Abstract
Pyrochar (PC) is always with high pH value, and improper application might increase rice paddy ammonia volatilization (PAV), which is the main nitrogen loss through air during rice production. Differently, hydrochar (HC) takes the advantages of high productive rate and always with lower pH value compared with PC. However, effect pattern and mechanism of HC on PAV are still unclear. In the present study, soil column experiments were conducted to investigate the effect of PC and HC application on PAV. In total, treatments with four types of biochar (WPC, SPC, WHC and SHC, i.e., PC and HC prepared with wheat straw and sawdust, respectively) and two application rates (0.5% and 1.5%, w/w) were set up and non-biochar application was used as control. Results showed that, application of HC with low pH value could not reduce PAV compared with PC. Total PAV increased significantly as the increase of HC application rate (especially for WHC). The increment of PAV under high rate HC application might be due to the strong buffer capacity of soil, the aging of biochar, the high nitrogen from HC. The results indicated that HC should be pretreatment before utilization in agricultural environment considering PAV reduction.
Similar content being viewed by others
Introduction
Ammonia (NH3) volatilization emission is a key incentive of air (fog and haze) and water pollution (eutrophication)1,2. Paddy ammonia volatilization (PAV) is the main nitrogen (N) losses through air, and occupies 10–40% of the total nitrogen (N) fertilizer3. Total NH3 emissions from global fertilizer use were estimated at approximately 11 Tg N year−14, and almost 1.7 Tg N year−1 from paddy fields in China5. Therefore, management to reduce PAV is of great importance for both environment and agriculture.
Pyrolysis biochar (PC, pyrochar) has been intensive studied in carbon sequestration, soil improvement and greenhouse gas mitigation6,7. However, PC always has a high pH value, usually ranged from 8–10, depends on different conditions8. Application of PC with high pH value could increase PAV by 19% on average9,10, especially under high rate PC application11,12. Hence, to reduce PAV, PC should be applied with appropriate rates13 or combined with other material such as wood vinegar14, and new biochar preparation methods should also be proposed.
Compared with PC by pyrolysis, hydrochar (HC) is produced by hydrothermal carbonization (HTC)15. During HTC, biomass is heated in the presence of water and autogenous pressure in an oxygen free environment16. HTC takes the advantages of higher char yield, lower amounts of energy during production17,18. Furthermore, HC is always with low pH value (3.5–6.5) and good absorption ability19,20, which showed a great potential to manage PAV21.
Most previous researches on HC are focus on the analysis in laboratory, such as comparison of physical and chemical properties of different types of HC22,23. Researches on HC application in agriculture environment are limited24,25. Considering the promising prospect for HTC technology in biomass conversion and utilization, the application of HC in agro-environment should be investigated before a comprehensive evaluation. A set of pot trials with barley, phaseolus bean, leek was conducted to determine the effects of HC on plant N availability and biomass production26, but without the observation of NH3 volatilization emission. Studies have been conducted to investigate the effects of HC on PAV, but the results were inconsistent for stimulating or inhibiting PAV, depending on the feedstock and preparation of HC27,28. By now, the pattern and mechanism of HC on PAV has seldom been reported, and whether HC could be an alternative of PC in consideration of PAV reduction are still unclear. Thus, soil column experiments were conducted to investigate the effect of PC and HC application on PAV in the present study. The purpose of this study is to (1) investigate and compare the effect of PC and HC on PAV; (2) investigate the correlation between properties of flood water, surface soil and PAV to understand the intrinsic mechanisms; and (3) evaluate the effects of biochar production method, feedstock and application rate to provide a reasonable biochar application scheme to manage PAV. The present study would offer a comprehensive evaluation on HC application in rice production for management of PAV.
Methods
Preparation and the basic properties of pyrochar and hydrochar
In the present study, two types of PC were generated from wheat straw and sawdust in an oxygen-limited pyrolysis system at 700 °C (named WPC and SPC, respectively). In detail, the temperature of the reactor was set at 700 ℃ for 8 h first. After the pyrolysis process completed, the temperature cooled to room temperature13. Two types of HC were also derived from wheat straw and sawdust (named WHC and SHC, respectively). Feedstock and water were mixed at a ratio of 100 g/L. The reactor was heated to 260 °C for 1 h, then cooled until reach the room temperature. The remained products were dried and passed through a 2 mm sieve. The basic properties of these 4 biochar were showed in Table 1.
To investigate the dissolved matter from biochar in the present study, 4 types of biochar (WPC, SPC, WHC and SHC) were dipped in cold (20 °C) and hot (95 °C) deionized water for 1 h at 1:20 char/water ratio (w/v), respectively. The mixture was stirred for 1 h at a speed of 600 r/min. Then, the extracted water was obtained after filtration by a 0.45 μm Millipore filter. TN and COD (Chemical oxygen demand) of extracted water were measured by a San++ Continuous Flow Analyzer (Skalar, Netherlands) and a COD analyser (DR1010 COD, HACH, USA), respectively. To simulate the aging process of hydrochar, WHC and SHC were dipped in 30% v/v H2O2 for 3 h at 1:10 char/water ratio (w/v). The remained material was obtained through filtration, drying and sieving as WHC-aged and SHC-aged, respectively. The pH value of these two aged hydrochar were measured in deionized water at a ratio of 1:10 w/v using combined reference electrodes and a Ф255 pH/temp/mV meter (Coulter Bechman Co., USA).
Soil column experiment design
Soils used for the column experiment was obtained from the surface layer (approximate to 20 cm) of a rice paddy (stagnic anthrosols) in Zhoutie Town, Jiangsu Province (31.4765° N, 119.9861° E). Sand, silt and clay contents were 8, 24 and 68%, respectively. The soil was with a pH value at 6.38, total nitrogen at 1.56 g kg−1, organic carbon at 2.28%11. Soil columns in the experiment were designed by polyvinyl chloride with a diameter of 30 cm and height of 50 cm. Drainage hole was designed at the bottom of each column for midseason drainage.
Four types of biochar were set at rates of 0.5% and 1.5% (w/w), respectively. In total, 8 biochar application treatments were set up (named as WPC-0.5%, WPC-1.5%, WHC-0.5%, WHC-1.5%, SPC-0.5%, SPC-1.5%, SHC-0.5% and SHC-1.5%, respectively). Non-biochar application treatments with N fertilizer (CKU) were set up as control. 35 kg soil were mixed up with relative biochar before filled into each soil column. Each treatment was conducted with three replicates.
Rice seedlings were transplanted on July 2, 2017, with a density of six seedlings per column, and a basal fertilizer (BF) of 96 kg N ha−1, 90 kg P2O5 ha−1, and 120 kg K2O ha−1 was applied. The tiller (TF) and panicle (PF) fertilizers were applied at a rate of 96 and 48 kg N ha−1 on Jul. 16 and Aug. 14, respectively. During the rice growing season, all the columns shared the same irrigation treatment, which were keeping with a 3 cm water layer, except a midseason drainage from Jul. 31 to Aug. 7, 2017. The harvested was conducted on Nov. 4, 2017.
Measurement of paddy NH3 volatilization
Continuous air-flow enclosure method was introduced for measurement of PAV fluxes by using a transparent Plexiglas chamber (with 15 cm inner diameter and 20 cm height) (Feng et al. 2016). The NH3 absorbent contained 80 mL boric acid (2%), and mixed with methyl red, bromocresol green, and ethanol as indicator. The absorbent solution was titrated against with 0.01 M H2SO4 after taken to laboratory. PAV measurement was conducted daily from 8:00 to 10:00 a.m. for 7 days after every fertilizer applied (BF, TF and PF, respectively). The cumulative PAV was calculated by daily emission summation over the relative measuring period.
Measurement of pH value, total nitrogen and NH4-N concentration for flood water and surface soil
Along with the PAV observation, the pH value and NH4-N concentration of flood water (accompanied with PAV observation) and surface soil (after PAV observation) was also measured. Flood water was sampled on 10:00 a.m. daily until the PAV measurement ended. Soils of each column were sampled after 7 days of each fertilizer application.
The pH value of flood water and soil were measured directly or in deionized water at a ratio of 1: 2.5 w/v using combined reference electrodes and a Ф255 pH/temp/mV meter (Coulter Bechman Co., USA), respectively. The TN and NH4-N concentration of surface flood water samples were measured by a San++ Continuous Flow Analyzer (Skalar, Netherlands), where those of surface soil samples were measured by sulfuric acid catalyzed digestion and boric acid titration and the similar method as water samples after extracted by 2.0 M KCl first.
Statistical analysis
Analysis of variance was conducted to determine significant differences between different treatments. Duncan's multiple range test was conducted using P < 0.05 as the standard for significance. Correlation analysis between properties of flood water, surface soil, biochar application rate and PAV was conducted. Statistical analysis and figures were performed by using the SPSS 19.0, R 3.4.1 and Origin 9.0.
Results
Paddy NH3 volatilization fluxes pattern after fertilizer application
Results of PAV fluxes showed that, after each fertilizer application, PAV rates of the 9 treatments all increased at the first 3 days and decreased later until a new balance was achieved. After a 7-day observation, PAV rate of all the 9 treatments were lower than 1 kg N ha−1 day−1 (Fig. 1).
Paddy NH3 Volatilization fluxes of the first week after basal fertilizer (BF), tiller fertilizer (TF) and panicle fertilizer (PF) application, respectively. WPC, SPC, WHC and SHC represent pyrochar and hydrochar prepared with wheat straw and sawdust, respectively; 0.5% and 1.5% represent biochar application rates; CKU represents treatment without biochar application but with N fertilizer; Error bars represent standard deviations.
Comparison showed that, PAV of SPC-1.5%, WHC-0.5%, WHC-1.5% and SHC-0.5% after BF were significantly higher than CKU, whereas other biochar application treatments showed no significant differences. After TF, only PAV of WPC-1.5% was significantly lower than CKU. While after PF, only PAV of SHC-1.5% was significantly higher than CKU (Table 2). Total PAV after 3 fertilizer application showed that, most biochar application treatment increased PAV. Only WPC-1.5% and SPC-0.5% showed a lower total PAV than CKU, though not significantly. HC application increased total PAV, and WHC-1.5% showed significantly higher total PAV than CKU.
Results of correlation analysis indicated that, chemical properties of flood water, e.g. pH value, TN, and NH4-N did not affect PAV significantly during the first week after three fertilizer application (Fig. 2). Most correlation coefficients between chemical properties of flood water and PAV in the same day (e.g. pH.BF1 and PAV.BF1) and different days (e.g. pH.BF1 and PAV.BF2) were not significant (Fig. 2). These results indicated that single daily properties of flood water could not predict daily PAV. However, daily PAV after fertilizer application were autocorrelated (e.g. PAV.BF5 and PAV.BF6). And the correlation was enhanced as the time of fertilizer application (BF ≈ TF < PF). After PF application, PAV of each single day was correlated with that next day.
Correlation between chemical properties of flood water and PAV during the first week after basal fertilizer, tiller fertilizer and panicle fertilizer application. pH, TN, NH4, and PAV represent pH value, total nitrogen, NH4-N, and paddy NH3 volatilization of flood water, respectively; BF, TF and PF represent basal fertilizer, tiller fertilizer and panicle fertilizer application; number 1–7 represent the day after each fertilizer application.
The effect of flood water on paddy NH3 volatilization
Properties of surface soil also showed limited impact on PAV, which was similar to that of flood water. PAV after BF and TF did not significantly correlate with its corresponding soil pH value, TN, and NH4-N (Table 2). In contrast, after PF, PAV were significantly correlated with soil pH value, TN and NH4-N. The result indicated that PAV after PF was more sensitive to surface soil properties than that after BF and TF.
The effect of biochar application rate on paddy NH3 volatilization
In general, biochar application could increase total PAV though not significantly (r = 0.29) (Table 3). When divided by biochar prepared method (PC and HC), results showed that HC was more likely to increase PAV as the increase of application rate compared with PC. This result was in accordance with total PAV observation (Table 2). HC application rate significantly increased PAV after PF and the total PAV (r = 0.61 and 0.52, respectively). However, PC application rate showed a weak correlation with PAV (r = 0.42 at most).
Furthermore, when feedstock of biochar was considered, WPC application rate showed no significant relationship with PAV after fertilizer application (r = − 0.26 to 0.06), whereas application rate of WHC, SPC and SHC all increased total PAV after fertilizer application (r = 0.51–0.80). By comparison, WHC application rate showed a significantly impact on PAV compared with other biochar.
Discussion
Relationship between pH value and paddy ammonia volatilization
Soil pH value is always considered as a key factor impacts NH3 volatilization, and a high soil pH value could increase the risk of N loss through NH3 volatilization4. Previous researches showed that PC (with high pH value) application caused higher PAV, but if at an appropriate application rate, the PAV increment could be limited13. HC always had a lower pH value compared with PC29, and HC application was assumed to have lower PAV. However, the results of the present study showed that PAV under HC application were higher than that of PC (Table 2).
Soil and water interface are very complex. When HC and PC applied to paddy soil, the strong buffer capacity of soil could exceed their impact on pH value of both flood water and surface soil. Moreover, soil microbe might decompose the acidic organic molecule30, which induced the pH value of HC increase to soil pH value. The result indicated that application of biochar with low pH value, might not show a reduction of PAV. Variation of PAV emission flux induced by high rate HC application should be more related on other reasons rather than its impact on pH value.
Relationship between nitrogen and paddy ammonia volatilization
Nitrogen of flood water and soil are the sources of NH3 and further affect PAV emission. Former researches showed that PAV was positively correlated with NH4-N of flood water31. However, this correlation was not observed in the present study (Fig. 2). N (TN and NH4-N) of flood water and soil after BF and TF showed no significant relationship with its corresponding PAV (Fig. 2 and Table 4). Only after PF, TN and NH4-N of soil were significantly correlated with PAV (Table 4).
The weak relationship between N (both flood water and surface soil) and PAV was mainly due to the complex interaction between soil and water. The intensive observation (each day during the first week) of the present study could not elucidate the intrinsic mechanism of biochar application on PAV. Researches showed that the inhibited activities of the NH3-oxidizers reduced the exhaustion of AOA on NH3 as a substrate, which possibly resulted in nitrification inhibition and an increase in soil NH4+-N retention32. For further study, soil microbial community should also be considered33,34.
The significant increase of PAV under WHC-1.5% treatment was mainly due to the extra nitrogen introduced from HC. The extracted water from HC had much more nitrogen and COD compared with that from PC (Fig. 3). Feedstocks showed limited effect on COD, but significant effect on TN. Wheat straw-based biochar (both WHC and WPC) showed a significantly higher TN than saw dust-based biochar. This might be due to the difference on the ratio of lignin and cellulose for different feedstocks35.
Why low pH hydrochar didn’t reduce PAV?
Biochar application had multiple effects to soil, and further to PAV. Most former researches showed that PC could increase PAV due to the high pH value and extra N input9. However, results from some researches also indicated PC could decrease PAV due to the high specific surface area and C/N ratio36,37. In the present study, PC application did not show a significant impact on PAV, mainly due to its contrary effects (adsorption effect and liming effect)9. HC was a new type of biochar and had considerable differences in properties compared with PC. Due to the low pH value, we assumed that PAV under HC application treatment would be lower than that under PC. However, the column experiment did not prove the hypothesis, mainly as a result of strong soil buffer capacity and biochar aging.
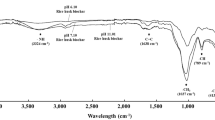
Though biochar was stable and could exist for centuries in soil38, aging of biochar should be considered for practical biochar application39. By simulating the aging process of hydrochar through H2O2 oxidation, the pH value of WHC and SHC turned to neutral from acidic, which was close to soil pH value (Fig. 4). In the present study, relationship between PAV and soil properties were different among three fertilizer application. After PF, pH value, TN and NH4-N showed significant relationship with PAV, but the same result was not observed after BF and TF. When applied to soil, biochar kept its function, e.g. high specific surface area and C/N ratio, at first. However, as the process of aging, the functionality of biochar was modified40,41. Therefore, significant relationship between PAV and soil properties were shown after PF. The effect of biochar aging on PAV also needed further study.
The higher PAV under high rate HC application might be due to several reasons. First, HC introduced more easily decomposed N42. Second, HC could release organic acid and phenol, which showed a negative effect on rice growth and its N uptake30. Moreover, HC application might have an impact on soil microbial community32, 43. Soil microbial community should be considered for further biochar application study focus on PAV. Thus, when considering PAV, biochar application should be more careful, and properties and rate of biochar should also be evaluated before application.
Conclusion
Application of hydrochar with low pH value to rice paddy did not show a reduction on PAV compared with high pH value pyrochar. As the increase of hydrochar application rate (especially for wheat straw based hydrochar), total PAV increased significantly. Intensive measurement of soil–water properties showed a weak correlation with PAV, which indicated that mechanism of PAV should be further investigated. The increment of PAV under high rate hydrochar application might be due to the aging process and the nitrogen introduced from hydrochar. For PAV reduction, pretreatment is needed before practical application of hydrochar.
Change history
13 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-88214-7
References
Zhang, Y. et al. Concentrations and chemical compositions of fine particles (PM2.5) during haze and non-haze days in Beijing. Atmos. Res. 174–175, 62–69 (2016).
Li, Y. et al. Observations of ammonia, nitric acid, and fine particles in a rural gas production region. Atmos. Environ. 83, 80–89 (2014).
Singh, J., Kunhikrishnan, A., Bolan, N. S. & Saggar, S. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Sci. Total Environ. 465, 56–63 (2013).
Sha, Z., Li, Q., Lv, T., Misselbrook, T. & Liu, X. Response of ammonia volatilization to biochar addition: A meta-analysis. Sci. Total Environ. 655, 1387–1396 (2019).
Wang, H. et al. Ammonia emissions from paddy fields are underestimated in China. Environ. Pollut. 235, 482–488 (2018).
Paustian, K. et al. Climate-smart soils. Nature 532, 49–57 (2016).
Huang, Y. et al. Methane and nitrous oxide flux after biochar application in subtropical acidic paddy soils under tobacco-rice rotation. Sci. Rep. 9, 17277 (2019).
Sheng, Y. & Zhu, L. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 622–623, 1391–1399 (2018).
Liu, Q. et al. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 426, 211–225 (2018).
Huang, M., Fan, L., Chen, J., Jiang, L. & Zou, Y. Continuous applications of biochar to rice: Effects on nitrogen uptake and utilization. Sci. Rep. 8, 11461 (2018).
Feng, Y. et al. Biochar applied at an appropriate rate can avoid increasing NH3 volatilization dramatically in rice paddy soil. Chemosphere 168, 1277–1284 (2016).
Sun, H., Zhang, H., Min, J., Feng, Y. & Shi, W. Controlled-release fertilizer, floating duckweed, and biochar affect ammonia volatilization and nitrous oxide emission from rice paddy fields irrigated with nitrogen-rich wastewater. Paddy Water Environ. 14, 1–7 (2015).
Sun, H., Lu, H., Chu, L., Shao, H. & Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 575, 820–825 (2017).
Sun, H. et al. Responses of ammonia volatilization from rice paddy soil to application of wood vinegar alone or combined with biochar. Chemosphere 242, 125247 (2020).
Nizamuddin, S. et al. Upgradation of chemical, fuel, thermal, and structural properties of rice husk through microwave-assisted hydrothermal carbonization. Environ. Sci Pollut. R. 25, 17529–17539 (2018).
Gronwald, M., Vos, C., Helfrich, M. & Don, A. Stability of pyrochar and hydrochar in agricultural soil—A new field incubation method. Geoderma 284, 85–92 (2016).
Liu, Y. et al. Effect of pyrochar and hydrochar on water evaporation in clayey soil under greenhouse cultivation. Environ. Res. Public Health 16, 2580 (2019).
Malghani, S., Gleixner, G. & Trumbore, S. E. Chars produced by slow pyrolysis and hydrothermal carbonization vary in carbon sequestration potential and greenhouse gases emissions. Soil Biol. Biochem. 62, 137–146 (2013).
Han, L. et al. New evidence for high sorption capacity of hydrochar for hydrophobic organic pollutants. Environ. Sci. Technol. 50, 13274–13282 (2016).
Hua, Y. et al. Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: Process and mechanism. Bioresour. Technol. 300, 122708 (2020).
Schimmelpfennig, S., Müller, C., Grünhage, L., Koch, C. & Kammann, C. Biochar, hydrochar and uncarbonized feedstock application to permanent grassland—Effects on greenhouse gas emissions and plant growth. Agric. Ecosyst. Environ. 191, 39–52 (2014).
Elaigwu, S. E. & Greenway, G. M. Microwave-assisted and conventional hydrothermal carbonization of lignocellulosic waste material: Comparison of the chemical and structural properties of the hydrochars. J. Anal. Appl. Pyrol. 118, 1–8 (2016).
Huang, R. & Tang, Y. Speciation dynamics of phosphorus during (hydro)thermal treatments of sewage sludge. Environ. Sci. Technol. 49, 14466–14474 (2015).
Donar, Y. O., Çağlar, E. & Sınağ, A. Preparation and characterization of agricultural waste biomass based hydrochars. Fuel 183, 366–372 (2016).
Mumme, J. et al. Hydrothermal carbonization of digestate in the presence of zeolite: Process efficiency and composite properties. ACS Sustain. Chem. Eng. 3, 2967–2974 (2015).
Bargmann, I., Rillig, M., Kruse, A., Greef, J. & Kücke, M. Effects of hydrochar application on the dynamics of soluble nitrogen in soils and on plant availability. J. Plant. Nutr. Soil. Sci. 177, 48–58 (2014).
Chu, Q. et al. Microalgae-derived hydrochar application on rice paddy soil: Higher rice yield but increased gaseous nitrogen loss. Sci. Total Environ. 717, 137127 (2020).
Chu, Q. et al. Sewage sludge-derived hydrochar that inhibits ammonia volatilization, improves soil nitrogen retention and rice nitrogen utilization. Chemosphere 245, 125558 (2020).
Xue, Y. et al. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 200–202, 673–680 (2012).
Yu, S. et al. Biowaste to treasure: Application of microbial-aged hydrochar in rice paddy could improve nitrogen use efficiency and rice grain free amino acids. J. Clean Prod. 240, 118180 (2019).
Ti, C., Xia, L., Chang, S. X. & Yan, X. Potential for mitigating global agricultural ammonia emission: A meta-analysis. Environ. Pollut. 245, 141–148 (2019).
Chu, Q. et al. Bentonite hydrochar composites mitigate ammonia volatilization from paddy soil and improve nitrogen use efficiency. Sci. Total Environ. 718, 137301 (2020).
Luo, S. et al. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 117–118, 10–15 (2017).
Zhu, X., Chen, B., Zhu, L. & Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 227, 98–115 (2017).
Rutherford, D. W., Wershaw, R. L., Rostad, C. E. & Kelly, C. N. Effect of formation conditions on biochars: Compositional and structural properties of cellulose, lignin, and pine biochars. Biomass Bioenerg. 46, 693–701 (2012).
Kastner, J. R., Miller, J. & Das, K. C. Pyrolysis conditions and ozone oxidation effects on ammonia adsorption in biomass generated chars. J. Hazard. Mater. 164, 1420–1427 (2009).
Zhao, L., Cao, X., Mašek, O. & Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 256–257, 1–9 (2013).
Lehmann, J. A handful of carbon. Nature 447, 143–144 (2007).
de la Rosa, J. M., Rosado, M., Paneque, M., Miller, A. Z. & Knicker, H. Effects of aging under field conditions on biochar structure and composition: Implications for biochar stability in soils. Sci. Total Environ. 613–614, 969–976 (2018).
Huang, Z. et al. Effect of aging on surface chemistry of rice husk-derived biochar. Environ. Prog. Sustain. Energy. 37, 410–417 (2017).
Mia, S., Dijkstra, F. A. & Singh, B. Aging induced changes in biochar’s functionality and adsorption behavior for phosphate and ammonium. Environ. Sci. Technol. 51, 8359–8367 (2017).
Zhou, B. et al. Impact of hydrochar on rice paddy CH4 and N2O emissions: A comparative study with pyrochar. Chemosphere 204, 474–482 (2018).
Sun, X., Zhong, T., Zhang, L., Zhang, K. & Wu, W. Reducing ammonia volatilization from paddy field with rice straw derived biochar. Sci. Total Environ. 660, 512–518 (2019).
Acknowledgements
This work was supported by the Natural Science Foundation of China (31800358), the Jiangsu Agricultural Science and Technology Innovation Fund (CX(19)3099), the Research Fund of Key Laboratory of Agro-Environment in Downstream of Yangtze Plain (AE2018006), the Key Project of Jiangsu Science and Technology Plan (BE2019378), and the Foundation of Jiangsu Vocational College of Agriculture and Forestry (2019kj014). The authors highly appreciated the contribution of Dr. Yanfang Feng from Jiangsu Academy of Agricultural Sciences to the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.L. and X.L.; methodology, Y.L., Y.W. and Y.C.; formal analysis, X.L., D.C., Y.C. and Y.C.; writing—original draft preparation, X.L., Y.C. and Y.W.; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Cheng, Y., Liu, Y. et al. Hydrochar did not reduce rice paddy NH3 volatilization compared to pyrochar in a soil column experiment. Sci Rep 10, 19115 (2020). https://doi.org/10.1038/s41598-020-76213-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76213-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.