Abstract
‘Filth flies’ facilitate the dispersal of pathogens between animals and humans. The objective was to study the intestinal colonization with antimicrobial resistant and enteropathogenic bacteria in ‘filth flies’ from Nigeria. Flies from Southern Nigeria were screened for extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), Staphylococcus aureus, Salmonella sp., Shigella sp., Campylobacter sp. and Yersinia enterocolitica by culture. ESBL-E were tested for blaSHV, blaCTX-M and blaTEM; S. aureus was screened for enterotoxins. Spa typing and multilocus sequence typing (MLST) was done for S. aureus and MLST for Escherichia coli. Of 2,000 flies, 400 were randomly collected for species identification. The most common species were Musca domestica (44.8%, 179/400), Chrysomya putoria (21.6%, 85/400) and Musca sorbens (18.8%, 75/400). Flies were colonized with S. aureus (13.8%, 275/2,000) and ESBL-E (0.8%, 16/2,000). No other enteropathogenic bacteria were detected. The enterotoxin sei was most common (26%, 70/275) in S. aureus, followed by sea (12%, n = 32/275). Four S. aureus isolates were methicillin resistant (mecA positive, t674 and t5305, ST15). The blaCTX-M (n = 16) was the most prevalent ESBL subtype, followed by blaTEM (n = 8). ‘Filth flies’ can carry antimicrobial resistant bacteria in Nigeria. Enterotoxin-positive S. aureus might be the main reason for food poisoning by ‘filth flies’ in the study area.
Similar content being viewed by others
Introduction
Diptera (true flies) are the most abundant and diversified endopterygota (or holometabola) of the insect order, with more than 180 families of about 158,000 described species1,2. ‘Filth flies’ (diptera) are universal, ubiquitous, coprophagic and synanthropic (living in close association with humans) insects that breed in garbage, animal and human faeces3. Known to serve as vectors of many pathogens, house flies can disperse pathogens through a flight distance of about 7 km between animals and humans4.
Flies transmit pathogens through three routes: mechanical translocation from the exoskeleton, regurgitation and defecation3. During feeding, flies can either pick up pathogens on its exoskeletal surfaces or ingest fluids contaminated with pathogens. Ingested pathogens can multiply in the crop (a blind sac of the digestive tract in higher flies) and after regurgitation which coined the term of “bioenhanced transmission”5.
Antimicrobial resistance (AMR) affects both humans and animals and antimicrobial resistant bacteria can be transmitted between animals and humans in both direction. This challenge is considered in the “one Health” approach. The most widespread resistance mechanisms in Enterobacterales is based on plasmid-mediated production of extended spectrum β-lactamases (ESBL) which hydrolyse β-lactam rings, thereby reducing the efficacy of cephalosporins and monobactams6. Flies are important reservoirs and vectors of antimicrobial resistant bacteria (such as methicillin resistant Staphylococcus aureus, ESBL-producing Enterobacterales [ESBL-E])3,7. One short report suggests that antimicrobial resistant bacteria can also be detected in flies (n = 25) in sub-Saharan Africa8. However, the true burden of AMR in ‘filth flies’ in Africa is unknown and it is currently unclear which fly species are the main vectors of ESBL-E and enteropathogenic bacteria. Therefore, the objective of this study was to analyse the colonization rates of ‘filth flies’ from Southern Nigeria with ESBL-E, S. aureus and other enteropathogenic bacteria and to identify those fly species which are mainly colonized with these target organisms.
Results
Sampling sites
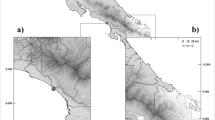
In total, 109 sites were sampled, with a total area cover of 15,075,000 m2 (Fig. 1). A total of 2,000 flies were captured with a mean number of 18.3 (± SD 5.8) flies per sampling spot. Approximately 40 flies were caught per hour. The mean (± SD) atmospheric parameters were a temperature of 26 °C (± 1.6), relative humidity of 86.5% (± 6.2), windfall of 5.6 Beaufort (± 1.4), air pressure of 1012.4 hPa (± 1.3) and 13 sunshine hours (± 0.4). The sampled sites were urban (8.3%, 9/109), semi-urban (61.4%, 67/109) and rural (30.3%, 33/109). Within a 10 m radius of each sampling site (n = 109), we recorded decomposing organic matter (79.8%, 87/109), refuse dump (84%, 92/109) and animal faeces (49%, 49/109, Table 1).
Study area and sampling sites. Each dot represents one of the 109 sampling sites. The size of each dot represents the number of flies. The size of the inner circle corresponds to the number of ESBL-E (black circle) or S. aureus colonized flies (light-grey circle) found in that specific site. Grey dots without inner black or light-grey circle indicate that no flies were colonized with ESBL-E or S. aureus. Sampling sites were positive for S. aureus (n = 45; 41%) or ESBL-E (n = 7; 6%). We used geodata from openstreetmap (https://www.openstreetmap.org) to render our own map using the package “ggplot2” as implemented in “R” (OpenStreetMap contributors)25.
Intestinal culturome of flies
The most common species of the intestinal culturome of 82 randomly selected flies were Bacillus cereus (73%, 60/82), followed by Enterococcus faecalis (27%, 22/82), Enterococcus faecium (24%, 20/82), Clostridioides tertium (21%, 17/82), Bacillus licheniformis (11%, 9/82), Bacillus subtilis (11%, 9/82), Enterococcus hirae (11%, 9/82) and others (70%, 57/82, Figure S1).
S. aureus
A total of 275 flies (13.8%) from 45 sites were colonized with S. aureus. Since S. schweitzeri is frequent in African wildlife, we tested if some isolates were misidentified by MALDI-TOF as S. aureus. All isolates were nuc positive and harboured the 160 bp-fragment of NRPS, thus ruling out S. schweitzeri in our collection9. The most predominant spa type was t674 (98%, 270/275) followed by singular occurrences of t1980, t5305 and t6313 (0.4%, 1/275 each, two isolates were not spa typeable). The four distinctive spa types belonged to MLST ST15 and showed similar spa repeat patterns (t674: 07-34-12-23-02-12-23, t1980: 07-34-12-23, t5305: 07-34-12-23-02-12, t6313: 07-34-12-23-12-23). Since the majority of isolates belonged to t674, WGS was used to increase the discriminatory power in order to distinguish these isolates (Fig. 2). All isolates belonging to t674 differed by ≤ 3 alleles from the most closely related isolate.
Minimum spanning tree of randomly selected Staphylococcus aureus belonging to spa type t674 (ST 15). The tree was constructed based on up to the 1,861 genes of the S. aureus core genome (cg)MLST. The first numbers in each node indicate the fly identification number while the second number indicates the spa type. The numbers on the lines connecting each node indicate the number of differing alleles.
The predominant antimicrobial resistance was against clindamycin (8.4%, 23/275) followed by tetracycline (2.2%, 6/275), penicillin (1.5%, 4/275), oxacillin (1.5%, 4/275), rifampicin (1.5%, 4/275), fusidic acid (0.8%, 2/275) and trimethoprim/sulfamethoxazole (0.4%, 1/275). All S. aureus isolates were susceptible to fluoroquinolones, macrolides, glycopeptides, gentamicin, linezolid, tigecycline and daptomycin. Four isolates were MRSA-ST15 (mecA positive, t5305 [n = 1], t674 [n = 3]). They were found in two different sampling spots (05°56′486" N, 007°25′448" E and 06°26′048" N, 007°28′805" E). All isolates were negative for lukF-PV/lukS-PV and all penicillin-resistant isolates (1.5%, 4/275) were positive for blaZ. S. aureus isolates were positive for chp (100%, 275/275) and scn (97%, 268/275) while none carried sak (0%, 0/275). The staphylococcal enterotoxin gene sei was predominant (26%, 70/275) followed by sea (12%, 32/275). Other staphylococcal enterotoxins (seb, sec, sed, see, sef, seg and seh) were not detected.
While S. aureus positive flies were less likely detected in semi-urban settings, a lower mean temperature, higher air pressure and a lower wind force were environmental factors that favoured the detection of flies with an intestinal colonization of S. aureus (Table 1).
The fly species that carried S. aureus (n = 275) were M. domestica (47.3%, 130/275), followed by Chrysomya putoria (20.4%, 56/275), Musca sorbens (19.6%, 54/275), Sacrophaga africa (8.0%, 22/275), Chrysomya megacephala (2.9%, 8/275), Lucilia cuprina (1.5%, 4/275) and M. crassirostris (0.3%, 1/275, Table S1). The randomly selected non-colonized fly species (n = 109) were M. domestica (41.3%, 45/109), C. putoria (26.6%, 29/109), M. sorbens (13.8%, 15/109), S. africa (9.2%, 10/109), Chrysomya albiceps (3.7%, 4/109), Lucilia porphyrina (2.8%, 3/109), Sacrophaga cultellata (1.8%, 2/109) and Sacrophaga crassipalsi (0.9%, 1/109). S. aureus was neither associated with a specific fly species (Table S1) nor with the genus of the fly (p ≥ 0.08).
ESBL-E
A total of 16 flies (0.8%) collected from seven sites (6.4%) carried ESBL-E (Fig. 1) and were found mostly within a radius of 2.0 km of the Abia State University Hospital (5° 49′ 278" N, 7° 23′ 772" E). ESBL-producing E. coli (n = 15) was predominant followed by Enterobacter cloacae complex (n = 1). ESBL-producing E. coli were resistant to ceftriaxone (100%, n = 15/15), ceftazidime (93%, n = 14/15), aztreonam (100%, n = 15/15), ciprofloxacin (53%, n = 8/15) and trimethoprim-sulfamethoxazole (100%, n = 15/15). All ESBL-E were susceptible to piperacillin/tazobactam, carbapenems, amikacin, tigecycline, fosfomycin and colistin.
The most frequent ESBL group was blaCTX-M (100%, 16/16), followed by blaTEM (33%, 8/16) with no occurrence of blaSHV. CTX-M-15 was the most predominant subgroup (10/16), followed by CTX-M-1 (5/16) and CTX-M-27 (1/16). Among TEM beta-lactamases, TEM 1 was predominant (7/8) followed by TEM 116 (1/8). All ESBL isolates were negative for the blaCMY-2 genes.
The majority of ESBL-producing E. coli were not grouped in any phylogroup and were consequently subjected to MLST (ST10 [n = 5], ST 617 [n = 2], and singular occurrences of ST40, ST44, ST46, ST218, ST443 and ST940, Fig. 3). Only two isolates belonged to phylogroup D (n = 1) and B2 (n = 1).
Minimum spanning tree of 13 non-phylogroupable ESBL-producing Escherichia coli. The tree was constructed based on the allelic profile of the up to 4671 genes included in the core genome multilocus sequence typing (cgMLST) scheme of the reference strain Sakai. The first number in each circle indicate the identifier of the fly while the second number indicates the MLST sequence type (ST). The numbers on the lines connecting each circle indicates the number of differing alleles.
The predominant fly species that carried ESBL-E were M. domestica (44.0%, 7/16), M. sorbens (38.0%, 6/16), C. putoria (12.0%, 2/16) and S. africa (6.0%, 1/16).
Enteropathogenic bacteria
None of the flies carried Salmonella sp., Shigella sp., Campylobacter sp. or Yersinia sp.
Discussion
A total of 2,000 flies from Southern Nigeria were screened for the intestinal colonization with antimicrobial resistant and enteropathogenic bacteria. Our main findings are a high proportion of S. aureus (13.8%, 275/2,000) and low occurrence of ESBL-E (0.8%, 16/2,000).
The analysis of the intestinal culturome was done to assess the occurrence of bacterial species independent of the resistance phenotype. In general, Enterobacterales were only rarely reported (e.g. K. pneumoniae, n = 1/82, Figure S1) which is in line with an overall low colonization rate with ESBL-E in flies (0.8%). The vast majority of isolates belonged to the genera Bacillus and Clostridioides suggesting a selection of spore-forming bacteria by the treatment with ethanol. However, we confirmed the observation that ethanol sanitation of the exoskeleton does not alter the intestinal colonization10. Although the culturome was assessed under aerobic conditions, some Clostridioides species were detected. This applies for those species, that are known to be aerotolerant (i.e. C. histolyticum, C. terium, Fig. S1)11.
The S. aureus intestinal colonization rate in our study (13.8%) is in stark contrast to a 0.4% colonization rate in flies in a comparable approach (i.e. same trap and bait) from Germany12. The differing proportions are most likely due to the different settings (tropical vs. temperate regions). All S. aureus from our study shared a very similar genetic background based on the spa repeat pattern, ST and cgMLST (Fig. 2). This is suggestive either for a common source for all isolates from flies or a cross-contamination between flies during sampling or an adaptation (due to e.g. fitness factors) of this ST15 lineage to the intestinal tract of the flies. An artificial contamination of flies would challenge the scientific significance of our work. However, the widespread detection of S. aureus from various sampling site (Fig. 1), both the absence and presence of mecA in isolates belonging to t674, different antimicrobial resistance rates, the high diversity of ESBL-E sampled simultaneously and the known effective sanitation of the exoskeleton by ethanol argues against a cross-contamination during sampling13. All isolates belonged to ST15, which is in line with a predominance of the clonal complex CC15 in isolates from community-acquired infection in sub-Saharan Africa14. In addition, CC15 is known to be well adapted to the human host15. However, the low antimicrobial resistance rates, the absence of lukF-PV/lukS-PV and sak might also suggest that S. aureus from flies are rather of animal (e.g. livestock or wildlife) than of human origin16,17,18. However, other factors not investigated in our study (e.g. adaptation of S. aureus to the gut of ‘filth flies’, fitness factors) could explain the predominance of ST15/t674-S. aureus in flies.
Among all S. aureus from ‘filth flies’, we only detected sea and sei. The superantigenic activity of both is superior to other staphylococcal enterotoxins (e.g. members of the SEB group) due to an additional high-affinity MHC II binding site19.
The intestinal colonization rate of ‘filth flies’ with ESBL-E was low (0.8%, 16/2,000) compared to a similar study from Germany (3.3%, 44/1346)12. This finding is surprising as ESBL-E rates are high both in asymptomatic carriers (33.6%, rectal) and among Enterobacterales from bloodstream infection (12.1–15%) in sub-Saharan Africa20,21,22.
Although our study has strengths (e.g. large sample size, broad bacterial culture), some limitations need to be addressed: first, culturome results revealed the abundance of Bacillus and Clostridioides suggesting a selection of spore-forming genera by ethanol. This might point towards a methodological bias of our work. However, others have also reported a higher prevalence of Bacillus cereus than Enterobacteriaceae23. Second, despite poor sanitation systems and access of flies to human and animal faeces, we did not detect any other enteropathogens (e.g. Salmonella sp., Shigella sp.). Since we were unable to culture the fly samples immediately, some isolates might have not stayed viable during storage and transport.
In conclusion, diptera-borne S. aureus food poisoning might be or become a health issue in the study region due to the high prevalence of enterotoxins (sea, sei) in S. aureus from ‘filth flies’. In contrast, a transmission of ESBL-E through flies by defecation and regurgitation does not seem to play a major role.
Materials and methods
Ethical statement
An ethical approval is not required for the analysis of invertebrates. All methods were carried out in accordance with relevant guidelines and regulations. The local Ph.D. committee, Medical Faculty, Westfälische Wilhelms-Universität Münster, approved all experimental protocols.
Study area/mapping
‘Filth flies’ were collected in Southern Nigeria between June and July 2017. Sampling sites were classified into “urban”, “semi-urban” and “rural” according to the European Union Methodological manual on territorial typologies24. GPS coordinates were taken for each sampling site (eTrex 10, Garmin, Olathe, Kansas, USA). For every sampling spot, key environmental conditions were documented (i.e. livestock/human faeces within 10 m radius, presence of refuse dump, presence of decomposing organic matters and setting [urban, semi-urban, rural]). Atmospheric conditions (i.e. humidity, temperature, air pressure, wind force, sunshine hours) were recorded as reported by the Nigerian Meteorological Agency (NiMet, https://nimet.gov.ng/).
The maps were downloaded as jpg-files from openstreetmap (https://www.openstreetmap.org) with bounding boxes (latitude, longitude) related to the region of interest and used as a background for the plots. All plots were produced with “R” (Version 3.6.2) using the package “ggplot2” (grammar of graphics, version 3.2.0)25. The data used for the plots were taken from the original files and, where necessary, transformed by methods from the package “dplyr” (grammar of data manipulation, version 0.8.3).
Diptera
Flies (n = 2,000) were collected using the Gaze trap method12. Insect bait, conventionally made from animal proteins, carbohydrates and sugar (Feldner, Waldsee, Germany) in a container covered with gaze and placed under the gaze trap was used to lure the flies. Trapped flies (approximately 20/sampling site) were collected and killed in 70% ethanol and dried in silica gel (2–4 mm, Carl Roth, Karlsruhe, Germany). Ethanol sanitizes the outer surfaces of the flies, avoiding cross-contamination during sampling without altering the intestinal microbiome of the flies10.
Each fly was sent for further analysis to Germany in 1 ml sterile sodium chloride (0.45%) at -18 °C (Peli Biothermal Credo Cube, UK).
Culturome of random flies
We analysed the culturome of 82 randomly selected flies in order to describe the overall colonization pattern of flies with bacteria independent of the AMR phenotype. Since our target bacterial species (e.g. Escherichia coli, Klebsiella sp.) are aerobic bacteria, we did not use specific anaerobic conditions for culture. After removal of the legs and wings for molecular species identification, the remaining body (head, thorax and abdomen) was mechanically homogenized and cultured in 1 ml BHI broth overnight (37 °C, ambient air). A total of 10 µl of overnight culture was sub-cultured on MacConkey Agar (Oxoid GmbH, Wesel, Deutschland), Columbia Blood Agar (Oxoid), Trypticase Soy Agar (BD, Heidelberg, Germany), Kimmig Agar (Oxoid), Chocolate Agar (BD) and Colistin-Aztreonam (CAP) Agar (Oxoid). All phenotypically different colonies were selected for species identification using Matrix-Assisted Laser Desorption/Ionization-Time-Of-Flight (MALDI-TOF, microflex LT, Bruker Daltonics, Bremen, Germany).
ESBL-E and enteropathogenic bacteria
After mechanical homogenization, flies (i.e. head, thorax and abdomen) were cultured in 1 ml BHI overnight (37 °C, ambient air). Of this overnight culture, 10 µl were sub-cultured each on Columbia Blood Agar (for growth control) and selective media for the detection of S. aureus (SAID, bioMérieux, Marcy l’Etoile, France), ESBL-E (chromID, bioMérieux), Shigella sp. and Salmonella sp. (SS agar, Oxoid), Campylobacter sp. (Campylobacter, Oxoid) and Yersinia sp. (CIN, Oxoid). For Salmonella sp., 10 µl of the overnight culture was additionally transferred to Müller-Kaufmann Tetrathionate-Novobiocin broth (Oxoid) for a second overnight enrichment step and then sub-cultured on Önöz Agar (Oxoid).
Identification and characterization
Species of suspected S. aureus was identified using MALDI-TOF and confirmed by the detection of a species specific polymorphism of the non-ribosomal peptide synthetase (NRPS)26 and the S. aureus specific thermostable nuclease (nuc)27. All S. aureus were screened for the immune evasion cluster (IEC)28, the Panton-Valentine leucocidin gene (lukS-PV/lukF-PV)29 and the enterotoxin genes sea, seb, sec, sed, see, sef, seg, seh and sei30,31.
Enterobacterales were identified with VITEK2 automated systems (bioMérieux) due to ambiguous delineation of E. coli and Shigella sp. using MALDI-TOF.
Antimicrobial resistance
The antimicrobial susceptibility testing was done with VITEK2 automated systems (bioMérieux) using EUCAST clinical breakpoints (Version 9.0). ESBL-E were confirmed using the double disc diffusion test (Mast diagnostics, Bootle, UK) and were screened for the presence of blaSHV, blaCTX-M, blaTEM and blaCMY-232,33. Subtypes of the detected beta-lactamase genes were determined by Sanger sequencing.
All S. aureus isolates were screened for the staphylococcal beta-lactamase blaZ34.
Genotyping
All S. aureus were spa typed and one isolate per spa type was selected for multilocus sequence typing (MLST)35,36. Using an online randomization tool (www.random.org), 13 S. aureus isolates were randomly selected for whole genome sequencing (WGS). This was done in order to understand the extent of similarity between the isolates on a whole genome level.
ESBL-producing E. coli were phylogrouped37. Non-phylogroupable strains were subjected to WGS to deduce the MLST sequence type (ST).
Whole genome sequencing
Genomes of selected isolates of ESBL-E and S. aureus were sequenced on an Illumina MiSeq platform (Illumina Inc., San Diego, USA) with a ≥ 75-fold coverage. Quality trimming and de novo assembly using the Velvet assembler was done with SeqSphere + (version 5.9.0; Ridom GmbH, Münster, Germany)38. Neighbor-joining trees were constructed based on the cgMLST scheme to assess the clonal relation of the tested isolates39,40.
Fly species identification
All flies being colonized with the target organisms (n = 291) plus 109 randomly selected non-colonized flies from each of the sampling spots were selected for species identification. Legs and wings of flies were crushed using a sterile pestle for DNA extraction (QIAamp DNA Mini Kit, Qiagen, Hilden, Germany). The washing and DNA purification were done according to the manufacturer’s instruction. After amplification, the cytochrome oxidase gene (coi) was Sanger sequenced and query sequences were aligned with sequences deposited at the BLAST database for nucleotides41. The best match (≥ 99% coverage, ≥ 99% identity) was selected for species identification.
Statistical analysis
Proportions of categorical variables (e.g. colonization with ESBL-E, S. aureus) were compared using the Pearson's chi-squared test or Fisher’s exact test where appropriate and normally distributed continuous variables were compared with student’s t-test. All environmental factors potentially associated with the detection of S. aureus in flies (p < 0.25) were entered in a multivariate logistic regression model with a stepwise backward elimination42. The significance level was set at 0.05. Analyses were done using “R”25.
The datasets generated during and/or analysed during the current study as well as all bacterial strains are available from the corresponding author on reasonable request.
References
Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 34, 1252–1255. https://doi.org/10.1042/BST0341252 (2006).
Wiegmann, B. M. & Richards, S. Genomes of Diptera. Curr. Opin. Insect Sci. 25, 116–124. https://doi.org/10.1016/j.cois.2018.01.007 (2018).
Onwugamba, F. et al. The role of `filth flies’ in the spread of antimicrobial resistance. Travel Med. Infect. Dis. https://doi.org/10.1016/j.tmaid.2018.02.007 (2018).
Graczyk, T. K., Knight, R. & Tamang, L. Mechanical transmission of human protozoan parasites by insects. Clin. Microbiol. Rev. 18, 128–132. https://doi.org/10.1128/cmr.18.1.128-132.2005 (2005).
Kobayashi, M. et al. Houseflies: not simple mechanical vectors of enterohemorrhagic Escherichia coli O157:H7. Am. J. Trop. Med. Hyg. 61, 625–629 (1999).
Madec, J. Y., Haenni, M., Nordmann, P. & Poirel, L. Extended-spectrum beta-lactamase/AmpC- and carbapenemase-producing Enterobacteriaceae in animals: a threat for humans?. Clin. Microbiol. Infect. 23, 826–833. https://doi.org/10.1016/j.cmi.2017.01.013 (2017).
Usui, M., Shirakawa, T., Fukuda, A. & Tamura, Y. The Role of Flies in Disseminating Plasmids with Antimicrobial-Resistance Genes Between Farms. Microbial drug resistance (Larchmont, N.Y.)21, 562–569. https://doi.org/10.1089/mdr.2015.0033 (2015).
Odetoyin, B., Adeola, B. & Olaniran, O. Resistance patterns of bacterial species isolated from the body surface of the housefly (Musca domestica) in Akure, Ondo State, Nigeria. J Arthropod Borne Dis. 14, 88–96 (2020).
Becker, K. et al. Implications of identifying the recently defined members of the Staphylococcus aureus complex S. argenteus and S. schweitzeri: a position paper of members of the ESCMID Study Group for Staphylococci and Staphylococcal Diseases (ESGS). Clin. Microbiol. Infect.https://doi.org/10.1016/j.cmi.2019.02.028 (2019).
Gupta, A. K. et al.Phylogenetic characterization of bacteria in the gut of house flies (Musca domestica L.). FEMS Microbiol. Ecol.79, 581–593. https://doi.org/10.1111/j.1574-6941.2011.01248.x (2012).
Wells, C. L. & Wilkins, T. D. in Medical Microbiology (ed S. Baron) (The University of Texas Medical Branch at Galveston, 1996).
Schaumburg, F. et al. A geospatial analysis of flies and the spread of antimicrobial resistant bacteria. Int. J. Med. Microbiol. 306, 566–571. https://doi.org/10.1016/j.ijmm.2016.06.002 (2016).
Sproston, E. L. et al. Multi-locus sequence types of Campylobacter carried by flies and slugs acquired from local ruminant faeces. J. Appl. Microbiol. 109, 829–838. https://doi.org/10.1111/j.1365-2672.2010.04711.x (2010).
Ruffing, U. et al. Community-associated Staphylococcus aureus from Sub-Saharan Africa and Germany: a cross-sectional geographic correlation study. Sci. Rep. 7, 154. https://doi.org/10.1038/s41598-017-00214-8 (2017).
Richardson, E. J. et al. Gene exchange drives the ecological success of a multi-host bacterial pathogen. Nat. Ecol. Evol. 2, 1468–1478. https://doi.org/10.1038/s41559-018-0617-0 (2018).
Olatimehin, A. et al. Staphylococcus aureus Complex in the Straw-Colored Fruit Bat (Eidolon helvum) in Nigeria. Front. Microbiol. 9, 162. https://doi.org/10.3389/fmicb.2018.00162 (2018).
Schaumburg, F. et al. Highly divergent Staphylococcus aureus isolates from African non-human primates. Environ. Microbiol. Rep. 4, 141–146. https://doi.org/10.1111/j.1758-2229.2011.00316.x (2012).
Senghore, M. et al. Whole-genome sequencing reveals transmission of Staphylococcus aureus from humans to green monkeys in The Gambia. Appl. Environ. Microbiol. 82, 5910–5917. https://doi.org/10.1128/aem.01496-16 (2016).
Fisher, E. L., Otto, M. & Cheung, G. Y. C. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 9, 436. https://doi.org/10.3389/fmicb.2018.00436 (2018).
Schaumburg, F. et al. High burden of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Gabon. J. Antimicrob. Chemother. 68, 2140–2143. https://doi.org/10.1093/jac/dkt164 (2013).
Flokas, M. E., Karanika, S., Alevizakos, M. & Mylonakis, E. Prevalence of ESBL-producing Enterobacteriaceae in pediatric bloodstream infections: a systematic review and meta-analysis. PLoS ONE 12, e0171216. https://doi.org/10.1371/journal.pone.0171216 (2017).
Toy, T. et al. Multicountry distribution and characterization of extended-spectrum beta-lactamase-associated gram-negative bacteria from bloodstream infections in Sub-Saharan Africa. Clin. Infect. Dis. 69, S449-s458. https://doi.org/10.1093/cid/ciz450 (2019).
Bahrndorff, S., de Jonge, N., Skovgard, H. & Nielsen, J. L. Bacterial communities associated with houseflies (Musca domestica L.) sampled within and between farms. PLoS ONE12, e0169753. https://doi.org/10.1371/journal.pone.0169753 (2017).
Regional statistics team. Updated urban-rural typology: integration of NUTS 2010 and the latest population grid, <https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Archive:Urban-rural_typology_update&oldid=209496> (2013).
R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Zhang, D. F. et al. Identification of Staphylococcus argenteus in Eastern China based on a nonribosomal peptide synthetase (NRPS) gene. Fut. Microbiol. 11, 1113–1121. https://doi.org/10.2217/fmb-2016-0017 (2016).
Brakstad, O. G., Aasbakk, K. & Maeland, J. A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30, 1654–1660 (1992).
van Wamel, W. J. B., Rooijakkers, S. H. M., Ruyken, M., van Kessel, K. P. M. & van Strijp, J. A. G. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on β-hemolysin-converting bacteriophages. J. Bacteriol. 188, 1310–1315. https://doi.org/10.1128/jb.188.4.1310-1315.2006 (2006).
Lina, G. et al. Involvement of panton-valentine leukocidin-producing staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis.https://doi.org/10.1086/313461 (1999).
Becker, K. et al. Prevalence of genes encoding pyrogenic toxin superantigens and exfoliative toxins among strains of Staphylococcus aureus isolated from blood and nasal specimens. J. Clin. Microbiol. 41, 1434–1439 (2003).
Becker, K., Roth, R. & Peters, G. Rapid and specific detection of toxigenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J. Clin. Microbiol. 36, 2548–2553 (1998).
Monstein, H. J. et al. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 115, 1400–1408. https://doi.org/10.1111/j.1600-0463.2007.00722.x (2007).
Souna, D., Amir, A. S., Bekhoucha, S. N., Berrazeg, M. & Drissi, M. Molecular typing and characterization of TEM, SHV, CTX-M, and CMY-2 beta-lactamases in Enterobacter cloacae strains isolated in patients and their hospital environment in the west of Algeria. Med. Mal. Infect. 44, 146–152. https://doi.org/10.1016/j.medmal.2014.01.008 (2014).
Kaase, M. et al. Comparison of phenotypic methods for penicillinase detection in Staphylococcus aureus. Clin. Microbiol. Infect. 14, 614–616. https://doi.org/10.1111/j.1469-0691.2008.01997.x (2008).
Enright, M. C., Day, N. P. J., Davies, C. E., Peacock, S. J. & Spratt, B. G. Multilocus sequence typing for characterization of methicillin resistant and methicillin susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38, 1008–1015 (2000).
Mellmann, A. et al. Automated DNA sequence-based early warning system for the detection of methicillin-resistant Staphylococcus aureus outbreaks. PLoS Med.https://doi.org/10.1371/journal.pmed.0030033 (2006).
Clermont, O., Christenson, J. K., Denamur, E. & Gordon, D. M. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 5, 58–65. https://doi.org/10.1111/1758-2229.12019 (2013).
Mellmann, A. et al. Real-time genome sequencing of resistant bacteria provides precision infection control in an institutional setting. J. Clin. Microbiol. 54, 2874–2881. https://doi.org/10.1128/jcm.00790-16 (2016).
Leopold, S. R., Goering, R. V., Witten, A., Harmsen, D. & Mellmann, A. Bacterial whole-genome sequencing revisited: portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J. Clin. Microbiol. 52, 2365–2370. https://doi.org/10.1128/JCM.00262-14 (2014).
Mellmann, A. et al. High interlaboratory reproducibility and accuracy of next-generation-sequencing-based bacterial genotyping in a ring trial. J. Clin. Microbiol. 55, 908–913. https://doi.org/10.1128/jcm.02242-16 (2017).
Zehner, R. et al. Genetic identification of forensically important flesh flies (Diptera: Sarcophagidae). Int. J. Legal Med. 118, 245–247. https://doi.org/10.1007/s00414-004-0445-4 (2004).
Schaumburg, F. et al. Acquisition and colonization dynamics of antimicrobial-resistant bacteria during international travel: a prospective cohort study. Clin. Microbiol. Infect. 25(1287), e1281-1287.e1287. https://doi.org/10.1016/j.cmi.2019.03.002 (2019).
Acknowledgements
Special thanks goes to the students Ojeh Emmanuel Uwadiegwu, Ekwu Chinonso Emmanuel, Nwaulu Onyekachi and Agwo Chinedu Emmanuel of Abia State University for their participation and assistance through the sampling period. This work was in part supported by institutional funds and the Deutsche Forschungsgemeinschaft (SCHA 1994/5-1).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.S. conceived and supervised the project and analysed data. F. O. performed the experiments and analysed data. A.M. performed and analysed whole genome sequencing. V.O.N. hosted F.O. during the field study in Nigeria, B.S. performed the geospatial analyses. F.O. and F.S. drafted the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onwugamba, F.C., Mellmann, A., Nwaugo, V.O. et al. Antimicrobial resistant and enteropathogenic bacteria in ‘filth flies’: a cross-sectional study from Nigeria. Sci Rep 10, 16990 (2020). https://doi.org/10.1038/s41598-020-74112-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74112-x
This article is cited by
-
Zoonotic sources and the spread of antimicrobial resistance from the perspective of low and middle-income countries
Infectious Diseases of Poverty (2023)
-
Higher seasonal temperature enhances the occurrence of methicillin resistance of Staphylococcus aureus in house flies (Musca domestica) under hospital and environmental settings
Folia Microbiologica (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.