Abstract
The purpose of this study was to analyze the correlation of renal function indices with sub-foveal choroidal thickness (SFChT) in treatment-naïve (naïve) eyes with retinal vein occlusion (RVO) using swept-source optical coherence tomography (SS-OCT) and systemic workup. Retrospective chart review was performed from Dec 2016 to Sep 2019 in patients newly diagnosed with treatment-naïve unilateral RVO. Ocular parameters, including SFChT, and systemic profiles, including renal function indices, were reviewed. Simple and multiple linear regression analyses were performed to check if there was a correlation between renal profiles and SFChT. A total of 56 patients were included in the study; 34 of them were branch RVO and 22 were central RVO patients. Multiple linear regression analysis revealed that SFChT was positively correlated with estimated glomerular filtration rate (eGFR) (P < 0.001). SFChT showed significant correlation with renal function indices. In addition, choroidal thickness may be considered as systemic biomarkers for renal function.
Similar content being viewed by others
Introduction
Retinal vein occlusion (RVO) is a common retinal disease in adults and has a prevalence of 0.52%–0.77%1,2. It is a major cause of visual impairment due to macular edema, retinal ischemia, and neovascular complications3. Epidemic studies have revealed that hypertension (HTN) is the strongest risk factor for RVO1,4. Moreover, RVO is a systemically important disorder because it itself is associated with increased risk of cardiovascular disease5,6.
Optical coherence tomography (OCT) is a useful noninvasive imaging tool for the in vivo visualization of the chorioretinal microstructure7. With recent technical developments, enhanced depth imaging OCT and swept-source OCT (SS-OCT) can visualize the deep choroid layer with accurate segmentation and high clarity8. Using these techniques, several studies have reported changes in sub-foveal choroidal thickness (SFChT) in eyes with RVO8,9. However, they have reported inconsistent findings. Du et al. reported that SFChT was significantly thinner in the RVO group than in the control group8. Tang et al. reported that SFChT was significantly thicker in eyes with RVO than in fellow eyes9. These inconsistent reports of SFChT in eyes with RVO suggest that it is affected by some confounding factors8,9. Moreover, Balmforth et al. reported that choroidal thickness is associated with renal function in patients with chronic kidney disease (CKD)10. Furthermore, Mule et al. also reported a correlation of SFChT with renal function in patients with HTN11,12.
RVO and CKD share a major independent risk factor, HTN1,13. Interestingly, the renal podocyte, which can be damaged by high blood pressure, has very similar microstructure and function as the vascular pericyte14. Thus, we hypothesized impaired kidney function caused by HTN to have a close correlation with SFChT in patients with RVO. Therefore, we analyzed the correlation of renal parameters with SFChT in patients with RVO.
Results
Baseline characteristics
A total of 415 patients with RVO visited the retina clinic, of whom the 56 patients (branched RVO, n = 34; central RVO, n = 22) who met the inclusion criteria and not the exclusion criteria were included in the present study. The mean age of the patients was 58.8 ± 11.9 years (range 18–85 years), mean body mass index (BMI) was 25.5 ± 3.5 kg/m2, and mean onset time for RVO was 32.4 ± 35.9 days (range 1–90 days). Fifty two out of 56 patients had hypertension with well-controlled blood pressure using medications. Six patients had hyperlipidemia. The mean arterial pressure was 93.2 ± 13.5 mmHg (81.1–108.3 mmHg), and the mean duration of hypertension was 6.8 ± 8.4 years (range, 0.5–30 years).
Comparison between RVO eyes and normal fellow eyes
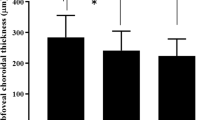
Central retinal thickness (RT) and mean RT were significantly higher in eyes with RVO than in normal fellow eyes (P < 0.001; P < 0.001). SFChT was significantly higher in eyes with RVO (316.1 ± 101.3 μm) than in normal fellow eyes (290.8 ± 100.5 μm) (P = 0.015). The mean choroidal thickness (ChT) was also significantly higher in eyes with RVO (285.6 ± 86.2 μm) than in normal fellow eyes (269.2 ± 59.4 μm) (P = 0.014). The interobserver reproducibility of ChT ranged from 0.986 to 0.993. Ocular parameters in eyes with RVO and normal fellow eyes are compared in Table 1.
Comparison between RVO eyes and normal control eyes
Eyes with RVO were compared with eyes in age-matched normal control group (n = 56). Age, BMI, and IOP were not significantly different between the two groups. Central and mean RT were also significantly higher in eyes with RVO than in normal control eyes (P < 0.001; P < 0.001). However, SFChT and mean ChT were not significantly different between eyes with RVO and normal control eyes (P = 0.167; P = 0.280). The comparison is shown in Table 2.
Simple and multiple linear regression analysis
In simple linear regression analysis, SFChT of normal fellow eyes was significantly associated with BMI (P = 0.028), age (P = 0.003), serum osmolarity (P = 0.035), creatinine (P = 0.002), estimated GFR (estimated glomerular filtration rate) (P < 0.001), and phosphorus levels (P = 0.016). Stepwise multiple linear regression revealed that SFChT had a significant positive correlation with eGFR (P < 0.001; Table 3, Fig. 1).
Discussion
In this study, we evaluated the correlation of renal function with choroidal thickness using SS-OCT. This study revealed that choroid thickness was closely correlated with eGFR in patients with RVO, independently of age and other potentially confounding factors.
The correlation of choroidal thickness with renal function has been rarely described with reports only by few studies10,11,12. Balmforth et al. reported that chorioretinal thinning in patients with CKD is associated with lower eGFR and greater proteinuria10. Mule et al. described a correlation between SFChT and eGFR in patients with HTN11,12. In patients with retinal disease, however, a close correlation has not been identified, even though patients with retinal disease frequently suffer from systemic diseases such as HTN or CKD. Moreover, RVO is a representative disease that is affected by the systemic condition. Thus, we investigated the systemic effect on choroidal thickness in eyes with RVO. Furthermore, there have been conflicting results for choroidal thickness in eyes with RVO. Du et al. reported that SFChT was significantly thinner in the RVO group than in the control group8. Tang et al. reported that SFChT was significantly thicker in eyes with RVO than in fellow eyes9. These inconsistencies in SFChT in eyes with RVO suggest that it is affected by some confounding factors8,9. Thus, these are the reasons why we have tried to reveal the effects of systemic factors in patients with RVO.
BRVO and CRVO are different diseases. However, they have similar phenotypes, similar pathophysiology, and common cardiovascular risk factors15,16. The primary purpose of this study is to reveal the association between choroidal thickness in normal contralateral eyes and systemic states because macular edema and RVO affect choroidal thickness. Thus, we considered BRVO and CRVO under the same category. CRVO usually involves the macula, whereas BRVO may not always involve the macula. Therefore, macular involvement by RVO may affect choroidal thickness. In this study, however, there were no BRVO patients in whom the macula was not involved.
Elevated arterial pressure is a major risk factor of both RVO and kidney damage3,13,17. The glomerulus is the target structure of hypertensive kidney damage due to its extensive vascular network18. The choroid is also a highly vascularized tissue and is very similar to the glomerulus in terms of anatomical structure and physiological function19,20. Moreover, both vascular structures are affected by the regulation of localized and systemic renin–angiotensin–aldosterone hormonal cascades14,21. The anatomic and physiologic similarity of the tissues may lead to simultaneous damage.
The retina has been considered an important organ for non-invasive evaluation of microvasculature through the diagnosis of diabetic or hypertensive retinopathy7,22,23. Our findings suggest that SFChT may be used as a potential biomarker for subclinical or clinical renal dysfunction, even though the parameters would require adjustment for age, axial length, and macular edema.
In addition, we found that SFChT was greater in eyes with RVO than in normal fellow eyes. Several studies reported that the choroid was significantly thicker in eyes with RVO or the occlusive area than in contralateral eyes or the non-occlusive area9,24,25,26. Our results were consistent with these studies. It is known that that the choroid is regulated by autonomic and sensory innervation27. Thus, the first possible explanation for thicker choroid in RVO eyes is that local autoregulatory myogenic mechanism (associated with vascular smooth muscles) caused by ischemic retina leads to compensatory increase in choroidal blood flow and consequently to choroidal thickening27. The second possible explanation is that the choroid becomes edematous passively by the intravitreal elevation of the vascular endothelial growth factor (VEGF) level. Several studies showed that choroidal thickness in eyes with RVO decreased after anti-VEGF injection24,28. From these previous studies, we can hypothesize that the choroid tissue is sensitive to intravitreal VEGF concentration. Accordingly, in this study, we used contralateral eyes without RVO to investigate if there is a correlation between SFChT and renal function. However, in a report by Du et al., SFChT was significantly thinner in the group with RVO than in the group without RVO in a large population-based study8. We additionally compared eyes with RVO and normal control eyes. SFChT was greater in eyes with RVO than in normal control eyes. However, the difference was not significant. In addition, SFChT was lesser in contralateral eyes than in normal control eyes. However, the difference was not significant here either. The systemic status of RVO patients may act as a confounding factor and lead to a large standard deviation in SFChT in patients with RVO. Consequently, this may lead to no significant statistics. Thus, in this study, when compared with contralateral eyes, eyes with RVO had a thicker choroid. However, eyes with RVO did not have a thicker choroid than normal control eyes. Interestingly, this discrepancy is similar to the previous conflicting results8,9. Given the effect of renal function on the choroid, the SFChT of eyes with RVO should be compared with that of contralateral eyes without RVO.
Additionally, Rayess et al. reported baseline SFChT as a predictor of improvement in visual acuity after anti-VEGF injection in both BRVO and CRVO29,30. The baseline SFChT of functional responder (BCVA gain ≥ 2 lines) was greater than that of nonresponders in BRVO and CRVO29,30. Although Rayess et al. did not consider renal function, we carefully suggest that functional recovery in patients with RVO is associated with renal function through the results of the present study.
This study has several limitations. First, this was a retrospective observational study. Thus, the timing of OCT was not controlled (diurnal variations were not considered). Second, this study has relatively few patients because systemic evaluation was not routinely performed in patients with RVO. Third, only patients whose systemic workup was performed within a span of four weeks from initial retinal evaluation were included. This interval could affect the systemic status. Fourth, this study did not investigate the relationship between functional outcome and renal dysfunction because of the cross-sectional nature of this study. Fifth, axial length was not considered although myopic and hyperopic eyes were excluded. Finally, retinal hemorrhage and edema may have affected the measurements of SFChT of eyes with RVO. However, multiple regression analysis for our key findings used normal contralateral eyes. A prospective future study should provide stronger evidence for correlation between SFChT and renal dysfunction.
In conclusion, SFChT was positively correlated with eGFR. It is the first glance to reveal the association between choroidal thickness and kidney function in treatment-naïve RVO patients.
Methods
Study design
This retrospective cross-sectional study was approved by the institutional review board committee of Chung-Ang University Hospital in Seoul, South Korea (IRB #1701-004-16029) and adhered to the tenets of the Declaration of Helsinki. Informed consent was waived by the institutional review board committee of Chung-Ang University Hospital in Seoul because of the retrospective nature of the study.
Study subjects
The medical records of newly diagnosed patients with treatment-naïve unilateral RVO (macular involving) at Chung-Ang University Hospital between Dec 1, 2016, and Sep 30, 2019, were retrospectively reviewed. Among them, only patients whose renal function was evaluated within a span of 4 weeks from retinal evaluation were selected. The following parameters were recorded from medical charts: age, gender, mean arterial pressure (mmHg), BMI (km/m2), HTN duration, serum level of glucose, serum osmolarity [(2 × (sodium + potassium)) + (blood urea nitrogen (BUN)/2.8) + (glucose/18)], and serum level of total cholesterol.
In addition, the following renal parameters were recorded from laboratory examination: BUN, creatinine, BUN-to-creatinine ratio (BUN/Cr), eGFR, phosphorus, and calcium. The exclusion criteria were as follows: other chorioretinal disorders including drusen, age-related macular degeneration, diabetic retinopathy, eyes with treatment history using laser and intravitreal injection, myopic or hyperopic eyes with refractive error > ± 3.0 D, and eyes with low-quality OCT images due to media opacity (i.e., low image quality index < 90). In addition, patients with a history of smoking, glaucoma, ocular trauma, ocular inflammation, ocular ischemic syndrome, or any type of intraocular surgery (except cataract surgery) were excluded as well. The patients with systemic disease including history of angina, acute myocardial infarction, acute coronary syndrome or coronary revascularization (with a stent or coronary artery bypass graft), or significant plaque on coronary angiography, acute or uncontrolled hypertension (systolic BP ≥ 150 mmHg, diastolic BP ≥ 90 mmHg), significant carotid artery stenosis, history of a carotid endarterectomy were excluded as well.
Ophthalmic examination and diagnosis of retinal vein occlusion
Patients had undergone a comprehensive ophthalmic evaluation, including slit-lamp biomicroscope examination, fundus examination, fundus photographs, and SS-OCT. The measurement of best-corrected visual acuity, refractive error, and intraocular pressure was also done. The determination of RVO presence was based on retinal examination, photographs, and SS-OCT.
Optical coherence tomography
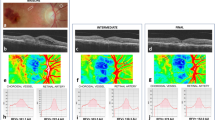
OCT imaging was done using Deep Range Imaging (DRI)-OCT Triton (Topcon, Tokyo, Japan). The device provided a 7 μm axial and 20-μm horizontal resolution and a scan speed of 100,000 A-scans per second using a swept wavelength light source. Central RT and ChT were defined and measured using the built-in software as previously described (Fig. 2)31. ChT was measured additionally at 1000-μm intervals from the fovea to 3000 μm to nasal and temporal area.
Representative swept-source optical coherence tomography image of a contralateral normal eye in patients with retinal vein occlusion. A. 66-year-old female patient with retinal vein occlusion. Estimated glomerular filtration rate (eGFR) was 105 mL/min/1.73 m2; subfoveal choroidal thickness was 278 μm. B. 62-year-old female patient with retinal vein occlusion. Estimated glomerular filtration rate (eGFR) was 20.3 mL/min/1.73 m2; subfoveal choroidal thickness was 145 μm. Arrow heads indicate chorioscleral interface.
Statistical analysis
Two independent masked observers (OJY and JTK) measured ChT, and the averaged values were used for statistical analyses. Data are presented as means ± standard deviations. Statistical analyses were performed using SPSS version 25.0 software (IBM Corp., Armonk, NY, USA). The baseline characteristics of patients were compared with those of normal subjects using independent t-tests. Ocular parameters of eyes with RVO were compared with those of normal fellow eyes (without RVO) using paired t-test. The correlations of SFChT with renal profile were analyzed using simple and multiple linear regression analyses. P values < 0.05 were considered statistically significant.
Data availability
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Song, P., Xu, Y., Zha, M., Zhang, Y. & Rudan, I. Global epidemiology of retinal vein occlusion: a systematic review and meta-analysis of prevalence, incidence, and risk factors. J. Glob. Health 9, 010427. https://doi.org/10.7189/jogh.09.010427 (2019).
Rogers, S. et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 117, 313 e311-319 e311. https://doi.org/10.1016/j.ophtha.2009.07.017 (2010).
Jonas, J. B., Mones, J., Glacet-Bernard, A. & Coscas, G. Retinal vein occlusions. Dev. Ophthalmol. 58, 139–167. https://doi.org/10.1159/000455278 (2017).
Wang, S. et al. Major eye diseases and risk factors associated with systemic hypertension in an adult Chinese population: the Beijing Eye Study. Ophthalmology 116, 2373–2380. https://doi.org/10.1016/j.ophtha.2009.05.041 (2009).
Wong, T. Y. et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology 112, 540–547. https://doi.org/10.1016/j.ophtha.2004.10.039 (2005).
Cugati, S. et al. Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology 114, 520–524. https://doi.org/10.1016/j.ophtha.2006.06.061 (2007).
Mule, G., Vadala, M., Geraci, G. & Cottone, S. Retinal vascular imaging in cardiovascular medicine: new tools for an old examination. Atherosclerosis 268, 188–190. https://doi.org/10.1016/j.atherosclerosis.2017.11.001 (2018).
Du, K. F. et al. Subfoveal choroidal thickness in retinal vein occlusion. Ophthalmology 120, 2749–2750. https://doi.org/10.1016/j.ophtha.2013.08.031 (2013).
Tang, F. et al. Comparison of subfoveal choroidal thickness in eyes with CRVO and BRVO. BMC Ophthalmol. 19, 133. https://doi.org/10.1186/s12886-019-1143-9 (2019).
Balmforth, C. et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight 1, e89173. https://doi.org/10.1172/jci.insight.89173 (2016).
Vadala, M. et al. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes Arch. Clin. Exp. Ophthalmol. 257, 1687–1698. https://doi.org/10.1007/s00417-019-04358-3 (2019).
Mule, G. et al. Association between early-stage chronic kidney disease and reduced choroidal thickness in essential hypertensive patients. Hypertens. Res. 42, 990–1000. https://doi.org/10.1038/s41440-018-0195-1 (2019).
Haroun, M. K. et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J. Am. Soc. Nephrol. 14, 2934–2941. https://doi.org/10.1097/01.asn.0000095249.99803.85 (2003).
Wong, C. W., Wong, T. Y., Cheng, C. Y. & Sabanayagam, C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. 85, 1290–1302. https://doi.org/10.1038/ki.2013.491 (2014).
Zhou, J. Q. et al. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology 120, 803–808. https://doi.org/10.1016/j.ophtha.2012.09.033 (2013).
Cugati, S., Wang, J. J., Rochtchina, E. & Mitchell, P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch. Ophthalmol. 124, 726–732. https://doi.org/10.1001/archopht.124.5.726 (2006).
Ponto, K. A. et al. Prevalence and risk factors of retinal vein occlusion: the Gutenberg Health Study. J. Thromb. Haemost. 13, 1254–1263. https://doi.org/10.1111/jth.12982 (2015).
Breyer, M. D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug. Discov. 15, 568–588. https://doi.org/10.1038/nrd.2016.67 (2016).
Izzedine, H., Bodaghi, B., Launay-Vacher, V. & Deray, G. Eye and kidney: from clinical findings to genetic explanations. J. Am. Soc. Nephrol. 14, 516–529. https://doi.org/10.1097/01.asn.0000051705.97966.ad (2003).
Zipfel, P. F., Heinen, S., Jozsi, M. & Skerka, C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 43, 97–106. https://doi.org/10.1016/j.molimm.2005.06.015 (2006).
Wilkinson-Berka, J. L., Agrotis, A. & Deliyanti, D. The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides 36, 142–150. https://doi.org/10.1016/j.peptides.2012.04.008 (2012).
Cheung, C. Y., Ikram, M. K., Sabanayagam, C. & Wong, T. Y. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 60, 1094–1103. https://doi.org/10.1161/HYPERTENSIONAHA.111.189142 (2012).
Wong, T. Y. et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 15, 2469–2476. https://doi.org/10.1097/01.ASN.0000136133.28194.E4 (2004).
Kim, K. H. et al. Regional choroidal thickness changes in branch retinal vein occlusion with macular edema. Ophthalmologica 234, 109–118. https://doi.org/10.1159/000437276 (2015).
Okamoto, M., Yamashita, M., Sakamoto, T. & Ogata, N. Choroidal blood flow and thickness as predictors for response to anti-vascular endothelial growth factor therapy in macular edema secondary to branch retinal vein occlusion. Retina 38, 550–558. https://doi.org/10.1097/iae.0000000000001566 (2018).
Esen, E., Sizmaz, S. & Demircan, N. Choroidal thickness changes after intravitreal dexamethasone implant injection for the treatment of macular edema due to retinal vein occlusion. Retina 36, 2297–2303. https://doi.org/10.1097/iae.0000000000001099 (2016).
Reiner, A., Fitzgerald, M. E. C., Del Mar, N. & Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 64, 96–130. https://doi.org/10.1016/j.preteyeres.2017.12.001 (2018).
Tsuiki, E., Suzuma, K., Ueki, R., Maekawa, Y. & Kitaoka, T. Enhanced depth imaging optical coherence tomography of the choroid in central retinal vein occlusion. Am. J. Ophthalmol. 156, 543-547.e541. https://doi.org/10.1016/j.ajo.2013.04.008 (2013).
Rayess, N. et al. Baseline choroidal thickness as a short-term predictor of visual acuity improvement following antivascular endothelial growth factor therapy in branch retinal vein occlusion. Br. J. Ophthalmol. 103, 55–59. https://doi.org/10.1136/bjophthalmol-2018-311898 (2019).
Rayess, N. et al. Baseline choroidal thickness as a predictor for treatment outcomes in central retinal vein occlusion. Am. J. Ophthalmol. 171, 47–52. https://doi.org/10.1016/j.ajo.2016.08.026 (2016).
Kim, J. T. & Park, N. Changes in choroidal vascular parameters following pan-retinal photocoagulation using swept-source optical coherence tomography. Graefes Arch. Clin. Exp. Ophthalmol. 258, 39–47. https://doi.org/10.1007/s00417-019-04475-z (2020).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. 2020R1F1A1072226). These funding sources had no role in the study design, writing of the manuscript, or collection, analysis, or interpretation of data.
Author information
Authors and Affiliations
Contributions
Drafting/writing: S.U.C., J.T.K. Study design: J.T.K. Analysis or interpretation of data: J.Y.O., Acquisition of data: J.Y.O., Statistical analysis: J.Y.O., Study supervision: J.T.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Choi, S.U., Oh, J.Y. & Kim, J.T. Correlations between choroidal thickness and renal function in patients with retinal vein occlusion. Sci Rep 10, 16865 (2020). https://doi.org/10.1038/s41598-020-74058-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74058-0
This article is cited by
-
Choroidal vascularity index of patients with coronary artery disease
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.