin. Microbiol. 47, 3294–3300. https://doi.org/10.1128/jcm.00355-09 (2009).
Horimoto, T., Ito, T., Alexander, D. J. & Kawaoka, Y. Cleavability of hemagglutinin from an extremely virulent strain of avian influenza virus containing a unique cleavage site sequence. J. Vet. Med. Sci. 57, 927–930. https://doi.org/10.1292/jvms.57.927 (1995).
Nobusawa, E. et al. Comparison of complete amino acid sequences and receptor-binding properties among 13 serotypes of hemagglutinins of influenza A viruses. Virology 182, 475–485. https://doi.org/10.1016/0042-6822(91)90588-3 (1991).
Nicholls, J. M., Bourne, A. J., Chen, H., Guan, Y. & Peiris, J. S. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir. Res. 8, 73. https://doi.org/10.1186/1465-9921-8-73 (2007).
Teng, Q. et al. A single mutation at position 190 in hemagglutinin enhances binding affinity for human type sialic acid receptor and replication of H9N2 avian influenza virus in mice. J. Virol. 90, 9806–9825. https://doi.org/10.1128/jvi.01141-16 (2016).
Colman, P. M. Influenza virus neuraminidase: structure, antibodies, and inhibitors. Protein Sci. 3, 1687–1696. https://doi.org/10.1002/pro.5560031007 (1994).
Wang, M. Z., Tai, C. Y. & Mendel, D. B. Mechanism by which mutations at his274 alter sensitivity of influenza a virus n1 neuraminidase to oseltamivir carboxylate and zanamivir. Antimicrob. Agents Chemother. 46, 3809–3816. https://doi.org/10.1128/aac.46.12.3809-3816.2002 (2002).
Matsuoka, Y. et al. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J. Virol. 83, 4704–4708. https://doi.org/10.1128/jvi.01987-08 (2009).
Dong, G. et al. Adamantane-resistant influenza a viruses in the world (1902–2013): frequency and distribution of M2 gene mutations. PLoS ONE 10, e0119115. https://doi.org/10.1371/journal.pone.0119115 (2015).
Manz, B., Schwemmle, M. & Brunotte, L. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J. Virol. 87, 7200–7209. https://doi.org/10.1128/jvi.00980-13 (2013).
Jiao, P. et al. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 82, 1146–1154. https://doi.org/10.1128/jvi.01698-07 (2008).
Peng, C. et al. Molecular epidemiological survey and complete genomic phylogenetic analysis of H6 subtype avian influenza viruses in poultry in China from 2011 to 2016. Infect. Genet. Evol. 65, 91–95. https://doi.org/10.1016/j.meegid.2018.07.023 (2018).
Ozaki, H., Guan, Y., Peiris, M., Webster, R. & Webby, R. Changing patterns of h6 influenza viruses in Hong Kong poultry markets. Influenza Res. Treat. 2011, 702092. https://doi.org/10.1155/2011/702092 (2011).
Ozaki, H. et al. Effect of the PB2 and M genes on the replication of H6 influenza virus in chickens. Influenza Res. Treat. 2014, 547839. https://doi.org/10.1155/2014/547839 (2014).
Wang, F. et al. Adaptation of avian influenza A (H6N1) virus from avian to human receptor-binding preference. EMBO J 34, 1661–1673. https://doi.org/10.15252/embj.201590960 (2015).
Ni, F., Kondrashkina, E. & Wang, Q. Structural and functional studies of influenza virus A/H6 hemagglutinin. PLoS ONE 10, e0134576. https://doi.org/10.1371/journal.pone.0134576 (2015).
Abolnik, C., Strydom, C., Rauff, D. L., Wandrag, D. B. R. & Petty, D. Continuing evolution of H6N2 influenza a virus in South African chickens and the implications for diagnosis and control. BMC Vet. Res. 15, 455. https://doi.org/10.1186/s12917-019-2210-4 (2019).
Castrucci, M. R. & Kawaoka, Y. Biologic importance of neuraminidase stalk length in influenza A virus. J. Virol. 67, 759–764 (1993).
Mitnaul, L. J. et al. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J. Virol. 74, 6015–6020. https://doi.org/10.1128/jvi.74.13.6015-6020.2000 (2000).
Radosevic, D. et al. Virtual screen for repurposing of drugs for candidate influenza a M2 ion-channel inhibitors. Front. Cell. Infect. Microbiol. 9, 67. https://doi.org/10.3389/fcimb.2019.00067 (2019).
Gillim-Ross, L. et al. Avian influenza h6 viruses productively infect and cause illness in mice and ferrets. J. Virol.. 82, 10854–10863. https://doi.org/10.1128/jvi.01206-08 (2008).
Simulundu, E. et al. The zoonotic potential of avian influenza viruses isolated from wild waterfowl in Zambia. Arch. Virol. 159, 2633–2640. https://doi.org/10.1007/s00705-014-2124-1 (2014).
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2016YFD0500204), and the Chinese Academy of Agricultural Sciences of Technology Innovation Project.
Author information
Authors and Affiliations
Contributions
Z.L. designed research; H.C. and W.L. performed research with the help of L.L., Y.S., X.L.; Q.T., J.Y., Q.L. and J.D. analyzed data; and Z.L., Q.L., W.L., H.C. wrote the main manuscript text. All authors reviewed and revised the drafts of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, W., Cui, H., Teng, Q. et al. Evolution and pathogenicity of H6 avian influenza viruses isolated from Southern China during 2011 to 2017 in mice and chickens. Sci Rep 10, 20583 (2020). https://doi.org/10.1038/s41598-020-76541-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76541-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.