Abstract
This study was performed to evaluate the long-term cardiovascular safety of gemigliptin in patients with type 2 diabetes mellitus (T2DM). After screening, eligible patients with T2DM were enrolled, received gemigliptin, and were followed up for a median of 2.50 years. The primary outcome was a composite of confirmed cardiovascular death, nonfatal myocardial infarction, or nonfatal ischemic stroke (3-point major adverse cardiovascular event [MACE]). The key secondary outcomes were incidence of all-cause mortality and any other cardiovascular events. A total of 5179 patients were included in the study and 5113 were treated with gemigliptin. Overall, the primary outcome occurred in 26 patients within 12 months (estimated incidence by Cox proportional hazard model 0.49%, 95% CI 0.29–0.69%) and in 54 patients within 54 months (estimated incidence from Cox proportional hazard model 1.35%, 95% CI 0.92–1.77%). During the study period, the incidence rates of each component of the primary composite outcome were 0.04% (0.2 events per 1000 person-years) for cardiovascular death, 0.51% (2.2 events per 1000 person-years) for nonfatal myocardial infarction, and 0.61% (2.5 events per 1000 person-years) for nonfatal ischemic stroke. The incidence of all-cause mortality was 0.82% (3.2 events per 1000 person-years) and the incidences of other cardiovascular events were all less than 0.3%. In conclusion, T2DM patients who received gemigliptin exhibited a low incidence of the primary composite MACE and all-cause mortality. Therefore, the use of gemigliptin is expected to be safe without an increase in cardiovascular risk.
Trial registration: The study was registered at ClinicalTrials.gov (identifier: NCT02290301).
Similar content being viewed by others
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has reached epidemic proportions globally and it is associated with cardiometabolic multimorbidity and mortality1,2. The goal of treatment is to achieve and maintain glycemic control to reduce the risks of macrovascular and microvascular complications associated with T2DM3. Given the heterogeneity of patients and the complementary mechanism of disease, different classes of antidiabetic agents have been developed and used for the management of hyperglycemia in T2DM4,5.
Dipeptidyl peptidase 4 (DPP-4) biologically activate a variety of bioactive peptides, including glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide6. DPP-4 inhibitors exhibit glucose-dependent insulinotropic effects and improve hyperglycemia with a low risk of hypoglycemia and other side effects7. Therefore, DPP-4 inhibitors have significantly changed the therapeutic options for patients in real-world practice around the world8,9. Gemigliptin, which is a DPP-4 inhibitor, stimulates the activity of incretin hormones by selectively inhibiting the activity of DPP-4 and exhibits 27,000- and 23,000- times greater selectivity than that DPP-8 and DPP-9, respectively10. The clinical effect of gemigliptin was shown to be superior to that of a placebo and non-inferior to comparable therapeutic agents in active use11,12,13.
Due to the issues related to the use of thiazolidinedione in 2007, the United States Food and Drug Administration and other regulatory authorities now require the results of cardiovascular (CV) outcome studies that investigate the long-term use of new diabetes drugs to confirm that they do not increase unexpected CV risks. Large-scale CV outcome studies were performed on the safety of DPP-4 inhibitors, and the results confirmed that these drugs do not increase CV risks14,15,16,17,18,19.
Important information supporting the CV safety of gemigliptin in patients with T2DM has been collected for the approved indications. However, data are still lacking on the long-term CV safety of gemigliptin, for usual care in patients with T2DM. This study was performed to evaluate CV events in patients with T2DM treated with gemigliptin monotherapy or in combination with other drugs.
Methods
Patients
Patients aged 19 years or older who were diagnosed with T2DM and scheduled to receive gemigliptin (single agent or fixed-dose combination) were included in this study. Key exclusion criteria included patients with type 1 diabetes, severe or end-stage heart failure (HF) (New York Heart Association class III or IV), or history of acute coronary syndrome or stroke within 3 months prior to screening, or who had taken any DPP-4 inhibitors or GLP-1 receptor agonists within 3 months prior to screening. Patients with any contraindications for gemigliptin were also excluded.
Study design
This multicenter, single-arm, prospective cohort study was conducted at 149 centers in the Republic of Korea from June 2013 to November 2017 to assess the long-term CV safety of gemigliptin in patients with T2DM. The study was conducted in compliance with the ethical guidelines of the Declaration of Helsinki and Good Epidemiological Practices and was approved by the institutional review board of Pusan National University Hospital and 54 other study sites according to the standards of a regulatory authority. Written informed consent was obtained from all participants and the study was registered at ClinicalTrials.gov (NCT02290301).
All eligible patients were enrolled on Day 1. They received gemigliptin (single agent or fixed-dose combination) alone or in combination with other antidiabetics, and the regimen and dosage were determined at the discretion of the investigator. The patients were then followed up according to routine medical practice. The addition and discontinuation of, and changes in antihyperglycemic agents, including gemigliptin, were allowed during the study at the discretion of the investigator, taking into consideration the patient’s condition. All of the patients were followed up until the end of the study whenever possible, regardless of whether they took gemigliptin or other diabetic medications.
Patients were recruited for about 30 months, and the planned study participation period for each patient was at least 24 months. Therefore, the last enrolled patient could be followed up for at least 24 months, and the first enrolled patient could be followed up for about 54 months. All data of interest were collected every 6 months from usual care data. Patient who did not visit the study site for more than 6 months, were contacted by telephone to collect CV safety information.
Outcomes
The primary composite major adverse CV event (MACE) endpoint was time to the first confirmed CV death, nonfatal myocardial infarction or nonfatal ischemic stroke. Investigators at the study site adjudicated all components of the primary composite endpoint and reported them as adverse events. All reported adverse events were coded by Medical Dictionary for Regulatory Activities (MedDRA) version 20.0. CV death was defined as death due to adverse events coded as myocardial infarction (narrow standardized MedDRA queries [SMQ]), central nervous system hemorrhage and cerebrovascular conditions (narrow SMQ), or unstable angina (preferred term [PT]). Nonfatal myocardial infarction was defined as an adverse event coded as myocardial infarction (narrow SMQ), and nonfatal ischemic stroke was defined as the adverse event term was coded as central nervous system hemorrhage and cerebrovascular conditions (narrow SMQ).
Secondary endpoints were classified as adverse events related to CV events and other adverse events. CV related endpoints included time to occurrence of the component of the primary composite MACE, the incidence of the primary composite MACE, incidence of each component of the primary composite MACE, incidence of all-cause mortality, and incidence of any other CV events (HF, hospitalization due to revascularization, peripheral vascular disease and unstable angina pectoris). Other endpoints related to adverse events included the incidences of any malignancies and any other specific adverse events (pancreatitis, increased blood amylase, increased lipase, arthralgia, bacteriuria, hypersensitivity and severe skin reactions such as Stevens-Johnson syndrome). Changes in glycated hemoglobin (HbA1c) levels from the baseline were included in other endpoints.
Statistical analysis
The sample size was calculated by using a one sample survival test. Assuming an annual incidence of 2% for the primary composite MACE in patients with T2DM and a dropout rate of 10% before the occurrence of MACE, the sample size of approximately 5000 patients was required to detect a 20% reduction of risk when treated with gemigliptin at a power of 95% and a significance level of 5%.
Statistical analyses were performed on the safety set, which included all participants who received gemigliptin at least once and who were followed up more than once.
Continuous and categorical data were summarized as descriptive statistics. The time to occurrence of the primary composite MACE was analyzed using a Cox proportional hazard model and Kaplan–Meier curves were plotted. The incidence of the primary composite MACE was calculated using a Cox regression model that included age, sex, duration of diabetes, and smoking status, which were expected to be related to MACE, as covariates. If the upper limit of the 95% CI of the annual incidence was less than 2%, gemigliptin was considered not to increase the incidence of the primary composite MACE. The incidence, 95% CI, and incidence rate (per 1000 person-years) were calculated for all endpoints except HbA1c level, and survival analysis was performed on the time to death. The paired t-test or Wilcoxon’s signed-rank test was performed to test for changes in HbA1c levels.
Additional subgroup analyses were performed based on the following treatment cohorts: gemigliptin monotherapy group, gemigliptin + metformin therapy group, gemigliptin + sulfonylurea therapy group, gemigliptin + sulfonylurea + metformin therapy group, gemigliptin + insulin ± other diabetic medications therapy group, and gemigliptin + other diabetic medications therapy group. These treatment cohorts were classified based on treatments received at the time of baseline, and treatment changes during the study were not considered. The chi-square test or Fisher’s exact test was performed to examine the differences in incidence among the treatment cohorts.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Patient disposition and baseline characteristics
A total of 5182 patients with T2DM were screened, 5,179 were enrolled in the study, and 5113 were treated with gemigliptin (Fig. 1). The majority of the patients (93.16%) received a dose of 50 mg of gemigliptin. In terms of treatment cohort, 346 patients received gemigliptin monotherapy (Gemi Mono group), 2177 received gemigliptin and metformin (Gemi + Met group), 252 received gemigliptin and sulfonylurea (Gemi + SU group), 1103 received gemigliptin, sulfonylurea and metformin (Gemi + SU + Met group), 365 received gemigliptin and insulin and/or other diabetic medications (Gemi + INS ± Others group), and 870 received gemigliptin and other diabetic medications (Gemi + Others group). The mean (standard deviation [SD]) duration of participation in the study was 2.47 (1.03) years, and the maximum period was 4.35 years. A total of 4896 patients who received gemigliptin at least once and were followed up more than once were included in the analysis.
Baseline characteristics are summarized in Table 1. The mean (SD) age of participants was 59.65 (11.82) years and males accounted for 54.21% of the group. The mean (SD) BMI was 25.33 (3.58) kg/m2 and the mean (SD) duration of diabetes was 6.05 (6.83) years. In terms of treatment cohort, the mean duration of diabetes was shortest in the Gemi Mono group (1.96 years) and longest in the Gemi + INS ± Others group (12.42 years). The mean (SD) CV risk calculated by the Framingham risk score20 was 22.04% (8.48%) and approximately three quarters of the patients had a CV risk > 15%. At baseline, the mean (SD) HbA1c was 8.29% (1.65%). Regarding concurrent diseases, 51.57% of the patients had hypertension, 42.95% had dyslipidemia, and 11.85% had cardiac disorders. As each treatment was determined at the discretion of the investigator taking the patient’s underlying disease and characteristics into consideration, there were significant differences in most of the baseline characteristics among the treatment subgroups.
Primary composite MACE
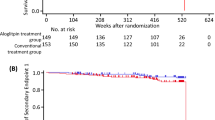
Overall, the primary composite MACE occurred in 26 patients within 12 months (estimated incidence 0.49%, 95% CI 0.29–0.69%) and in 54 patients within 54 months (estimated incidence 1.35%, 95% CI: 0.92–1.77%) (Fig. 2, Supplementary Table S1). Because the upper limit of the 95% CI of the annual incidence was < 2%, it was confirmed that gemigliptin did not significantly increase the incidence of the primary composite MACE. In the subgroup analysis based on treatment cohort, the estimated incidence in the Gemi Mono group at 54 months was the lowest at 0.04%, whereas that in the Gemi + SU group was the highest at 1.69% (Supplementary Table S1 and Fig. S1). Overall, for each component of the primary composite MACE, CV death occurred in two patients within 54 months, nonfatal myocardial infarction occurred in 25 patients, and nonfatal ischemic stroke occurred in 30 patients (Supplementary Table S2). The incidence of the composite MACE was 1.10% (4.7 events per 1000 person-years) during the study period and differences in incidence among treatment cohorts were not significant (p = 0.3221) (Table 2).
All-cause mortality and other cardiovascular events
Overall, the incidence of all-cause mortality was 0.82% (3.2 events per 1,000 person-years) and differences in incidence among treatment cohorts were not significant (p = 0.0762). The Kaplan–Meier curve for overall survival is presented in Fig. 3. The incidence of HF was 0.22% (1.1 events per 1000 person-years), the incidence of peripheral vascular disease was 0.02% (0.1 events per 1000 person-years), the incidence of unstable angina pectoris was 0.29% (1.2 events per 1000 person-years), and there were no cases of hospitalization due to revascularization (Supplementary Table S3).
Malignancies and other adverse events
The incidence of overall adverse events was 28.70% (231.7 events per 1000 person-years) and most adverse events were mild to moderate in severity. The most frequently reported adverse event was hyperlipidemia (2.25%, 8.9 events per 1000 person-years). The incidence of adverse events of special interest (any malignancies, arthralgia, hypersensitivity, or severe skin reactions) was less than 2%, and there were no cases of pancreatitis, increased blood amylase, increased lipase, or bacteriuria (Table 3). Of the adverse events of special interest, only three cases of arthralgia and one case of malignancy were reported as adverse drug reactions, and only four cases of MACE were reported as adverse drug reactions. Most adverse drug reactions were resolved during the study period.
HbA1c changes and other results
After 6 months of gemigliptin administration, HbA1c levels were reduced by 0.94% compared to the baseline (p < 0.0001). The mean change (SD) in HbA1c levels relative to the baseline at 24 months was − 0.83% (1.61%), and reduction in HbA1c levels was also observed at 48 months (mean change − 0.40%; Fig. 4).
After participating in this study, the treatments for 27.41% of the patients were adjusted―e.g., gemigliptin was discontinued and/or other antidiabetic agents were administered. Additionally, 22.39% of the patients received other antidiabetic agents after administration of gemigliptin, and of the antidiabetic agents, sulfonylureas (38.87%) and metformin (25.18%) were the most commonly administered. The gemigliptin dose was also changed or discontinued for 8.70% of the patients.
Discussion
In this observational study, the estimated incidence of MACE at 12 months of follow-up, which was a primary endpoint, was 0.49%, and that at 54 months was 1.35%. In addition, the incidence of MACE at every time point in 6-month interval was all < 2%, which was statistically significant. Therefore, the use of gemigliptin (as monotherapy or part of combination therapy) did not significantly increase MACE incidence.
DPP-4 inhibitors which are drugs that regulate blood glucose by increasing insulin secretion and inhibiting the degradation of GLP-1 by reacting with blood glucose have strong anti-hypoglycemic effects6. DPP-4 inhibitors had been expected to confer beneficial effects on CV disease (CVD) due to pleiotropic effects as they show positive effects on CV risk factors in T2DM patients, such as weight loss, reduced blood pressure, improved postprandial dyslipidemia, and reduced inflammation21. In addition, DPP-4 inhibitors may have a direct protective effect against CVDs, which may be mediated by the inhibition of endoplasmic reticulum (ER) stress in cardiomyocytes, vascular calcification, and vascular remodeling22. Gemigliptin effectively inhibited ER stress-induced apoptosis and inflammation via the Akt/protein kinase RNA-like endoplasmic reticulum kinase (PERK)/C-EBP homologous protein (CHOP) and inositol-requiring enzyme 1 α (IRE1α)/c-Jun N-terminal kinase (JNK)-p38 pathways in H9C2 cardiomyocytes in vitro22. In addition, gemigliptin attenuated vascular calcification in vitro and in vivo and osteogenic transdifferentiation of vascular smooth muscle cells (VSMCs) by reducing PiT-1 expression, attenuating phosphate-induced oxidative stress, phospho-AKT/PI3K signaling, and Wnt signaling23. Although DPP4 inhibitors have been considered to have beneficial effects on CVD from experimental studies, there was no evidence of beneficial effects on CVD with DPP4 inhibitors from large-scale CV outcome studies14,15,16,17,18,19.
In the results of the subgroup analyses, the incidence of MACE was lowest in the Gemi Mono and Gemi + Met groups and highest in the Gemi + SU group. The differences of these findings among the treatment subgroups are likely primarily due to differences of the baseline characteristics among subgroups. On the other hand, we can assume the reason from the studies that revealing that fewer CV events occurred in patients treated with metformin compared to patients treated with sulfonylurea or a combination of metformin and sulfonylurea24.
The incidence of MACE was 8.4% in a large-scale CV outcome study of sitagliptin14, another DPP-4 inhibitor, 11.3% in a study of alogliptin16, 12.4% in a study of linagliptin17, and 7.3% in a study of saxagliptin15, all of which are values higher that reported in this study for gemigliptin. The incidence of MACE in this study was lower upon simple comparison with the results of the previous studies outlined above. However, while these previous reports presented results of randomized placebo-controlled studies, the present study had different design as a non-interventional study, and there were differences in the methods of evaluation, adjudication and analysis of MACE. In addition, considering the different baseline characteristics of the subjects, particularly the younger age of subjects enrolled in this study, and the lower rate of subjects with a medical history of CVD compared to that of the cohorts in previous studies, it was determined that the incidence of MACE was lower than in previous studies as our subjects exhibited relatively fewer CV risk factors.
In a retrospective analysis with data from several nationwide registries in Denmark, sitagliptin monotherapy was not associated with any significant increase in risk of a composite endpoint of stroke, acute myocardial infarction and all-cause mortality compared with metformin monotherapy25. In the Danish retrospective study, subjects using sitagliptin had a relatively short duration of monotherapy (0.9 year) and the incidence of the composite endpoint was 5.0%. Another retrospective analysis of nationwide data from Taiwan’s National Health Insurance Research Database demonstrated that DPP-4 inhibitors (sitagliptin, vildagliptin and saxagliptin), compared with sulfonylureas, were associated with lower risks of all-cause death and MACEs (ischemic stroke and myocardial infarction) as add-ons to metformin therapy26. In this retrospective cohort study, 209 MACEs occurred during a 3.3-year follow-up period in patients using DPP-4 inhibitors (10.4 events per 1000 person-years). As prospective observational study, this current study provided additional information of CV safety in the gemigliptin as one of new DPP-4 inhibitors (MACE, 4.7 events per 1000 person-years).
The incidences of each component of MACE and other CV events (HF, hospitalization due to revascularization, peripheral vascular disease, unstable angina pectoris) were low with values < 1%. Previous large-scale CV outcome studies of different DPP-4 inhibitors did not provide clear data regarding HF risk27. However, the potential for increased risk of HF with DPP-4 inhibitors was reported in the saxagliptin study, in which patients with T2DM and either a history of CVD or multiple CV risk factors were randomized to receive saxagliptin or placebo15. We also evaluated the incidence of HF, which was 0.22% for the whole patients and ≤ 0.50% for all subgroups in this study.
In this study, the incidence of all-cause mortality was 0.82%. According to the analysis of National Health Insurance data27, the all-cause mortality risk was lower for patients receiving metformin and DPP-4 inhibitors compared to patients taking metformin and sulfonylurea (hazard ratio [HR] 0.84, 95% CI 0.66–1.07)28. The risk of all-cause mortality was compared among five types of DPP-4 inhibitors, and CV risk was shown to be lower for gemigliptin compared to sitagliptin (HR 0.84, 95% CI 0.80–0.88)29.
DPP-4 inhibitors are relatively well-tolerated and have fewer side effects compared to other antidiabetic agents. However, they must be prescribed carefully, as adverse events, such as angioedema, anaphylaxis and Stevens-Johnson syndrome, have been reported albeit rarely30. In addition, a previous study showing increases in number of pancreatic duct cells in response to incretin-based treatment suggested that DPP-4 inhibitors may be associated with pancreas-related safety issues, such as pancreatitis and pancreatic cancer31. In large-scale CV outcome trials, sitagliptin was associated with incidence of 0.3% for pancreatitis and 0.1% for pancreatic cancer, and saxagliptin was associated with incidence of 0.3% and 0.06%, respectively14,15. However, in this large-scale prospective observational study, pancreatitis and increased blood amylase and lipase levels were not reported. The incidence of all types of cancer was 1.25%, which was lower than the reported incidence for other DPP-4 inhibitors (3.7% for sitagliptin14, 3.3% for linagliptin17, and 3.9% for saxagliptin15). One case of pancreatic cancer was reported in this study. However, it was not considered to be related to gemigliptin as the patient had a medical history of acute pancreatitis prior to enrollment in this study and the duration of gemigliptin treatment was short (less than 1 month). In addition, gemigliptin is considered to be safe for long-term use as the incidences of other events such as arthralgia, hypersensitivity, and severe skin reactions were low.
This study had limitations. This non-interventional observational study did not involve comparisons with a placebo or other DPP-4 inhibitors. It also had the limitation that it was difficult to collect accurate data via self-reporting of MACE from the patients. In addition, the methods of evaluation, adjudication and analysis of MACE were different and patients with relatively low CV risk (younger age, lower rate of concurrent hypertension and history of CVDs, and shorter duration of diabetes) were enrolled in this study compared to previous large-scale CV outcome studies conducted as placebo-controlled randomized studies, and these factors may have influenced the results of this study, such as the incidences of MACE and other adverse events. The subgroup analyses of this study were performed for exploratory purposes based on the treatment cohorts, but this also had some limitations. Participants were allowed to change or discontinue their treatment during the study period at the discretion of the investigator, but treatment changes were not considered in the MACE analysis. In addition, some of the baseline characteristics, such as the history of CVD, had significant differences among the treatment subgroups, but were not included as covariates in a Cox regression model, and HbA1c level was also not adjusted in the MACE analysis due to insufficient data collection. Despite these limitations, this study yielded meaningful information regarding the safety of DPP-4 inhibitors through a large-scale long-term prospective cohort study with a wide range of T2DM patients who used gemigliptin alone or as combination therapy.
In conclusion, this study showed that gemigliptin use was associated with low incidences of the primary composite MACE, all-cause mortality, and other adverse events in patients with T2DM in real practice. In addition, it was also confirmed that combination therapy of gemigliptin with other antidiabetic agents (metformin, insulin and other agents except sulfonylurea) did not increase the incidence of CV events. Thus, the clinical use of gemigliptin may safe, and it also may not increase CV risk in real practice.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Kim, J. H., Kim, D. J., Jang, H. C. & Choi, S. H. Epidemiology of micro- and macrovascular complications of type 2 diabetes in Korea. Diabetes Metab. J. 35, 571–577 (2011).
Collaboration, E. R. F. et al. Association of cardiometabolic multimorbidity with mortality. JAMA 314, 52–60 (2015).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412 (2000).
Davies, M. J. et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41, 2669–2701 (2018).
Kim, M. K. et al. 2019 clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab. J. 43, 398–406 (2019).
Mulvihill, E. E. & Drucker, D. J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 35, 992–1019 (2014).
Aroor, A. R., Sowers, J. R., Jia, G. & DeMarco, V. G. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 307, H477-492 (2014).
Montvida, O., Shaw, J., Atherton, J. J., Stringer, F. & Paul, S. K. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 41, 69–78 (2018).
Ko, S. H. et al., Trends of antidiabetic drug use in adult type 2 diabetes in Korea in 2002–2013 Nationwide population-based cohort study. Medicine 95 (2016).
Kim, S. H. et al. Pharmacological profiles of gemigliptin (LC15-0444), a novel dipeptidyl peptidase-4 inhibitor, in vitro and in vivo. Eur. J. Pharmacol. 788, 54–64 (2016).
Ahn, C. H. et al. Efficacy and safety of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24-week, multicentre, randomized, double-blind, placebo-controlled study (TROICA study). Diabetes Obes. Metab. 19, 635–643 (2017).
Rhee, E. J. et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes. Metab. 15, 523–530 (2013).
Yang, S. J. et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes. Metab. 15, 410–416 (2013).
Green, J. B. et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 373, 232–242 (2015).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013).
Rosenstock, J. et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA 321, 69–79 (2019).
Khalse, M. & Bhargava, A. A review on cardiovascular outcome studies of dipeptidyl peptidase-4 inhibitors. Indian J. Endocrinol. Metab. 22, 689–695 (2018).
Chin, H. J., Nam, J. H., Lee, E. K. & Shin, J. Y. Comparative safety for cardiovascular outcomes of DPP-4 inhibitors versus glimepiride in patients with type 2 diabetes: a retrospective cohort study. Medicine (Baltimore) 96, e7213 (2017).
D’Agostino, R. B. et al. General cardiovascular risk profile for use in primary care—the Framingham heart study. Circulation 117, 743–753 (2008).
Scheen, A. J. Cardiovascular effects of gliptins. Nat. Rev. Cardiol. 10, 73–84 (2013).
Hwang, H. J. et al. Dipeptidyl petidase-IV inhibitor (gemigliptin) inhibits tunicamycin-induced endoplasmic reticulum stress, apoptosis and inflammation in H9c2 cardiomyocytes. Mol. Cell Endocrinol. 392, 1–7 (2014).
Choi, S. Y. et al. Dipeptidyl peptidase-4 inhibitor gemigliptin protects against vascular calcification in an experimental chronic kidney disease and vascular smooth muscle cells. PLoS ONE 12, e0180393 (2017).
Evans, J. M., Ogston, S. A., Emslie-Smith, A. & Morris, A. D. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49, 930–936 (2006).
Scheller, N. M., Mogensen, U. M., Andersson, C., Vaag, A. & Torp-Pedersen, C. All-cause mortality and cardiovascular effects associated with the DPP-IV inhibitor sitagliptin compared with metformin, a retrospective cohort study on the Danish population. Diabetes Obes. Metab. 16, 231–236 (2014).
Ou, S. M. et al. Effects on clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann. Intern. Med. 163, 663–672 (2015).
Filion, K. B. & Suissa, S. DPP-4 inhibitors and heart failure: some reassurance some uncertainty. Diabetes Care 39, 735–737 (2016).
Ha, K. H., Kim, B., Choi, H., Kim, D. J. & Kim, H. C. Cardiovascular events associated with second-line anti-diabetes treatments: analysis of real-world Korean data. Diabet. Med. 34, 1235–1243 (2017).
Ha, K. H. et al. Comparative cardiovascular risks of dipeptidyl peptidase-4 inhibitors: analyses of real-world data in Korea. Korean Circ. J. 48, 395–405 (2018).
Pathak, R. & Bridgeman, M. B. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. Pharm Ther 35, 509–513 (2010).
Perfetti, R., Zhou, J., Doyle, M. E. & Egan, J. M. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141, 4600–4605 (2000).
Acknowledgements
This study was supported by LG Chem, Ltd. The funding body was involved in the design of the study, data analysis and writing the manuscript, but played no role in the collection and interpretation of data. The authors gratefully acknowledge the patients and OPTIMUS Study Group for their participation in this study, Younghwan Jang of LG Chem, Ltd. for study management, Eun Youn Kim of LG Chem, Ltd. for providing statistical support, and Yunae Eom of LG Chem, Ltd. for editing assistance in preparing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors conducted the study and contributed toward acquisition of data. E.H.K.: Methodology, software, data curation, formal analysis, investigation, resources, validation, writing–original draft, writing–review and editing, and visualization. S.S.K.: Conceptualization, software, data curation, methodology, validation, formal analysis, investigation, resources, visualization, supervision, project administration, writing–original draft, and writing–review and editing. D.J.K., Y.S.C., C.W.L., B.J.K., K.S.C., K.H.S., D.K.K.: Data curation, validation, and writing–review and editing. I.J.K.: Conceptualization, methodology, validation, writing–review and editing, visualization, supervision, and project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, E.H., Kim, S.S., Kim, D.J. et al. A prospective cohort study on effects of gemigliptin on cardiovascular outcomes in patients with type 2 diabetes (OPTIMUS study). Sci Rep 10, 19033 (2020). https://doi.org/10.1038/s41598-020-75594-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75594-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.