Abstract
Optimized fluid management is a key component of enhanced recovery (ERAS) pathways. Implementation is challenging for pancreatoduodenectomy (PD) and clear guidance is missing in the respective protocol. The aim of this retrospective study was to evaluate the influence of perioperative intravenous (IV) fluid administration on postoperative complications. 164 consecutive patients undergoing PD within ERAS between October 2012 and June 2017 were included. Perioperative IV fluid and morbidity (Clavien classification and comprehensive complication index (CCI)) were assessed. A threshold of more than 4400 ml IV fluid during the first 24 h could be identified to predict occurrence of complications (area under ROC curve 0.71), with a positive and negative predictive value of 93 and 23% respectively. More than 4400 ml intravenous fluids during the first 24 h was an independent predictor of overall postoperative complications (adjusted odds ratio 4.40, 95% CI 1.47–13.19; p value = 0.008). Patients receiving ≥ 4400 ml were associated with increased overall complications (94 vs 77%; p value < 0.001), especially pulmonary complications (31 vs 16%; p value = 0.037), as well as a higher median CCI (33.7 vs 26.2; p value 0.041). This threshold of 4400 ml intravenous fluid might be a useful indicator for the management following pancreatoduodenectomy.
Similar content being viewed by others
Introduction
The outcomes following pancreatoduodenectomy (PD) have improved, with a drastic fall of postoperative mortality from 15 to 20% in the 80s1 to less than 2% in high-volume centres2 nowadays. These interventions still have a high morbidity and patients may need a delay up to 6 month to reach their preoperative quality of life3,4. In an effort to improve those outcomes, the concept of enhanced recovery (ERAS) is increasingly recommended for pancreatic surgery. ERAS pathway aims to reduce the surgical stress in order to shorten patient’s recovery and works in pancreatic surgery5,6. One of the cornerstone of enhanced recovery is balanced fluid therapy with avoidance of fluid overload. Uncontrolled perioperative fluid administration has potential deleterious impact on postoperative outcome. While fluid overload may lead to interstitial edema, hypovolemia may result in renal dysfunction. For these reasons, implementation of an enhanced recovery protocol for PD is challenging7 and specific guidance on defining fluid balance for PD are required.
This study aimed to assess the potential impact of perioperative fluid administration on postoperative outcomes after PD within an ERAS protocol.
Results
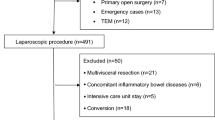
A total of 178 consecutive patients underwent pancreatoduodenectomy in our institution during the study period. As 14 patients refused the use of their data, 164 patients were included in the analysis. Patients demographics and surgical characteristics are detailed in Table 1. The final diagnosis was a primary adenocarcinoma in most of the cases (82%), with 11 patients among them who underwent neoadjuvant chemotherapy. All operations were open PD without pylorus preservation. The overall compliance to the ERAS pathway was 62%, with 4% of missing data. The mean compliance was 99% for preoperative items (100% no oral bowel preparation, 98% oral carbohydrate drinks, 95% avoidance of long-acting sedatives, 99% thromboprophylaxis, 100% antibioprophylaxis, 100% postoperative nausea and vomiting prophylaxis), 87% for intraoperative items (99% epidural when not contraindicated, 100% upper-body heating cover, 88% removal of nasogastric tube at end of surgery, 62% early drain removal), and 32% for postoperative items (2% termination of urinary drainage on Postoperative Day (POD) 3, 83% of stimulation of gut motility, 99% postoperative use of epidural if applicable, 12% < 3500 ml IV fluids on POD 0, 7% termination of IV fluids on POD 2, 25% patient weight on POD1, 4% mobilization on day of surgery, 42% mobilization more than 4 h on POD1, 25% mobilization more than 6 h on POD 2, 34% mobilization more than 6 h on POD3, 17% 30 day follow up).
Perioperative fluid and overall complications
The median total amount of IV fluids POD 0 was 5005 ml (IQR 3963–6124). The median intraoperative IV fluid was 3500 ml (IQR 2575–4500) and was composed by crystalloids (median 3000 ml, IQR 2175–3500) and colloids (median 500 ml, IQR 0–1000). The median intraoperative infusion rate was 9 ml/kg/h (IQR 7–11). The amount of IV fluids POD 0 was composed by the addition of intraoperative, and postoperative IV fluids, and both were higher in patients with complications (3500 ml (IQR: 2712–4500) vs 3000 ml (IQR 2000–3687); p value 0.034, and 1400 ml (IQR 1100–1860) vs 1165 (IQR 770–1605); p value 0.024, respectively).
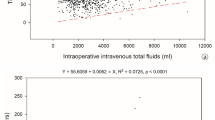
As postoperative complications were higher in patients with increased perioperative fluids, the potential correlation between perioperative fluids and postoperative complications was assessed. As presented in Fig. 1, the amount of IV fluids POD 0 weakly correlated with CCI (r = 0.168, p value = 0.020).
Correlation between intravenous (IV) fluids on postoperative day (POD) 0 fluids and Comprehensive Complication Index (CCI). Spearman’s rank correlation coefficient (Rho = 0.168, p value = 0.020) between the volume of intravenous fluid within the first postoperative day (IV fluids POD 0) and the Comprehensive Complication Index (CCI).
Fluid threshold
In order to identify a critical threshold, a ROC curve analysis of IV fluids POD 0 (ml/24 h) and overall complications was performed (Fig. 2). The Area Under the Curve (AUC) was 0.71 (95% CI 0.59–0.83), which is above the 0.70 cut-off indicating moderate accuracy8. The optimal threshold of IV fluids POD 0 as predictor of complications was set at 4400 ml. This threshold provided a sensitivity of 69% and a specificity of 63% with a positive predictive value of 93% and a negative predictive value of 23%. The ROC curve analysis for IV fluids POD 0 and major complications was not contributive (AUC = 0.55). Further ROC curve analyses of IV fluids on POD taking account the body weight (ml/kg/24 h) found an AUC of 0.69 for overall complications and an AUC of 0.49 for major complications. When considering the infusion rate of intraoperative fluids (ml/kg/h) the AUC for overall and major complications were 0.58 and 0.42, respectively.
Predictors for overall and major complications
In order to evaluate the potential predictive role of perioperative fluid on postoperative complications, a binary logistic regression analysis with preoperative and perioperative characteristics including the identified 4400 ml threshold of IV fluids on POD 0 was performed and presented in Tables 2 and 3. On multivariable analysis, the 4400 ml IV fluids POD 0 was an independent predictor of overall postoperative complications (adjusted OR 4.40, 95% CI 1.47–13.19; p value = 0.008), but not of major complications.
Postoperative outcomes according to critical fluid threshold
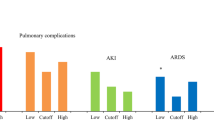
According to this 4400 ml of IV fluids POD 0, postoperative outcome was compared as shown in Table 4. There were 61 patients who received less than 4400 ml and 103 patients who received 4400 ml or more. The overall complication rate and the CCI were significantly higher in the ≥ 4400 ml group (94 vs 77%; p value = 0.001, and 33.7 vs 26.2; p value = 0.041, respectively). The 90 days after surgery mortality was not different in both groups: 3% in the < 4400 ml group (one patient died of hemorrhagic shock and one patient of septic shock) and 2% in the ≥ 4400 ml group (one patient died of bleeding shock and one patient of disease progression with carcinosis after 61 postoperative days); p value = 0.629. The rate of postoperative pulmonary complications was significantly increased in patients receiving higher perioperative volume (31 vs 16%; p value = 0.037). The increased rate of respiratory complications in patients receiving more than 4400 ml was especially observed for minor (Clavien I-II) complications (21 vs 5%; p value = 0.006). There was no significant difference among detailed pulmonary complications, such as atelectasis (8 vs 5%; p value = 0.748), pneumonia (18 vs 10%; p value = 0.179), pleural effusion (12 vs 10%; p value = 0.800), respiratory failure (10 vs 5%; p value = 0.332), and pneumothorax (1 vs 0%; p value = 1.000). Regarding hemodynamic related complications, there was no significant difference in terms of cardio-vascular dysfunction (13 vs 10%; p value 0.500), and of renal failure (3 vs 5%; p value = 0.322). Between the two groups. In the < 4400 ml group, one patient had a renal failure classified AKIN (Acute Kidney Injury Network) 1 and one patient AKIN. 3. In the group with more than 4400 ml, two patients were classified AKIN 1 and three patients AKIN 3. No significant difference in pancreatic surgery specific complications such as delayed gastric emptying or pancreatic fistula was observed between both groups. The use of postoperative nasogastric tube was 3% (2/61) in the < 4400 ml group compared to 17% (18/103) (p value = 0.007). The rate of nasogastric tube reinsertion was comparable (43% vs 42%; p value = 0.913). The rate of postoperative parenteral nutrition started in the postoperative period was not different between both groups (55 vs 49%; p value = 1.000). The number of relapartomy needed for postoperative hemorrhage was 8 (8%) in patients with ≥ 4400 ml compared to 5 (8%) in patients with < 4400 ml (p value = 1.000). The proportion of patients requiring intensive care unit was 21% in the < 4400 ml group and 19% in the ≥ 4400 ml group (p value = 0.841).
Discussion
This study described a higher administration of perioperative IV fluids among patients presenting with postoperative morbidity. The correlation of perioperative IV fluids and postoperative cumulative complications expressed by CCI was weak and meant that a small proportion of complications could be correlated to the amount of IV fluids. However, a critical threshold of 4400 ml IV fluid administration during the first 24 h was identified as an independent predictor of postoperative complication. More than 4400 ml IV fluids POD 0 was especially associated with increased respiratory complications, but neither pancreatic fistula nor delayed gastric emptying.
The issue on optimal fluid management in abdominal surgery is still open to debate. Previous studies assessing fluid therapy and outcome in PD perfectly reflects this situation. A fair amount of published studies including PD, predominantly retrospective cohort, described an increased rate of postoperative complications with increased fluids9,10,11,12,13. However, most randomized controlled studies14,15,16 failed to show any significant difference in postoperative outcome when comparing liberal to restrictive fluid therapy.
Comparison between studies on fluid therapy in PD is difficult because of the various definition of fluids (units, measure, and duration) and heterogeneous data. Moreover, most studies did not encompass the use of a systematic enhanced recovery protocol. Nevertheless, our center has a more restrictive fluid administration as in some other centers with a median intraoperative fluid administration of 3500 ml in comparison to 6000 ml for Kulemann et al.10 and 5000 ml for Weinberg et al.9. This is reflected in the intraoperative infusion rate too, as we have a median intraoperative infusion rate of 9 ml/kg/h (IQR 7–11), in comparison of 14 ml/kg/h for Eng et al., and corresponding to a restrictive fluid administration (< 10 ml/kg/h)11,13, although no standard definition exists. The rate of renal failure in the present study (3 and 5% in the < 4400 ml and ≥ 4400 ml groups, respectively) was in accordance to the 7% rate reported by Weinberg et al.9. Other parameters were comparable with the literature: similar demographics in term of age (mean age 65, going from 53.5 to 69.0 in a systematic review17), gender (male 52% versus 50.3%10 to 59%9), mean BMI (25.6 kg/m2 versus 25.8 kg/m212 to 26 kg/m29). As in most studies, a large majority of patients had malignant tumor as indication for PD (86% versus 73.4%10 to 88%12). The ASA score may be lower in the present study with 33.5% of patients with ASA-score of 3 or 4, which ranged from 34.510 to 89.5%12 in other studies. In term of operative parameter, the mean operative time tend to be lower in our data (335 min versus 420 min9 to 445 min11) but with similar vascular resection rate (22% versus 24% for Behman et al.12) and blood loss (median blood loss 440 ml versus 350 ml9 to 909 ml11).
As avoiding fluid overload might improve postoperative outcome after PD, the way to achieve this euvolemia is still matter of debate. Goal directed fluid therapy requires cardiac output monitoring that may be invasive and lacks easily identifiable target. For these reasons it is a challenging measure to implement in daily clinical practice. Further guidance are awaited from the OPTIMISE II trial18, a multicenter international trial of cardiac output-guided fluid therapy with low-dose inotrope infusion compared with usual care in patients undergoing major elective gastrointestinal surgery. Meantime, a threshold of perioperative IV fluid is a simple and reproducible way to estimate the fluid balance. The ROC analysis identified a threshold of 4400 ml perioperative fluid for complications. Previously, specific thresholds for perioperative IV fluid were identified, for example for open colorectal surgery (> 3500 ml)19 or loop ileostomy closure (> 1700 ml)20. Although the above mentioned threshold of 3500 ml for perioperative fluids was determined for open colorectal surgery, it was previously used by extrapolation for pancreatodudenectomy7. Data for pancreatic resection are sparse and Bruns et al. identified a ratio (infusion rate/glomerula function rate) of 0.15 as predictive of pancreatic fistula21. Available data for liver surgery identified a threshold of an increased weight of 3.5 kg on the second postoperative day which was associated with increased major complications22, but no specific data on fluids were provided. In the present study, the weight difference did not vary between patients with or without complications, possibly indicating that fluid balance evaluation is a complex process.
Noteworthy, the 4400 ml threshold was related to increased complications and length of stay. Especially postoperative minor pulmonary complications were increased in patients receiving 4400 ml or more. Similarly, Eng et al. reported an increased rate of pulmonary complications (29 vs 5%, p < 0.01) related to intraoperative infusion when comparing patients receiving more or less than 13.95 ml/kg/h11. The meta-analysis of Garland et al23 found no difference between restrictive and liberal fluid therapy on overall morbidity (Odds Ratio 1.17, 95% CI 0.92–1.50) and pulmonary complications (Odds Ratio 0.69, 95% CI 0.43–1.10). While causes of postoperative respiratory complications are multifactorial, a recent systematic review24 identified enhanced recovery (risk ratio 0.35, 95% CI 0.21–0.58) and goal directed therapy (risk ratio 0.87, 95% CI 0.77 to 0.98) among other interventions as effective measure to reduce their occurrence. Postoperative pulmonary complications are probably also related to other complications, but the study design as well as the lack of date of occurrence of each complications prevented to conduct a separate detailed analysis of predictive factors, including other complications, of pulmonary complications. As enhanced recovery also encloses goal-directed fluid therapy, the direct effect of fluid management is difficult to isolate. The threshold of 4400 ml would be more on the restrictive side as opposed to the liberal side when considering the various cut-offs reported in the systematic review on fluid regimens in pancreatoduodenectomy by Garland et al23. A fear of a too restrictive fluid regimen is potential tissue hypoperfusion leading to an increased rate of renal failure or altered wound healing. For example, the RELIEF (Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery) trial observed an increased rate of acute kidney injury associated with restrictive fluid therapy (8.6% in the restrictive fluid group and 5.0% in the liberal fluid group) among high risk patient undergoing major abdominal surgery25. Of notice, in the RELIEF trial the median intravenous fluid during and up-to 24 h surgery was 3700 ml in the restrictive group and 6100 ml in the liberal group. In the present study no increased renal failure was observed in patients receiving less than 4400 ml.
Regarding pancreatic-specific complications such as delayed gastric emptying and pancreatic fistula, the present study did not observed increased risk associated with increased perioperative fluid infusion. This is in line with Garland et al.23 who reported in a meta-analysis no significant difference in occurrence of delayed gastric emptying or pancreatic fistula when comparing restrictive to liberal fluid therapy in PD patients. However, some retrospective studies not included in the abovementioned meta-analysis26,27,28 were suggesting an increase in pancreatic fistulas associated with excessive fluid. Moreover, a study from Bannone et al. reported an increased incidence of postoperative acute pancreatitis and pancreatic fistula in high-risk patients after pancreatoduodenectomy with an intraoperative fluid administration of ≤ 3 ml/kg/h29. In the present study, the median intraoperative fluid administration was 9 ml/kg/h and no increased rate of pancreatic fistula was observed between both groups.
Limitations of the present study are inherent to the retrospective analysis and to the limited study sample obtained in a single center. Moreover, enhanced recovery relies on compliance to protocol elements, including fluid management, which has altogether a strong influence on the postoperative outcome7. However, in order to avoid redundancy with previously published results7, this study focused on fluid management and impact of ERAS compliance was not assessed here. In addition, the amount of IV fluid administered in the first 24 h of surgery is not an entirely modifiable factor, as it is closely related to patient’s comorbidity, disease’s prognosis and surgery’s extent.
In order to validate and generalize this threshold of 4400 ml iv fluids on POD 0 and associated findings, further study including PD performed within an enhanced recovery setting need to be conducted. Once available, this will provide useful guidance on how to refine fluid management of patients undergoing PD to avoid both fluid overload and kidney insufficiency.
In conclusion, the present study emphasizes the potential deleterious consequence of fluid overload especially on pulmonary complications. A threshold for perioperative intravenous fluid at 4400 ml might be a useful indicator in the management after pancreatoduodenectomy within an enhanced recovery pathway.
Methods
Study design
This retrospective cohort study included all consecutive patients undergoing elective PD within an ERAS program in a tertiary referral centre (Lausanne University Hospital (CHUV), Switzerland) between October 2012 and June 2017. This cohort of patients was previously included and merged with data of three other institutions in a multicentre study7. Patients younger than 18 years old, and those who were opposed to the use of their data were not included. The study protocol was approved by the Institutional Review Board (CER-VD # 2016-01815) and patients gave informed consent. This study was reported in accordance with the STROBE guidelines30.
Enhanced recovery protocol, fluid management and data collection
The enhanced recovery protocol for PD was initiated in October 2012 according to the ERAS guidelines5 and was previously detailed7. During the surgery, an infusion of Ringer Lactate at a rate of 3-5 ml/kg/h was initiated and bolus of 500 ml were given in case of decreased cardiac output (assessed by pulse pressure variation, oesophageal Doppler or transoesophageal echocardiography) or increased lactate with a minimal diuresis set at 0.5 ml/kg/h. Vasopressors were liberally used during and after the operation to counterbalance the vasodilator effect of epidural. In the postoperative period, an infusion of 1000 ml/24 h of Ringer Lactate was instilled, with 250 ml bolus of Physiogel in case of diuresis less than 2 ml/kg/4 h. Free oral drinks were encouraged from the day of surgery. Preoperative and demographic characteristics, intraoperative data and postoperative outcomes were prospectively collected using the ERAS Interactive Audit System (EIAS). The postoperative follow-up was 30 days.
Fluids assessment and outcomes
Perioperative fluids were defined as intravenous (IV) infusion, including crystalloids, colloids and blood products given during the first 24 h of surgery (postoperative day 0: POD 0). The total IV volume administration on POD 0 (IV fluids POD 0) was used as parameter to define critical threshold.
Postoperative complications were graded using the Clavien classification31. Grade I and II were defined as minor and grade IIIa to IVb as major complications. Grade V was reported as mortality. The Comprehensive Complication Index32 (CCI) was calculated to obtain a continuous morbidity scale. Complications, including postoperative pulmonary complications were reported according to the EPCO (European Perioperative Clinical Outcome) definitions33. Accordingly, postoperative pulmonary complications included respiratory infection (antibiotics for suspected respiratory infection and with one or more criteria: sputum, lung opacities, fever, with blood cell > 12 × 109/l), respiratory failure (postoperative PaO2 < 8 kPa, PaO2: FIO2 ratio < 40 kPa, or arterial saturation < 90% requiring oxygen therapy), pleural effusion (demonstrated by chest radiograph), atelectasis (lung opacification with shift toward affected area and compensatory over-inflation in the adjacent non-atelectatic lung), pneumothorax (air in the pleural space), bronchospasm (newly detected expiratory wheezing treated with bronchodilators) and aspiration pneumonitis (acute lung injury after the inhalation of regurgitated gastric content.
Primary outcome was overall postoperative morbidity. Secondary outcomes were major complications, CCI, and length of stay. Primary length of stay was the number of days spent in the hospital from the primary operation until discharge. Total length of stay was the addition of primary length of stay with the number of days following hospital readmission. Specific complications related to pancreas surgery were reported according to the respective ISGPS guidelines34,35,36,37.
Statistical analysis
Descriptive statistics for categorical variables were reported as numbers and percentages, while continuous variables reported as median and interquartile ranges (IQR) or mean and standard deviations (SD). Categorical variables were compared with the Chi-Square or Fisher’s exact test. The Student’s t test and Mann–Whitney U test were used for continuous normally and non normally distributed data, respectively. Correlation between CCI and perioperative fluids was assessed by the Spearman’s test. The diagnostic ability was illustrated by a receiver operating characteristic (ROC) curve. An area under the curve (AUC) of > 0.7 was considered statistically significant. Threshold was identified according to the Youden index. Potential predictors of overall and major complications were assessed by binary logistic regression analysis and multiple logistic regression analysis was further performed including all previous independent variables with a p value < 0.1. All p values were two-sided and p < 0.05 was considered statistically significant. Data analysis was performed with SPSS version 25 (IBM Corp, Amonk, NY).
References
Herter, F. P., Cooperman, A. M., Ahlborn, T. N. & Antinori, C. Surgical experience with pancreatic and periampullary cancer. Ann. Surg. 195, 274–281 (1982).
Gooiker, G. A. et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br. J. Surg. 101, 1000–1005 (2014).
Eaton, A. A. et al. Health-related quality of life after pancreatectomy: results from a randomized controlled trial. Ann. Surg. Oncol. 23, 2137–2145 (2016).
Heerkens, H. D. et al. Health-related quality of life after pancreatic resection for malignancy. Br. J. Surg. 103, 257–266 (2016).
Lassen, K. et al. Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (ERAS®) Society recommendations. World J. Surg. 37, 240–258 (2013).
Melloul, E. et al. Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J. Surg. 44, 2056-2084 (2020).
Roulin, D. et al. Feasibility of an enhanced recovery protocol for elective pancreatoduodenectomy: a multicenter international cohort study. World J. Surg. 44, 2761–2769 (2020).
Fischer, J. E., Bachmann, L. M. & Jaeschke, R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 29, 1043–1051 (2003).
Weinberg, L. et al. The impact of fluid intervention on complications and length of hospital stay after pancreaticoduodenectomy (Whipple’s procedure). BMC Anesthesiol. 14, 35 (2014).
Kulemann, B. et al. Complications after pancreaticoduodenectomy are associated with higher amounts of intra- and postoperative fluid therapy: a single center retrospective cohort study. Ann. Med. Surg. 2012(16), 23–29 (2017).
Eng, O. S. et al. Intraoperative fluid administration is associated with perioperative outcomes in pancreaticoduodenectomy: a single center retrospective analysis: fluid outcomes pancreaticoduodenectomy. J. Surg. Oncol. 108, 242–247 (2013).
Behman, R. et al. Impact of fluid resuscitation on major adverse events following pancreaticoduodenectomy. Am. J. Surg. 210, 896–903 (2015).
Healy, M. A. et al. Intraoperative fluid resuscitation strategies in pancreatectomy: results from 38 hospitals in Michigan. Ann. Surg. Oncol. 23, 3047–3055 (2016).
Grant, F. et al. Prospective randomized controlled trial of liberal vs restricted perioperative fluid management in patients undergoing pancreatectomy. Ann. Surg. 264, 591–598 (2016).
Weinberg, L. et al. Restrictive intraoperative fluid optimisation algorithm improves outcomes in patients undergoing pancreaticoduodenectomy: a prospective multicentre randomized controlled trial. PLoS ONE 12, e0183313 (2017).
Fischer, M. et al. Relationship between intraoperative fluid administration and perioperative outcome after pancreaticoduodenectomy: results of a prospective randomized trial of acute normovolemic hemodilution compared with standard intraoperative management. Ann. Surg. 252, 952–958 (2010).
Chen, B. P. et al. Systematic review and meta-analysis of restrictive perioperative fluid management in pancreaticoduodenectomy. World J. Surg. 42, 2938–2950 (2018).
Edwards, M. R. et al. Optimisation of Perioperative Cardiovascular Management to Improve Surgical Outcome II (OPTIMISE II) trial: study protocol for a multicentre international trial of cardiac output-guided fluid therapy with low-dose inotrope infusion compared with usual care in patients undergoing major elective gastrointestinal surgery. BMJ Open 9, e023455 (2019).
Pache, B. et al. Receiver operating characteristic analysis to determine optimal fluid management during open colorectal surgery. Colorectal Dis. 21, 234–240 (2019).
Grass, F. et al. Stringent fluid management might help to prevent postoperative ileus after loop ileostomy closure. Langenbecks Arch. Surg. 404, 39–43 (2019).
Bruns, H., Kortendieck, V., Raab, H.-R. & Antolovic, D. Intraoperative fluid excess is a risk factor for pancreatic fistula after partial pancreaticoduodenectomy. HPB Surg. 2016, 1–6 (2016).
Labgaa, I. et al. Impact of postoperative weight gain on complications after liver surgery. HPB. 22, 744–749 (2020).
Garland, M. L., Mace, H. S., MacCormick, A. D., McCluskey, S. A. & Lightfoot, N. J. Restrictive versus liberal fluid regimens in patients undergoing pancreaticoduodenectomy: a systematic review and meta-analysis. J. Gastrointest. Surg. 23, 1250–1265 (2019).
Odor, P. M., Bampoe, S., Gilhooly, D., Creagh-Brown, B. & Moonesinghe, S. R. Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ 368, m540 (2020).
Myles, P. S. et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N. Engl. J. Med. 378, 2263–2274 (2018).
Winer, L. K. et al. Perioperative net fluid balance predicts pancreatic fistula after pancreaticoduodenectomy. J. Gastrointest. Surg. 22, 1743–1751 (2018).
Han, I. W. et al. Excess intraoperative fluid volume administration is associated with pancreatic fistula after pancreaticoduodenectomy: a retrospective multicenter study. Medicine 96, e6893 (2017).
Wang, S. et al. The effect of intraoperative fluid volume administration on pancreatic fistulas after pancreaticoduodenectomy. J. Investig. Surg. Off. J. Acad. Surg. Res. 27, 88–94 (2014).
Bannone, E. et al. Postoperative acute pancreatitis following pancreaticoduodenectomy: a determinant of fistula potentially driven by the intraoperative fluid management. Ann. Surg. 268, 815–822 (2018).
von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int. J. Surg. Lond. Engl. 12, 1495–1499 (2014).
Dindo, D., Demartines, N. & Clavien, P.-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240, 205–213 (2004).
Slankamenac, K., Graf, R., Barkun, J., Puhan, M. A. & Clavien, P.-A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann. Surg. 258, 1–7 (2013).
Jammer, I. et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 32, 88–105 (2015).
Bassi, C. et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138, 8–13 (2005).
Wente, M. N. et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142, 20–25 (2007).
Wente, M. N. et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142, 761–768 (2007).
Bassi, C. et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161, 584–591 (2017).
Acknowledgements
The authors of this manuscript would like to thank Valérie Addor, enhanced recovery-dedicated clinical nurse at Lausanne University Hospital. This study was presented at the Swiss Surgery Congress (SGC) 2018 and International Hepato-Pancreato-Biliary Association (IHPBA) World Congress 2018.
Author information
Authors and Affiliations
Contributions
N.H., M.H., N.D. and D.R. conceived the study, J.G. and D.R. collected and analyzed the data, J.G., N.H., M.H., N.D. and D.R. interpreted the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gilgien, J., Hübner, M., Halkic, N. et al. Perioperative fluids and complications after pancreatoduodenectomy within an enhanced recovery pathway. Sci Rep 10, 17898 (2020). https://doi.org/10.1038/s41598-020-74907-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74907-y
This article is cited by
-
Framework, component, and implementation of enhanced recovery pathways
Journal of Anesthesia (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.