Abstract
Fungal infections represent a worrisome complication in hematologic cancer patients and in the absence of disease specific symptoms, it is important to establish new biological indicators, which can be used during mould-active prophylaxis. Recently, miRNAs have appeared as candidate diagnostic and prognostic markers of several diseases. A pilot clinical study was performed to evaluate the diagnostic utility of 14 microRNAs which can be related to invasive fungal infections. Based on our data miR-142-3p, miR-142-5p, miR-26b-5p and miR-21-5p showed significant overexpression (p < 0.005) due to invasive aspergillosis in hemato-oncology patients with profound neutropenia. A tetramiR assay was designed to monitor peripheral blood specimens. Optimal cut-off was estimated by using the median value (fold change 1.1) of the log10 transformed gene expressions. The biomarker panel was evaluated on two independent sample cohorts implementing different antimicrobial prophylactic strategies. The receiver operating characteristic analysis with area under the curve proved to be 0.97. Three miRNAs (miR-142-5p, miR-142-3p, miR-16-5p) showed significant expression alterations in episodes with sepsis. In summary, the tetramiR assay proved to be a promising diagnostic adjunct with sufficient accuracy and sensitivity to trace invasive aspergillosis in hemato-oncology patients.
Similar content being viewed by others
Introduction
In the developed countries life-threatening invasive fungal diseases (IFD) represent crude morbidity and mortality1. Members of the Aspergillus and Candida genera are predominant etiological agents2,3. In the past decades the incidence of aspergillosis has increased4,5,6,7. Currently, other relevant fungi are also increasingly identified, such as Cryptococcus spp., Fusarium spp. and, Zygomycetes8. Invasive aspergillosis (IA) can be frequently associated mainly with acute myeloid and lymphoid (AML, ALL) leukemia and occurs primarily in the lung9. The well-established risk factors include underlying malignancy, immunosuppression and profound neutropenia10,11,12.
Without timely initiated antifungal therapy the mortality of IA related hospitalization can top 70%, whereas in bone-marrow transplant patients, it has been reported to be even higher13,14,15. As a result of prolonged hospital stay, the burden of aspergillosis-related IFDs remains an immense public health problem, with an estimated treatment cost above $600 million/year in the US13.
In high-risk hematology patient groups, the first-line prophylaxis is preferred16. Timely initiation of treatment critically affects the outcome of the disease however diagnostic driven strategy remains a challenge6. Identification of IA relies on the combination of cultivation and non-conventional microbiological tests such as fungal antigen testing, as well as radiology and histopathology4.
MicroRNAs (miRNAs) are among the class of small, conserved, non-coding RNAs. By estimates, approximately 50% of the human transcriptome is influenced by miRNAs17. MiRNAs emerged as important endogenous regulators of virtually all basic biological processes18. In response to pathological processes miRNAs are expressed by all cell types and tissues into the circulation where their free forms are protected from RNAse mediated degradation19. Recent studies estimate the potential of free circulating miRNAs as prognostic disease biomarkers in diagnosis. During infection, lung hypoxia established by an ischemic microenvironment, vascular invasion, thrombosis, antiangiogenic factors such as gliotoxin, are considered to be a significant virulence factor of A. fumigatus, therefore hypoxia related miRNAs (miR26a, miR26b, miR21 and miR101) were also considered20,21,22. Further data revealed the participation of miR26a, miR26b in relation to apoptosis and autophagy23. The regulatory role of miR132 and miR155 was elucidated during A. fumigatus infection on human dendritic cells suggesting a strong involvement of these microRNAs in the anti-fungal response of the host20. MiR140, miR16 and miR494 were related to immune responses, anti-inflammatory processes accompanying infection, and cell apoptosis during C. albicans infection23,24,25,26. Furthermore, miR155, miR26, miR16 and miR21 were found to modulate immune response against Gram-positive bacteria in human, mouse and murine models27,28,29. However, due to the lack of consensus in methodology, as well as processing and normalization, the actual number of clinical studies which establish the contribution of certain miRNAs to IFDs is surprisingly low20,21,30,31,32,33,34,35.
To ameliorate success and accuracy of diagnosis, a definite proof-of-concept was set to capture invasive aspergillosis specific miRNA expression patterns in a hemato-oncology patient population. 14 miRNAs were chosen which are principally related to hypoxic microenvironmental stress conditions, inflammation, apoptosis and, autophagy20,21,22,24,25,26,34,35. All miRNAs are potentially obtainable from the peripheral blood circulation.
To our present knowledge, this is a pioneer clinical study which could lead to the implementation of miRNAs supporting the diagnosis of IA among patients with hematologic malignancies and profound neutropenia.
Results
Characteristics of patient cohort
The vast majority of participants suffered from acute myeloid leukemia (C1 45%, C2 65%) and chronic lymphocytic leukemia (C1 25%, C2 10%) (Fig. 1a). 16 patients (40% of patients in both C1 and C2) died during the study period. Patients of these study groups were well balanced according to age (mean 60 ± 16.2, p = 0.229) and gender (mean 53 ± 15.8 p = 0.638). Overall, 95% of the onco-hematologic (OH) patients (except P2, P36) developed systemic inflammatory response syndrome (SIRS). 26 patients developed sepsis while 35% of patients had non-infected SIRS. Postmortem histology (PMH) was pursued in 56.25% of fatalities. In the case of four C1 patients (P4, P6, P11, P23), histological evaluation clearly proved the existence of IA. Descriptive Periodic Acid Schiff (PAS) and Hematoxylin and Eosin (H&E) stained hyphae supported the fungal invasion and the diagnosis of invasive aspergillosis. There was one case (P4) where pathology confirmed the presence of the fungus Aspergillus in the central nervous system. In C1 15.78% of patients suffered from both diabetes and hypertonia. Altogether, there were 11 patients (45% in C1, 10% in C2) with recurrent fever, refractory to antibiotic treatment and among these 4 patients (2 in C1, 2 in C2) were diagnosed with co-infections (CO: aspergillosis and bacteremia), 6 patients (all in C1) with invasive aspergillosis (IA) and 1 patient with bacteremia (B) (Fig. 1b). There were no significant differences in the inflammation status of the two study groups. Patient C-reactive protein levels in C1 (CRP: 107.57 ng/ul; inter quartile range (IQR) 55.89–159.25) and in C2 (CRP: 108.43 ng/ul; IQR 55.43–161.43). Patients were retrospectively categorized with P(1) proven IA, P(2) probable IA (25% cases) versus P(3) possible IA (having no evidence of infection). 100% of P(1) patients (4 cases) were diagnosed in C1 with 66.7% of P(2); 4 of 6 probable IA cases.
Shortlisting of parameters of our patient recruitment strategy with regard to baseline demographics and center specific characteristics. Demographic and clinical characteristics of patients with regard to their EORTC/MSG classification are described in (a) Center specific characteristics are shown in (b). C1 (center 1 Debrecen); University of Debrecen, Faculty of Medicine, Institute of Internal Medicine, Debrecen, Hungary. C2 (center 2 Nyíregyháza); Institute of András Jósa County and Teaching Hospital, Division of Hematology, Nyíregyháza, Hungary. (P1) proven IA, (P2) probable IA, (P3) possible IA-having no evidence of infection. AML Acute Myeloid Leukemia, ALL Acute Lymphoblastic Leukemia, CLL Chronic Lymphocytic Leukemia, MDS Myelodysplastic syndrome, HL Hodgkin Lymphoma, NHL Non-Hodgkin Lymphoma. EORTC European Organization for the Research and Treatment of Cancer/Mycosis Study Group, NF neutropenic fever, LOS Length of hospital stay, PMH Postmortem Histology, PAS Periodic acid–Schiff, CT Thoracic Computed Tomography, SIRS Systemic Inflammatory Response Syndrome.GM galactomannan serology, B bacteremia, IA invasive aspergillosis, CO co-infection, BAL bronchoalveolar lavage.
First-line antifungal prophylaxis was associated with lower IA prevalence
In C2, primary antifungal prophylaxis was initiated in the case of OH patients. Relevant endpoints of this prophylactic strategy manifested with a sharp decrease in the number of IA cases (40% vs. 10%; p < 0.001) with no observation of proven IA (Fig. 1). On the contrary, in C2, significantly higher proportion of patients (13 patients, 65%) developed bacteremia in comparison to C1 (15%). The number of co-infections was balanced (10%) between the two centers.
Identification of normalizer miRNAs
Initial miRNA testing was performed to identify an appropriate miRNA normalizer showing consistent gene expressions in the members of this study. MiR191 was found to be optimal across patients and controls (Fig. 2).
Testing of four potential miRNA normalizers. Hsa-miR191 was found to be optimal normalizer for estimating relative miRNA expression fold changes. (a) Heatmap represents arrayed Cq values for the normalizers on this cohort. P stands for onco-hematology patients of this study (Cq 21.34 ± 4.57), C stands for healthy controls (Cq 21.71 ± 3.07). (b) represents mean has-miR191 Cq values (± SD) in healthy controls (C) with no malignancies in comparison to OH patients (Cq 20.94 ± 5.1) developing B bacteremia (Cq 20.54 ± 2.84), IA invasive aspergillosis (Cq 20.34 ± 3.95) and CO co-infections (Cq 21.45 ± 2.56).
Calculation of miRNA expression fold changes
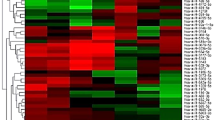
Real-time quantitative PCR (qRT-PCR) was used to measure the relative gene expression levels of miRNAs in OH patients. Figure 3 displays the study timeline and the date of recruitments in the case of two Hungarian centers; C1 and C2 with a cluster heatmap demonstrating normalized relative gene expression levels of miRNA targets measured in the peripheral blood. All 14 miRNAs were expressed in the peripheral blood samples of patients and were generally overexpressed in comparison to healthy controls.
Relative expression levels of selected miRNAs in patients with hematologic malignancies. The expression levels of selected miRNAs detected by qRT-PCR were normalized to hsa-miR191 and presented as fold-changes (2−ΔΔCt). Black colors indicate no difference in gene expressions in comparison to the healthy controls, green scale represents the fold changes of under-, while pink the overexpressions.
Circulating miRNAs showed aberrant expressions in patients with sepsis
We managed to establish miRNAs showing significant expression alterations in infected SIRS patients. Based on our data three miRNAs were related to sepsis in OH patient population on the basis of significant changes in their relative gene expressions in the blood; miR142-3p (p < 0.01), miR142-5p (p < 0.05), miR16-5p (p < 0.01).
Identification of aspergillosis specific miRNAs
Nine miRNAs (miR142-3p, miR21-5p, miR142-5p, miR26a-5p, miR26b-5p, miR101-3p, miR16-5p, miR140-5p, miR28-5p) were overexpressed presenting statistically significant fold changes (p < 0.0001) in comparison to non-infected SIRS. In the case of miR16-5p significant overexpression was observed in both aspergillosis and bacteremia, thus it was omitted from further analysis (Fig. 4a1–a2). In the case of three miRNAs (miR26b-5p, miR21-5p, miR142-3p), bacteremia did not obscure the significant overexpressions indicating aspergillosis in diseased patients (Fig. 4a3). Only two microRNAs (miR28-5p, miR140-5p) showed significant overexpressions in cases with co-infections (Fig. 4a3). In Fig. 4b1 heatmap represents area under the curve (AUC) values estimated for 8 pre-selected miRNAs showing significant associations with infections in OH patients. Six microRNAs; miR26a-5p, miR26b-5p, miR142-3p, miR142-5p, miR101-3p, miR21-5p demonstrated good discriminatory property with high AUC values indicating aspergillosis. In Fig. 4b2 receiver operator curves (ROC) are also shown for miRNA discriminators, displaying AUC values greater than 82% in patients with aspergillosis. Reassuringly, the relative fold changes of these miRNAs proved to be specific for aspergillosis and bacterial co-infections did not interfere with the expression profiles (p-values 0.54–0.99) (data not shown). Violin plots with Kernel density estimations were also made to visualizing the distribution and probability density of relative fold changes of the miRNAs in different patient groups (Fig. 4c). In the case of miR26a-5p and miR101-3p we did not observe statistically significant (p ≥ 0.05) gene expression alterations between OH patients whether aspergillosis was diagnosed or not. With the considerable heterogeneity in the distribution of results mir142-5p provided significant overexpression in comparison to the non-infected patients (×80 ± 12; p < 0.0001), and its expression was significantly higher due to aspergillosis in comparison to bacteremia (×70 ± 14; p < 0.001). MiR26b-5p proved to be a promising aspergillosis specific biomarker (p < 0.0001).
miRNAs showing statistically significant overexpressions indicating aspergillosis. Volcano plots were made to identify differentially expressed miRNAs due to aspergillosis using the LIMMA statistical model (a). Red spotted miRNAs represent statistically significant (p < 0.001) gene expressions indicating bacteremia (a1), aspergillosis (a2), bacterial and fungal co-infections (a3). Vertical lines represent miRNAs with a fold change of > 1.5. Red spotted microRNAs situated towards the right top quadrant represent values of increasing magnitude fold changes. CO stands for co-infection, IA invasive aspergillosis, B bacteremia, OH onco-hematology, HC healthy controls. (a) Volcano plots show log2 transformed fold changes in relation to − log10 transformed p-values. On the y-axis dashed lines indicate the p-value = 0.05, miRNAs with statistically significant expressions are shown with red spots. (b) ROC (Receiver Operating Characteristics) curves with AUC (Area Under the Curve) were made to check and visualize the performance of the 10 pre-selected miRNA assays. To measure the diagnostic accuracy of the miRNAs relative fold changes were converted to qualitative (proven IA, probable IA vs. possible IA) indexes. (c) Horizontal violin plots visualizing the distribution of the relative fold changes with probability density in different patient groups. *p ≤ 0.05; **p ≤ 0.005; and ***p ≤ 0.0005.
Diagnostic performance of the miRNA assays
Differential expression matrices represent the values of alterations in relative miRNA fold changes (fc) when comparing different patient groups. MiR142-3p showed the highest association with aspergillosis (Δlog2fc 6.81) in OH patients compared to healthy controls (Fig. 5a). All miRNAs were assessed to be more abundant during aspergillosis and showed only a mild correlation with bacteremia. Noticeably, miR26b-5p (Δlog2fc: 4.28) and miR21-5p (Δlog2fc: 4.27) were expressed with a lesser extent during bacteremia. All miRNAs showed remarkable expression alterations due to aspergillosis in comparison to bacteremia. We observed moderate fold changes in the expression of several miRNA targets due to aspergillosis (mir142-5p Δlog2fc: 1.92, miR26b-5p Δlog2fc: 2.02; miR21-5p Δlog2fc: 1.67), in comparison to other infections, however mir142-3p was over-represented as per co-infections (Δlog2fc 6.37). Log10 transformed fold changes (log10 fc) for cases (proven IA, probable IA) and controls (possible IA) were dichotomized by mapping the sensitivity values in relation to 1—specificity in the case of mir142-5p, miR26b-5p, miR21-5p, miR142-3p to estimate optimal cut-off values for these biomarkers (Fig. 5b). MiR-21-5p (log10 transformed fold change: 1.3) showed the lowest, while miR-26b-5p (log10 transformed fold change: 2,9) showed the highest sensitivity (93%) and specificity (89%). To mitigate interpatient variability tetramiR diagnostic panel was established for a more reliable diagnosis of IA by the combined application of miR142-3p, miR142-5p, miR26b-5p, miR21-5p (Fig. 5c). This model relies on the median gene expression values (log10 fold change: 1.1) of these four miRNAs achieving higher sensitivity (96%) and specificity (95%).
Log-2 transformed miRNA expression fold changes were used to exploit expression alterations between patient groups. (a) OH onco-hematology, IA invasive aspergillosis, B bacteremia, CO co-occurrence of bacteremia and aspergillosis. Extents of logarithmic (Δlog2fc) transformed fold differences are designated by dots. A dot colored red is more abundantly expressed by the condition designated in the columns while green colored dots show more abundant fold changes designated by the rows. (b) Optimal cut-point values were also estimated for mir142-5p (log10 fold change 0.9), miR26b-5p (log10 fold change 0.2), miR21-5p (log10 fold change 1.3), miR142-3p (log10 fold change 2.9) biomarker assays. (c) Estimating the optimal cut-point for tetramiR panel with ROC curve and with AUC to visualize performance of the method.
The diagnostic power of the tetramiR panel compared to the gold-standard Platelia Aspergillus GM-EIA
To compare the performance of the tetramiR panel to the gold-standard Platelia Aspergillus GM-EIA test sensitivity, specificity values were estimated for both classifiers on this patient population. Distribution of the test results of cases and controls are also shown with significances (Fig. 6a). Sample concordance was also assessed between the two classifiers; Platelia Aspergillus GM-EIA and tetramiR panel showed a fair agreement with an observed ratio of 77.5% (agreement; 31 of 40) and a kappa statistic of 0.41 (100% confidence interval from 0.1672 to 0.6713). We did not observe significant difference in the distribution of test results between cases and controls. Out of the 10 patients diagnosed with IA 30% proved to be true positive (TP) with both of the methods. GM-EIA failed to detect IA in 7 of 10 (TP 30%) and tetramiR panel 1 of 10 (TP 90%) cases (Fig. 6b). None of them provided false negative (FN) results. 60% of IA cases were detected only by tetramiR assay. The specificity of GM-EIA was 100% providing no single FP results among controls whereas the true positivity rate was 93% in the case of the tetramiR assay.
Diagnostic utility of the tetramiR panel when compared to the Aspergillus GM-EIA method. (a) Values of the test results categorized to proven IA, probable IA, possible IA-having no evidence of infection and their distributions shown with significances. *p ≤ 0.05; **p ≤ 0.005; and ***p ≤ 0.0005. In the case of tetramiR panel the median of the log10 transformed gene expression data was used. (b) Venn-diagrams with section-analysis to compare the outcomes of Platelia Aspergillus GM-EIA and tetramiR qRT-PCR panel diagnosing invasive aspergillosis. TP true-positive, FN false-negative, TN true-negative, FP false-positive. Cases (proven and probable IA) with positive GM-EIA and tetramiR panel results were regarded as true positive (TP) while those with negative outcomes were considered to be false negative (FN). Controls (unclassified patients with no EORTC/MSG evidence of IA) with negative GM-EIA and tetramiR panel results were coded to true negative (TN) and those with positive outcomes false positive (FP).
Discussion
With the significant developments achieved in the field of oncology, nosocomial etiologies are continuously associated with high mortality and increased susceptibility to IFDs12. The prophylactic use of available azoles during anticancer therapy has led to a reduction of Candida blood stream infections however the growing antifungal resistance especially in the case of Aspergillus fumigatus and A. terreus has emerged as a global health issue12,36,37,38,39,40,41. In Europe the genus Aspergillus accounts for 12–28% of all fungal isolates41,42,43,44. Aspergillus-related pulmonary diseases can encompass various clinical syndromes moreover immunosuppressed patients may develop invasive pulmonary aspergillosis (IPA)45,46.
Despite the immense number of published guidelines, no obvious recommendations are available addressing diagnosis and managing infections in these patient groups47,48,49,50,51,52.
This pilot study was performed to capture the expression of specific free circulating miRNAs in blood to support the diagnosis of invasive aspergillosis. Among patients with hematologic malignancies and profound neutropenia IA was diagnosed in 14% of episodes and 55% of these cases were diagnosed post-mortem only. Owing to the fact, that the vast majority of patients suffered by AML and CLL (in total: 72.5%) this study population can be considered homogenous in terms of underlying malignancies.
In the case of severely immunocompromised patients the escalation in the lengths of hospital stay (LOS) appreciably raises the odds of nosocomial co-infections. Patients with fungal septicaemia show significantly higher mean hospital costs thus duration of hospitalization was positively correlated with disease occurrence53. According to our results none of the study participants with LOS < 29 developed IA. Whereas the highest disease prevalence (80%) was observed in patients with LOS ≥ 44, while IA was diagnosed in 27% of patients with LOS ≥ 29, LOS < 36.
Sepsis is a systemic inflammatory response syndrome (SIRS) driven by infection and is a leading cause of mortality in patients after intensive cytotoxic therapy significantly decreasing the survival rate with more than 50%12,54,55. The prevention of infection has become a major goal however there is no widely accepted standard for antimicrobial prophylaxis17,27,56. The risk appreciably rises in severe neutropenic episodes having persistent fever with more than 7 days. Based on overlapping data of previous studies the induction of miR132, miR155, miR26, miR16 and miR21 was found to modulate immune response to different bacterial stimuli28,29,57,58,59,60,61,62. These estimations did not manifest in our data with one exception. The contribution of hsa-miR16 to bacterial infections was strengthened showing significant expression alterations.
A difference was shown in the distribution (C1 47.36%, C2 only 10.52%) of episodes in whom fever persisted despite antibiotics. An appreciable rise was observed in the number of aspergillosis patients who did not receive antifungal prophylaxis. In C1 remarkably higher proportion of SIRS episodes (77%) developed sepsis, out of which the vast majority (85.71%) was diagnosed with IA.
Antimicrobial prophylaxis has already been estimated as an important prognostic factor of bacteremia in several studies12,63,64,65,66,67,68,69,70. Due to a more intense antifungal prophylactic strategy in C2 there were only two episodes (P35, P32) with recurrent fever developing non-infected SIRS. A paradox was found when comparing the number of infection related SIRS between C1 and C2 and the onset of diagnosed bacterial infection and invasive aspergillosis. The prevalence of aspergillosis proved to be remarkably lower in the case of C2, in comparison to C1 (10% vs. 60%; p < 0.001), which can be explained by the more intensive prophylaxis management followed by C2. On the contrary, in C2, significantly higher proportion of patients (13 patients, 65%) developed bacteremia in comparison to C1 (15%).
Numerous studies have been conducted in recent years to establish expression levels of certain miRNAs in different disease conditions4,53. With the fact that miRNAs are expressed by all cell types and tissues they are often present at very low concentrations in peripheral blood thus their quantification requires highly sensitive and specific methods. Diverse technical biases such as initial sample volume, collection modality and, storage condition can be introduced during the experimental steps which profoundly obscure the true biological response71,72,73,74,75. Additionally, miRNA family members exhibit a high degree of homology having rather low absolute copy numbers in body fluids. Peripheral blood samples are enriched with both free circulating, membrane enclosed and intracellular miRNAs as well.
The choice of a reference gene remains problematic, having serious impact on the available transcript levels and consequently, on the adequate interpretation of data. We therefore applied and tested different assays to find a reliable normalizer to analyze circulating miRNA expression profiles. Hsa-miR191 proved to be an appropriate reference miRNA to mitigate inter-patient variabilities. Exogenous influences and the genetic heterogeneity which is concomitant with haematological malignancies can obscure the miRNA regulome. To mitigate these accidental effects, we recommend to draw conclusions from the expression alterations of multiple biomarkers. In the case of four miRNAs (miR142-3p, miR142-5p, miR26b-5p and miR21-5p), a significant association to IA was confirmed. We therefore decided to label this set of miRNAs as a novel biomarker panel (tetramiR panel) supporting diagnosis of invasive aspergillosis. We found, that the expression of these key indicators was not confounded by age, gender, prophylactic drug choice, underlying malignancy, other diseases (diabetes, hypertonia) and sepsis. Considering the fact that our sampling data reflect only single time points conclusions cannot be drawn about how the mirRNA expression profiles correlate with disease progression.
The diagnostic power and the accuracy were estimated for the tetramiR panel and compared to the Platelia Aspergillus GM-EIA method. In 66.66% of the patients with pulmonary infiltrates the Platelia Aspergillus GM-EIA showed a favorable diagnostic accuracy which is in line with observations of other studies76. In the case of the galactomannan-enzyme immunoassay (GM-EIA) studies steadily report about a various test performance (positive predictive values: 25–62% and negative predictive values: 92–98% using 0.5 as the cut-off)77,78. By monitoring the hemato-oncology episodes with profound neutropenia the results of the GM-EIA exerted poor sensitivity in comparison to the tetramiR panel (GM-EIA Se: 30% vs. tetramiR panel: 90%).
Diagnosis of invasive aspergillosis still constitutes a major issue in the severely immunocompromised patient groups, especially in those undergoing intensive cytotoxic therapy. Based on our data, the tetramiR panel which relies on the expression of four concomitant miRNAs (miR142-3p, miR142-5p, miR26b-5p and miR21-5p) can serve as a good adjunct in the diagnosis of aspergillosis. Although the optimal diagnostic cut-off point of this method might be biased by the limited size of the patient population, we underpin its suitability and utility in real diagnostic practice.
Materials and methods
Patient population
This retrospective case–control study was performed from May 2017 to February 2019, involving two hematology centers in Hungary: University of Debrecen, Faculty of Medicine, Institute of Internal Medicine, Debrecen, Hungary (C1) and Institute of András Jósa County and Teaching Hospital, Division of Hematology, Nyíregyháza, Hungary (C2). Patient population comprised 40 adult admissions; 26 males with the median age of 52 (range 21–83) and 14 females with the median age of 54 (range; 24–83) having different hematological malignancies (mainly acute leukemia—42%) receiving stem cell transplantation and intensive chemotherapy (neutrophil count < 0.5 × 109 cells/L). Patients developing neutropenic fever (NF); temperature > 38 °C of fever recorded twice or > 38.5 °C recorded once were recruited with two exceptions (P2, P36). Children < 17 years were excluded from the study. There were 15 healthy controls included with no previous history of onco-hematology (OH) diseases or SIRS with the median age of 42.5 (range 24–75).
Diagnosis of sepsis
Patients were considered to develop SIRS presenting minimum 2 of the following clinical findings; (1) body temperature less than 36 °C (96.8 °F) or greater than 38 °C (100.4 °F), (2) heart rate greater than 90 beats per minute, (3) tachypnea greater than 20 breaths per minute; or, an arterial partial pressure of carbon dioxide less than 4.3 kPa (32 mmHg), (4) white blood cell count less than 4000 cells/mm3 (4 × 109 cells/L) or greater than 12,000 cells/mm3 (12 × 109 cells/L). Bacterial and/or fungal sepsis was defined using clinical and laboratory parameters such as culture-based pathogen detection with the isolation of viable microorganisms from blood cultures79,80,81,82,83.
First-line choices for antimicrobial prophylaxis
C1 (Debrecen) and C2 (Nyíregyháza) followed two different antimicrobial prophylactic strategies. Both of the centers initiated antiviral and antibacterial prophylaxis in hematology and oncology patients in parallel with the administration of chemotherapeutics84,85,86. In C2, antifungal therapy was uniformly initiated immediately at the onset of neutropenia while in C1 only 50% of the cases received prophylactic antifungals, and in the rest of the cases, therapy was administered only in the case of patients with fever refractory to broad-spectrum antibiotics (≥ 6 days of fever).
Stratification of episodes
Patients were retrospectively stratified using standard criteria according to revised European Organization for the Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) to define a case-group of “P(1) proven” or “P(2) probable” IA (10 patients, 25%) and a group of patients comprising P(3) possible IA (30 patients 75% with no EORTC/MSG with no evidence of infection)87,88.
Sampling
Patient samples came from 40 prospectively enrolled hemato-oncology patients from two centers; C1 and C2 of Hungary. For galactomannan testing and PCR analyses peripheral blood samples were collected from every patient at the onset (day 1) of fevered neutropenia. In the case of P2 and P36 samples were collected from non-fevered neutropenic patients suspected to develop fungal infections. In 95% of the cases (except P22, P26), hemocultures (75%) or BAL samples (20%) were also obtained in parallel the collection of blood samples.
Cultures
There were 30 cases where hemocultures were obtained. Adult patients having bacteremia typically have low quantities of bacteria in the blood, therefore multiple blood culture examinations were performed (C1: 2.07 ± 1.98, C2: 3.33 ± 3.2) to detect blood stream bacterial infections. Classical mycology of bronchoalveolar lavage (BAL) was performed in the case of 8 patients in whom there was a suspicion of bacteremia or aspergillosis (infected SIRS).
Biomarker analyses
Liquid biopsy samples were parallelly screened for the presence of galactomannan (GM) antigens and for microRNA. Intact EDTA serum tubes were drawn from patients routinely for GM analysis in parallel with intact EDTA whole blood tubes for microRNA analyses and sent to the Department of Medical Microbiology, Debrecen, Hungary.
Platelia Aspergillus GM-EIA
The GM-EIA assays required 300 μl of serum specimens. The Platelia Aspergillus GM-EIA (Bio-Rad Laboratories, Hungary) was used for GM screening. GM-EIA cut-off values were determined using the OD450/620 values. While evaluating single specimens any value above the OD (optical density) 450/620 ≧ 0.5 cut-off value was considered positive as requested for in vitro testing.
miRNA-based biomarker assays
Whole blood tubes were forwarded to the Department of Human Genetics, Debrecen, Hungary. MicroRNA analyses were carried out in a class II laminar-flow cabinet to avoid environmental contamination. Total RNA was extracted from the peripheral blood using the MagMax mirVana according to manufacturer’s instructions (Thermo Fisher Scientific, Maryland) by recovering on average 46.3 ± 42.8 SD ng RNA/µl from 50 µl peripheral blood specimens. 3 ng of RNA was constantly used for the miRNA specific reverse transcription using TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific). To identify a stabile miRNAs endogenous control in whole blood samples of healthy patients and in study participants four candidate miRNAs (RNU6B, miR520-6d, hsa-let7a, miR191) were selected on the basis of literature data73,89. TaqMan quantitative real-time PCR (miRNA assay) was used to detect miRNA expression profiles in 3 independent technical repeats including negative controls (no-template from the RT reaction), using a LightCycler 96 Real-Time PCR System (Roche Diagnostics, Risch-Rotkreuz Switzerland). Normalized miRNA expression levels of 14 miRNAs (miR26a-5p, miR26b-5p, miR21-5p, miR142-3p, miR142-5p, miR16-1-3p, miR16-5p, miR155-5p, miR140-5p, miR101-3p, miR494-3p, miR28-5p, miR132-3p and miR204-5p) were measured with quantitative real-time PCR (qRT-PCR) assays. Relative mRNA expressions were calculated by using hsa-miR191 for normalization.
Statistical analyses
Expression levels of the selected miRNAs detected by qRT-PCR were normalized to miR-191 and presented as the fold change (2−ΔΔCt) above the controls (non-HM): ΔCt = assay Cq—normalizer Cq. Results for non-normally distributed continuous variables are summarized as medians (interquartile ranges) and were compared by Mann–Whitney U test. Statistical comparison among multiple groups were performed with Kruskal–Wallis test, and intergroup differences were tested with Dunn test. Significance was accepted at p-value < 0.05. Levels of significance were assigned as: *p ≤ 0.05; **p ≤ 0.005; and ***p ≤ 0.0005. All statistical analyses were performed using GraphPad Prism statistical software. To measure the diagnostic accuracy for the GM-EIA and the tetramiR panel, results (GM-OD and normalized Ct) were converted to qualitative (positive, negative) indexes. In the case of the qRT-PCR based tetramiR panel every single run was evaluated independently prior to calculating the median of the Ct values to take diagnosis. Area under the ROC curve (AUC) was used as an accuracy index for evaluating the diagnostic performance of the selected miRNAs. After calculating the series of sensitivity (Se) and specificity (Sp) reports at every single decision threshold, the receiver operating characteristic (ROC) curve was edited by plotting the true positive values (sensitivity values on y axis) versus the false positive rates (1—specificity values on x-axis). Area under the ROC curve (AUC) was estimated with 95% confidence intervals (95% CIs) and with standard errors (± SD)90.
Postmortem histology
The specificity of Aspergillus infection morphology via Periodic acid–Schiff (PAS) staining was addressed due to the open lung biopsy, performed via postmortem thoracotomy91. Histological samples were taken from the major organs according to a standard protocol. Lung sampling was performed from three independent parts of the potentially infiltrated lung parenchyma.
Ethical statement
The study protocol was approved by the ethics committee of the University Hospitals of Debrecen, Hungary (MK-JA/50/0096-01/2017) and carried out in accordance with the approved guidelines. In the case of postmortem histology informed consent was obtained from the donor’s next of kin. Written consent was obtained from all participants. Measuring of miRNA expression levels were performed in parallel with Aspergillus GM-EIA.
References
Webb, B. J. et al. Epidemiology and clinical features of invasive fungal infection in a US health care network. Open Forum Infect Dis. 5, ofy187 (2018).
Samonis, G. et al. A prospective study of characteristics and outcomes of bacteremia in patients with solid organ or hematologic malignancies. Support Care Cancer 9, 2521–2526 (2013).
Dix, D. et al. Association between corticosteroids and infection, sepsis, and infectious death in pediatric acute myeloid leukemia (AML): results from the Canadian infections in AML research group. Clin. Infect. Dis. 12, 1608–1614 (2012).
Pagano, L. et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study-Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin. Infect. Dis. 9, 1161–1170 (2007).
Badiee, P. & Hashemizadeh, Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J. Med. Res. 2, 195–204 (2014).
Ruhnke, M. et al. Diagnosis of invasive fungal diseases in haematology and oncology: 2018 update of the recommendations of the infectious diseases working party of the German society for hematology and medical oncology (AGIHO). Mycoses 11, 796–813 (2018).
Bone, R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 6, 1644–1655 (1992).
Salih, Z. et al. Prognostic factors for mortality with fungal blood stream infections in patients with hematological and non-hematological malignancies. South Asian J. Cancer 4, 220–224 (2013).
Taccone, F. S. et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit. Care 19, 7 (2015).
Sun, K. S., Tsai, C. F., Chen, S. C. & Huang, W. C. Clinical outcome and prognostic factors associated with invasive pulmonary aspergillosis: an 11-year follow-up report from Taiwan. PLoS ONE 12, e0186422 (2017).
Stubljar, D. & Skvarc, M. Effective strategies for diagnosis of systemic inflammatory response syndrome (SIRS) due to bacterial infection in surgical patients. Infect. Disord. Drug Targets 1, 53–56 (2015).
Di Marco, M. et al. MicroRNAs in autoimmunity and hematological malignancies. Int. J. Mol. Sci. 19, 3139 (2018).
Zilberberg, M. D., Nathanson, B. H., Harrington, R., Spalding, J. R. & Shorr, A. F. Epidemiology and outcomes of hospitalizations with invasive aspergillosis in the United States, 2009–2013. Clin. Infect. Dis. 5, 727–735 (2018).
Dasbach, E. J., Davies, G. M. & Teutsch, S. M. Burden of aspergillosis-related hospitalizations in the United States. Clin. Infect. Dis. 6, 1524–1528 (2000).
Lin, S. J., Schranz, J. & Teutsch, S. M. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 3, 358–366 (2001).
Cornely, O. A. et al. Primary prophylaxis of invasive fungal infections in patients with hematologic malignancies. Recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Haematologica 1, 113–122 (2009).
Shanxin, P. et al. Endogenous cellular MicroRNAs mediate antiviral defense against influenza A virus. Mol. Ther. Nucl. Acids 10, 361–375 (2018).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2, 281–297 (2004).
Huang, W. MicroRNAs: biomarkers, diagnostics, and therapeutics. Methods Mol. Biol. 1617, 57–67 (2017).
Dix, A. et al. Specific and novel microRNAs are regulated as response to fungal infection in human dendritic cells. Front Microbiol. 23, 8–270 (2017).
Saliminejad, K., Khorram Khorshid, H. R. & Ghaffari, S. H. Why have microRNA biomarkers not been translated from bench to clinic?. Future Oncol. 8, 801–803 (2019).
Xu, X., Liu, C. & Bao, J. Hypoxia-induced hsa-miR-101 promotes glycolysis by targeting TIGAR mRNA in clear cell renal cell carcinoma. Mol. Med. Rep. 3, 1373–1378 (2017).
Caserta, S. et al. Circulating plasma microRNAs can differentiate human sepsis and systemic inflammatory response syndrome (SIRS). Sci. Rep. 6, 28006 (2016).
Gupta, M. et al. Aspergillus fumigatus induces microRNA-132 in human monocytes and dendritic cells. Int. J. Med. Microbiol. 304, 592–596 (2014).
Muhammad, S. A. et al. MicroRNA expression profiling of human respiratory epithelium affected by invasive Candida infection. PLoS ONE 10, e0136454 (2015).
Wu, C. X. et al. Microrna expression profiling of macrophage line raw 264.7 infected by Candida albicans. Shock 47, 520–530 (2017).
Springer, J. et al. Multicenter comparison of serum and whole-blood specimens for detection of Aspergillus DNA in high-risk hematological patients. J. Clin. Microbiol. 5, 1445–1450 (2013).
Ceppi, M. et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. USA 8, 2735–2740 (2009).
Cremer, T. J. et al. MiR-155 induction by F. novicida but not the virulent F. tularensis results in SHIP down-regulation and enhanced pro-inflammatory cytokine response. PLoS ONE 4, e8508 (2009).
Dumache, R. et al. Use of miRNAs as biomarkers in sepsis. Anal. Cell Pathol. 2015, 186716 (2015).
Reithmar, M. et al. Cellular and extracellular miRNAs are blood-compartment-specific diagnostic targets in sepsis. J. Cell Mol. Med. 21, 2403–2411 (2017).
Correia, C. N. et al. Circulating microRNAs as potential biomarkers of infectious disease. Front. Immunol. 8, 118 (2017).
Juan, J. G. P. Current roles of microRNAs in infectious diseases—advancing into healthcare. Redni broj lanka 781, 1331–2820 (2016).
Drury, R. E., O’Connor, D. & Pollard, A. J. The clinical application of MicroRNAs in infectious disease. Front. Immunol. 25, 8–1182 (2017).
Lacedonia, D. et al. MicroRNA expression profile during different conditions of hypoxia. Oncotarget 9, 35114–35122 (2018).
Bodey, G. et al. Fungal infections in cancer patients: an international autopsy survey. Eur. J. Clin. Microbiol. Infect. Dis. 2, 99–109 (1992).
Edmond, M. B. et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 2, 239–244 (1999).
Verweij, P. E., Chowdhary, A., Melchers, W. J. & Meis, J. F. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles?. Clin. Infect. Dis. 3, 362–368 (2016).
Marr, K. A., Seidel, K., White, T. C. & Bowden, R. A. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 1, 309–316 (2000).
Ullmann, A. J. Review of the safety, tolerability, and drug interactions of the new antifungal agents caspofungin and voriconazole. Curr. Med. Res. Opin. 4, 263–271 (2003).
Daniel, P. R. et al. Impact of bloodstream infection on outcomes among infected surgical inpatients. Ann. Surg. 4, 549–555 (2001).
Wichmann, M. W., Inthorn, D., Andress, H. J. & Schildberg, F. W. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2, 167–172 (2000).
Zaoutis, T. E. et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect Dis. 9, 1232–1239 (2005).
Morgan, J. et al. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect. Control Hosp. Epidemiol. 6, 540–547 (2005).
Pasupneti, S., Manouvakhova, O., Nicolls, M. R. & Hsu, J. L. Aspergillus-related pulmonary diseases in lung transplantation. Med. Mycol. 1, 96–102 (2017).
Nina, S. & David, L. P. Aspergillus Infections in transplant recipients. Clin. Microbiol. Rev. 1, 44–69 (2005).
Rhodes, A. et al. Surviving sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 3, 304–377 (2017).
Dellinger, R. P. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 1, 296–327 (2008).
Green, R. S. et al. Critical Care Interest Group. Canadian Association of Emergency Physicians Sepsis Guidelines: the optimal management of severe sepsis in Canadian emergency departments. CJEM 5, 443–459 (2008).
Marik, P. E. et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit. Care Med. 6, 1937–1949 (2008).
Pottecher, T., Calvat, S., Dupont, H., Durand-Gasselin, J. & Gerbeaux, P. Haemodynamic management of severe sepsis: recommendations of the French Intensive Care Societies (SFAR/SRLF) Consensus Conference, 13 October 2005, Paris France. Crit. Care 10, 4–311 (2006).
Reinhart, K. et al. Prevention, diagnosis, therapy and follow-up care of sepsis: 1st revision of S-2k guidelines of the German Sepsis Society (Deutsche Sepsis-Gesellschaft e.V. (DSG)) and the German Interdisciplinary Association of Intensive Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (DIVI)). Ger. Med. Sci. 59, 347–370 (2010).
Xie, G. H. et al. Impact of invasive fungal infection on outcomes of severe sepsis: a multicenter matched cohort study in critically ill surgical patients. Crit. Care 12, R5 (2008).
Bos, M. M., Smeets, L. S., Dumay, I. & de Jonge, E. Bloodstream infections in patients with or without cancer in a large community hospital. Infection 5, 949–958 (2013).
Wisplinghoff, H., Seifert, H., Wenzel, R. P. & Edmond, M. B. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 29, 1103–1110 (2003).
Raad, I. I. et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin. Infect. Dis. 12, 1726–1734 (2006).
Kumar, H., Kawai, T. & Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34 (2011).
Taganov, K. D., Boldin, M. P., Chang, K. J. & Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 33, 12481–12486 (2006).
O’Connell, R. M., Taganov, K. D., Boldin, M. P., Cheng, G. & Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 5, 1604–1609 (2007).
Tili, E. et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 8, 5082–5089 (2007).
Zhou, X., Li, X. & Wu, M. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct. Target Ther. 25, 3–14 (2018).
He, Y. et al. miR-26 induces apoptosis and inhibits autophagy in non-small cell lung cancer cells by suppressing TGF-β1-JNK signaling pathway. Front Pharmacol. 9, 1509 (2019).
Viscoli, C. et al. Association between antifungal prophylaxis and rate of documented bacteremia in febrile neutropenic cancer patients. Clin. Infect. Dis. 11, 1532–1537 (2001).
Pfaller, M. A. & Diekema, D. J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 1, 133–163 (2007).
Syrjälä, H. et al. Blood stream infections during chemotherapy-induced neutropenia in adult patients with acute myeloid leukemia: treatment cycle matters. Eur. J. Clin. Microbiol. Infect. Dis. 10, 1211–1218 (2010).
Orasch, C. et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 3, 521–526 (2010).
Nesher, L. & Rolston, K. V. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 1, 5–13 (2014).
Ibrahim, K. Y. et al. Health care-associated infections in hematology-oncology patients with neutropenia: a method of surveillance. Am. J. Infect. Control 11, 1131–1133 (2013).
Viscoli, C. et al. Factors associated with bacteraemia in febrile, granulocytopenic cancer patients. The International Antimicrobial Therapy Cooperative Group (IATCG) of the European Organization for Research and Treatment of Cancer (EORTC). Eur. J. Cancer 4, 430–437 (1994).
Faraldi, M. et al. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci. Rep. 9, 1584 (2019).
Vickers, K. C. et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 4, 423–433 (2011).
Miotto, E. et al. Quantification of circulating miRNAs by droplet digital PCR: comparison of EvaGreen- and TaqMan-based chemistries. Cancer Epidemiol. Biomark. Prev. 12, 2638–2642 (2014).
Moldovan, L. et al. Methodological challenges in utilizing miRNAs as circulating biomarkers. J. Cell Mol. Med. 3, 371–390 (2014).
Kreth, S., Hübner, M. & Hinske, L. C. MicroRNAs as clinical biomarkers and therapeutic tools in perioperative medicine. Anesth Analg. 2, 670–681 (2018).
Xiao, B. et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J. Infect. Dis. 6, 916–925 (2009).
Penack, O., Rempf, P., Graf, B., Blau, I. W. & Thiel, E. Aspergillus galactomannan testing in patients with long-term neutropenia: implications for clinical management. Ann. Oncol. 5, 984–989 (2008).
Pfeiffer, C. D., Fine, J. P. & Safdar, N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin. Infect. Dis. 15, 1417–1427 (2006).
Sarrafzadeh, S. A. et al. The accuracy of serum galactomannan assay in diagnosing invasive pulmonary aspergillosis. Iran. J. Allergy Asthma Immunol. 3, 149–155 (2010).
Kaukonen, K. M., Bailey, M., Pilcher, D., Cooper, D. J. & Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 17, 1629–1638 (2015).
Chambliss, A. B., Butler-Wu, S. M. & Dien Bard, J. Diagnosis and management of sepsis and blood stream infections. J. Appl. Lab. Med. 4, 525–526 (2019).
Peters, R. P., van Agtmael, M. A., Danner, S. A., Savelkoul, P. H. & Vandenbroucke-Grauls, C. M. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 12, 751–760 (2004).
Mancini, N. et al. The era of molecular and other non-culture-based methods in diagnosis of sepsis. Clin. Microbiol. Rev. 1, 235–251 (2010).
Gee, H. E. et al. The small-nucleolar RNAs commonly used for microRNA normalisation correlate with tumour pathology and prognosis. Br. J Cancer. 7, 1168–1177 (2011).
Cornely, O. A. et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 4, 348–359 (2007).
Dykewicz, C. A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2, 139–144 (2001).
Goodman, J. L. et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N. Engl. J. Med. 13, 845–851 (1992).
De Pauw, B. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 12, 1813–1821 (2008).
Bone, R. C. et al. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit. Care Med. 5, 389–393 (1989).
Peltier, H. J. & Latham, G. J. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 5, 844–852 (2008).
Paholcsek, M. et al. Combining standard clinical methods with PCR showed improved diagnosis of invasive pulmonary aspergillosis in patients with hematological malignancies and prolonged neutropenia. BMC Infect. Dis. 15, 251 (2015).
Lehrnbecher, T. et al. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 3, 259–265 (2010).
Acknowledgements
We thank to the National Excellence Program NTP-NTÖ-19-B-0190.
Funding
This study was financially supported by the GINOP-2.3.2–15–2016-00042 project of the Széchenyi 2020 Programme given by the European Union and the Hungarian Government.
Author information
Authors and Affiliations
Contributions
M.P. reviewed and interpreted the results and drafted the manuscript. M.P., E.T., S.B., L.R., R.S.Z. designed the study plan and coordinated the study protocol. E.T. and K.O.N. performed the miRNA isolations, RT-qPCR analysis. G.F. prepared the figures. S.B. reviewed manuscript and conducted critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tolnai, E., Fidler, G., Szász, R. et al. Free circulating mircoRNAs support the diagnosis of invasive aspergillosis in patients with hematologic malignancies and neutropenia. Sci Rep 10, 16532 (2020). https://doi.org/10.1038/s41598-020-73556-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73556-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.