Abstract
This work compares the electrochemical windows of polished and unpolished boron doped diamond (BDD) electrodes with hydrogen and oxygen terminations at a series of temperatures up to 125 °C. The experiment was run at 5 bar pressure to avoid complications due to bubble formation. An alternative method for determining the electrochemical window is compared to the most commonly used method, which defines the window at an arbitrary current density cut-off (Jcut-off) value. This arbitrary method is heavily influenced by the mass transport of the electrolyte and cannot be used to compare electrodes across literature where different Jcut-off values have been used. A linear fit method is described which is less affected by the experimental conditions in a given measurement system. This enables a more accurate comparison of the relative electrochemical window from various diamond electrode types from reported results. Through comparison of polished and unpolished BDD electrodes, with hydrogen and oxygen surface terminations, it is determined that the electrochemical window of BDD electrodes narrows as temperature increases; activation energies are reported.
Similar content being viewed by others
Introduction
Boron doped diamond (BDD) can act as an exceptional electrode material, with the widest electrochemical window of any known material—that is the potential range that can be applied across a working electrode before the onset of either oxidation or reduction of the electrolyte at its surface1,2. To date, there has not been a systematic study reported on how the electrochemical window of BDD is affected by temperature. This has particular relevance to the use of BDD electrodes that are employed in extreme environments, such as oil wells, where the electrode can be exposed to temperatures exceeding 150 °C3. This work aims to give an initial insight into how temperature impacts the electrochemical window of BDD electrodes.
Experimentally, the electrochemical window of an electrode is determined by measuring a polarisation curve over a potential range wide enough to observe the anodic and cathodic decompositions of the electrolyte at the working electrode; the electrochemical window is the region between these two points4. There are several methods that can be employed to define the electrochemical window of an electrode from experimental data. The most commonly used method is to plot the current density (J) in mA/cm2 against the applied potential, then to read off the potential at a defined current density cut off (Jcut-off). The arbitrary choice of the Jcut-off value, reported in the range 0.01–5.0 mA/cm2, can result in electrochemical windows for the same electrode being quoted to differ by as much as 0.9 V4,5,6,7. This method is heavily influenced by mass transport of the electrolyte, meaning that changing the concentration of the electrolyte will affect the electrochemical window recorded4. Therefore, it is not possible to accurately compare electrochemical windows that have been determined using different Jcut-off values. An alternative approach introduced by Olson and Bühlmann has been considered, which was designed to address the inconsistencies that occur when defining the electrochemical window of an electrode by using Jcut-off values8. In this method, linear fits are made of the three sections of the CV curve, before and after the oxidation and reduction of the electrolyte at the working electrode. The intersections of the linear fits are taken to define the electrochemical window4,8. The benefit of this technique is that the defined electrochemical window is less sensitive to the concentration of the electrolyte than when the Jcut-off method is used. Also, this method more closely resembles the method of defining the limits of detection of ion-selective electrodes recommended by IUPAC8,9. Here, these methods are compared and contrasted with the aim of setting a standard approach for the determination of electrochemical windows from experimental data. To facilitate investigation of the methods described and to define an electrochemical window from experimental results, heavily boron doped diamond electrodes ([B] > 1020 atoms/cm3) have been used here with two roughnesses: RA ~ 50 nm and RA ~ 50 µm, half of which are hydrogen terminated (BDDH) and half of which are oxygen terminated (BDDO). BDDH has been shown to have a slightly narrower electrochemical window than BDDO, however, there are some sensing applications for which BDDH is more suitable10,11. The CV measurements are repeated with each electrode over the temperature range 21–125 °C, to identify how the electrochemical window of BDD is affected by temperature. Although pH will also influence the electrochemical window this is outside the scope of this work. Through use of a buffer system, pH 7, we have fixed the pH throughout the experiments.
Experimental methods
Electrochemical grade BDD ([B] > 1020 atoms/cm3) substrates (10 × 10 × 0.5 mm) were purchased from Element Six Ltd. (e6cvd.com). Half of the substrates were unpolished polycrystalline BDD, with surface roughness RA ~ 50 µm. The remaining substrates were polished polycrystalline BDD (pBDD), with surface roughness RA ~ 50 nm. The substrates were laser cut into 3 mm diameter pieces at Laser Micromachining Ltd. (lasermicromachining.com). All chemicals, unless otherwise stated, were purchased from Sigma-Aldrich. Milli-pure water, resistivity 18 MΩ-cm was used throughout (0.22 µm membrane filter).
BDD surface preparation
Prior to processing, the 3 mm diameter BDD and pBDD substrates were cleaned with a highly oxidising acid to remove adventitious carbon, hydrocarbons, and graphitic carbon on the surface of the diamonds. During the acid clean, the substrates were heated to 200 °C for 10 min in a cleaning solution [ammonium persulfate (20 g) and concentrated sulfuric acid (20 g)] before being placed in the rinsing solution [ammonium hydroxide (10 ml) and hydrogen peroxide (10 ml)] for 10 min12. The substrates were rinsed thoroughly with water and dried under N2 gas.
The graphitic carbon content at the surface of the electrodes was assessed with a Renishaw micro-Raman spectrometer (532 nm laser source). The microscope was calibrated using a silicon substrate and the Raman analysis was performed with 20 × magnification, 10 s exposure and an average was taken over ten accumulations. WiRE (v 2.0) software was used for data acquisition.
One each of the BDD and pBDD substrates were hydrogen terminated in an AX5010 Seki Technotron Inc. reactor with H-plasma for 10 min, at 400 °C platen temperature (Williamson Dual Wavelength pyrometer), 700 W power, 35 Torr pressure. One BDD and one pBDD substrate were oxygen terminated via ozone treatment in an Ozone Cleaner NL-UV253, under 10–6 mbar vacuum, at ozone generation of 10 g/h for one hour. The extent of the hydrogen and oxygen terminations at the substrate surfaces was assessed with contact angle measurements, conducted with a Kruss DSA1 contact angle goniometer, using 4 µl water droplets. Kruss DSA1 v1.80 drop shape analysis software was used to determine the contact angle at the three-phase contact point between the water droplet and the electrode surfaces.
Electrode preparations
The 3 mm diameter BDD pieces were metallised with Ti–Pt–Au and soldered to Be–Cu pins to form the working electrode. The electrode construction process involved temperatures sufficient for the formation of the required carbide within the diamond-ohmic contact stack given the duration of the process. The bulkhead into which the electrode was placed was machined from PEEK (polyether ether ketone) in-house. A steel counter electrode (4 mm diameter) and a silver wire reference electrode (1.5 mm diameter) were used. The three electrodes were sealed into the bulkhead body using Loctite Hysol 9483 epoxy (rated to 150 °C), which was injected into the channels from behind the pins while applying constant pressure to the front face to avoid leakage of the epoxy to the BDD surface (which would require vigorous polishing to remove, thus destroying the terminations). The bulkhead did require some polishing before the electrochemical measurements to remove dust and other residues left on the electrode surfaces following the sealing of the bulkhead with epoxy. Contact angle measurements were made after the electrodes were polished with 3 µm diamond slurry (Kemet International Ltd.) on a PSU-M polishing pad (Kemet International Ltd.) to assess the extent of the hydrogen and oxygen terminations following this polishing procedure. These measurements were made with a Kruss DSA1 contact angle goniometer, using 4 µl water droplets, and Kruss DSA1 v1.80 drop shape analysis software was used to determine the contact angle at the three-phase contact point between the water droplet and the electrode surfaces.
Electrochemical measurements
Cyclic voltammetry (CV) measurements, were used to determine the electrochemical window of each BDD working electrode in a 1 M phosphate buffer electrolyte (0.025 M K2HPO4, 0.025 M KH2PO4, 0.1 M KCl) doped with 0.5 mM 3-ferrocenophane sulfonate (prepared in-house at SCR) as a pseudo reference system. Before the CV measurements, the electrodes were polished with a 3 µm diamond slurry (Kemet International Ltd.) on a PSU-M polishing pad (Kemet International Ltd.) and rinsed thoroughly with water.
The bulkhead was placed in a PEEK flow cell using Viton o-rings to seal it into place, then the cell was placed in a steel box (to aid heat transfer). A thermocouple was inserted into the bottom of the cell to measure the temperature. The cell was connected to a two-channel syringe pump system (Syrris Asia pump) via Hastelloy fittings inside an oven. The flow line to the electrode was held at 5 bar pressure, to prevent the electrolyte from boiling at the elevated temperatures measured. The solution was injected into the cell, via a heating coil inside the oven, to fill the sensing chamber above the electrode (approx. 1 mm high). Measurements were made after the temperature in the cell was stable for 10 min. The flow was diverted to waste just before measurement, so that static conditions were achieved at the electrode surface whilst taking a scan and reopened after measurement to flush the sensing chamber. CV staircase scans with upper vertex potential 1.5 V, lower vertex potential − 1.9 V and 0.0085 V step potential were used to determine the electrochemical windows. The CV scans were repeated at the scan rates 01, 0.5, and 1 V/s for each temperature measured (21, 50, 75, 100, and 125 °C) with each electrode. As the electrochemical window is independent of the scan rate, the data from each scan rate was combined with the repeats made using each electrode to determine the error in the observed current.
Electrochemical window determination
Two methods for determining the electrochemical window from the experimental data have been explored, as illustrated in Fig. 1, in which the vertical dotted lines represent where the electrochemical window is defined. Figure 1a shows the current/potential curve obtained experimentally, to which three linear fits have been applied. The electrochemical window is defined as the potential window between the two intersections of the linear fits. Figure 1b illustrates the Jcut-off method for the Jcut-off values 1.0 mA/cm2 and 5.0 mA/cm2. Here the electrochemical window is defined as the potential window between the points where the Jcut-off value intersects with the current density/potential curve.
Comparison of the two methods identified for determining the electrochemical window of an electrode from experimental data (BDD working electrode in a 1 M phosphate buffer electrolyte (0.025 M K2HPO4, 0.025 M KH2PO4, 0.1 M KCl) doped with 0.5 mM 3-ferrocenophane sulfonate as a pseudo reference system). The methods are: (a) taking the intersection of the linear fits of the CV curve (the crossing points of the dashed lines)4,9 and (b) the well-established Jcut-off method that determines the electrochemical window by a predefined current density (J) value of 1.0 or 5.0 mA/cm2.
Results
BDD characterisation
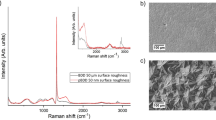
The proportion of non-diamond carbon and sp3 diamond carbon on the BDD surfaces was assessed with Raman spectroscopy before and after the acid cleaning process described in “BDD surface preparation” section (Fig. 2).
The extent of the hydrogen and oxygen terminations imparted by the processes described in “BDD surface preparation” section were qualitatively assessed with contact angle measurements. The measurements were repeated after the electrodes were polished with 3 µm diamond slurry. These give an insight into the hydrophilic/hydrophobic nature of the substrate surfaces before and after they were polished (Table 1).
Electrochemical measurements
The CV measurements made with each electrode were repeated four times at each temperature measured (21, 50, 75, 100, and 125 °C). The average observed current from these repeats with the standard deviation for these values is plotted in Fig. 3.
Combined spectra of the CV average scans and standard deviation across the full temperature range measured for each electrode (a) unpolished BDDH, (b) polished BDDH, (c) unpolished BDDO and (d) polished BDDO at a scan rate of 0.5 V/s (similar results obtained at scan rates 0.1 V/s and 1.0 V/s are in the Supplementary Information).
The activation energy for the oxidation potential for each electrode (Table 2) was determined from the slopes of Arrhenius plots of ln(current density) against the inverse temperature in Kelvin at the applied potential − 1.4 V (Fig. 4), which is part way through the oxidative curve for the electrodes at each temperature measured. By plotting the current densities as a function of inverse temperature we can determine the activation energies of the hydrogen evolution reaction with the Arrhenius equation (Eq. 1) where J is the current density, A is the Arrhenius pre-exponential factor, EA is the activation energy, kb is the Boltzmann constant and T is the absolute temperature.
Electrochemical window determination
The electrochemical window of each electrode was determined by the two methods described in “Electrochemical window determination” section. A comparison of how the electrochemical windows of the electrodes were affected by temperature with each of these methods is shown in Fig. 5. The electrochemical windows determined with the Jcut-off method are heavily influenced by the choice of Jcut-off value. In Fig. 5 this is demonstrated by comparison of the electrochemical windows determined for each electrode with the linear fit method and the Jcut-off method using the Jcut-off values 0.5, 1.0, and 5.0 mA/cm2.
Comparison across the temperature range 21–125 °C of the electrochemical window of each electrode (a) unpolished BDDH, (b) polished BDDH, (c) unpolished BDDO and (d) polished BDDO, as determined by the J cut off method at 0.5 mA/cm2, 1.0 mA/cm2, 5.0 mA/cm2 and the intersection of linear fits method described in “Electrochemical window determination” section.
Discussion
The proportion of non-diamond carbon and sp3 diamond carbon at the BDD surfaces before and after the acid cleaning process was analysed with Raman spectroscopy (Fig. 2). Before the substrates were acid cleaned (inset Fig. 2) the characteristic 1332 cm−1 diamond carbon peak is clearly seen in the centre of the BDD disks. However, at the edge of the disks the 1332 cm−1 peak is broad and has a lower intensity than the non-diamond carbon G peak at 1575 cm−1. This indicates that when the BDD disks were laser-cut from the 10 × 10 cm2 squares purchased from Element Six Ltd. non-diamond carbon was most likely sputtered onto the edge of the diamond surface as a result of the laser ablation12. The acid cleaning procedure removed a significant proportion of this non-diamond carbon from the BDD surfaces, as indicated by the presence of the characteristic 1332 cm−1 diamond carbon peak at both the centre and edge of each substrate, at a significantly greater intensity than the 1575 cm−1 peak. The shape of the 1332 cm−1 peak after the acid clean is indicative of the crystalline quality of the BDD substrates, as a lower quality diamond that contains more defects would have a shorter phonon lifetime and broader line width than seen in Fig. 21.
Comparison of the 1332 cm−1 and 1575 cm−1 peaks can be used to assess the relative proportions of diamond and non-diamond carbon at the substrate surfaces. Although, as the relative intensities of the two peaks are dependent on the grain size, film stress, doping density, and excitation wavelength used, we can only qualitatively compare the two peaks; in the visible region, the sensitivity to sp2 materials is typically 100 times that of sp313. Following the acid clean, the proportion of sp2 carbon at the centre of the substrates is very low when compared to literature values where Raman spectroscopy is supported by other techniques14,15. The intensity of the 1575 cm−1 peak remains high in relation to the 1332 cm−1 diamond peak at the edge of the polished BDD substrate, even after the acid clean. This is likely due to the polishing process introducing damage to the diamond surface, which has not been completely removed during the acid clean.
The broad peaks between 500 cm−1 and 1030 cm−1 in the Raman spectra for both the polished and unpolished BDD substrates indicate a high boron doping concentration (> 1020 boron atoms/cm3) within the diamond structures (Fig. 2)16. The Fano resonance (asymmetry at the base of the 1332 cm−1 peak) further confirms the high concentration of boron in the diamond lattices, as this relates to the onset of metal-like conductivity in the diamond, which is a result of the boron impurity band transitioning into a continuum state17.
The average surface termination across the electrodes was assessed with contact angle measurements (Table 1), after the terminating processes described in “BDD surface preparation” section and again after the electrodes were polished with 3 µm diamond slurry, before the CV measurements. Determination of the exact wetting angle from inspection of contact angle measurements is open to error, however, the values for the contact angle on the polished and unpolished BDDH electrodes after termination are consistent with a strongly hydrophobic and therefore hydrogen terminated surface, when compared to literature18. After polishing with 3 µm diamond slurry the contact angles on the unpolished and polished BDDH were reduced to 78° and 55° respectively, which corresponds to the hydrogen termination being damaged and the surfaces therefore becoming more hydrophilic. Both sets of contact angles for the unpolished and polished BDDO electrodes are indicative of a hydrophilic and therefore predominantly oxygen terminated surface19. The contact angle for a fully oxidised diamond surface would be expected to be < 30°, although, diamond is considered to be predominantly oxygen terminated when the contact angle is between 0 and 601,20.
Contact angle measurements are heavily influenced by the environment of the measurement, not just the electrode itself, especially in the case of small electrode sizes. This means that absolute contact angle measurements contain too much error for direct interpretation. However, relative measurements can still be informative. Here, BDDO contact angles were considerably smaller than for BDDH, as expected. The high temperature measurement did not, at least for the duration of the experiments carried out here, lead to any significant change in either. BDDH with a contact angle of 78° ± 1.0° after polishing was subsequently measured at 78° ± 2.0° after the high temperature measurements; BDDO (31° ± 1.0° after polishing) was subsequently measured at 30° ± 1.5°.
As the temperature of the CV measurements was increased from 21 to 125 °C the onset of oxidation and reduction of the electrolyte at the working BDD electrodes occurred at smaller positive and negative potentials. Therefore, the electrochemical window for each electrode narrowed as the temperature was increased (Fig. 3).
The Arrhenius plots in Fig. 4 show that the current densities of the hydrogen evolution reaction (reduction of the electrolyte) recorded at − 1.4 V for each electrode are temperature dependent. The anomalous peaks at − 0.5 V, − 0.2 V, and 1.25 V lead to analogously high current densities for the hydrogen evolution peak at − 1.4 V for both polished electrodes (BDDH and BDDO), so these points were omitted from the data used to make the Arrhenius plots. The activation energies derived from the Arrhenius plots are shown with the standard error in these values in Table 2. The activation energies are higher and with smaller errors for the unpolished electrodes compared to the polished electrodes, which corresponds to the wider electrochemical windows reported for the unpolished electrodes in Fig. 5, for both methods of determination (with Jcut-off 0.1 mA/cm2).
The unpolished electrodes have a lower proportion of sp2 to sp3 carbon at their surface (Fig. 2) than the polished electrodes, meaning that a wider electrochemical window is expected21. When the proportion of sp2 carbon is higher, as with the polished electrodes, the oxidation mechanism at the BDD surfaces is affected, leading to changes in the electrocatalytic properties of the electrode surface and reducing the barrier activation to the evolution of hydrogen and reduction of oxygen at the electrode surface, therefore reducing the electrochemical window21,22. The activation energies are slightly higher for the BDDO electrodes than the BDDH electrodes (both polished and unpolished), as reported previously10. However, the different terminations resulted in significantly smaller changes in the activation energy and electrochemical windows reported than the different surface roughness of the electrodes.
In Fig. 3b,d there are analogous peaks at − 0.5 V, − 0.2 V, and 1.25 V for the CV spectra at 125 °C for both polished electrodes (BDDO and BDDH). As nothing changed in the system other than BDD electrode used, these peaks must be a result of the changes induced by the higher sp2/sp3 carbon ratio at the surfaces of the polished electrodes21,22. In existing literature there are no reports of CV spectra for similar electrodes above 100 °C, so we are unable to make comparisons between this change in the CV spectra and other results. Further investigation is needed.
The linear fit method resulted in electrochemical windows that were apparently narrower at room temperature, than when the Jcut-off method was used, and less impacted by increasing temperature (Fig. 5). As the temperature of the CV measurements increased and the electrochemical windows narrowed, the linear fits of the curve remained similar. The significant change is that the curves at the beginning of oxidation and reduction of the electrolyte become shallower as the temperature increases (Fig. 6). The change in curvature is more subtle to the linear fit method, meaning that for the same experimental results the electrochemical window will appear to undergo less change with increasing temperature than when the Jcut-off method is used.
To demonstrate the extent to which the arbitrary choice of the Jcut-off value affects the determined electrochemical window, we compared three Jcut-off values: 0.5, 1.0, and 5.0 mA/cm2 with the linear fit method (Fig. 5). In Fig. 5c the electrochemical window determined with Jcut-off 0.5 mA/cm2 is 2.31 V, but when determined with Jcut-off 5 mA/cm2 it is 0.88 V lower, at 1.43 V. This is a significant difference. It is not possible to compare the electrochemical windows quoted across literature where different Jcut-off values have been used and as this method is heavily influenced by the mass transport of the electrolyte it is only accurate to compare electrochemical windows for electrodes that have been determined under the same experimental conditions.
Particularly for the unpolished BDDH and BDDO electrodes (Fig. 5a,c), the linear fit method has a similar trend to the Jcut-off method using 5.0 mA/cm2. This highlights the importance of choosing an appropriate Jcut-off value because it is shown here that the linear fit method is less sensitive to changes in the curvature of the hydrogen evolution or oxygen reduction reactions (Fig. 6), so a less accurate method of determining the electrochemical window. This overshadows the aim of the linear fit method, to determine electrochemical windows by a method that is less influenced by the concentration of the electrolyte than the Jcut-off method, meaning that comparison between literature is possible4.
The electrochemical windows reported in these results were shown to narrow as the temperature of the measurements increased for each of the electrodes. This means that when using these electrodes in high temperature environments the user would need to consider the range of ions that it would be possible to identify, as redox peaks outside the electrochemical window would not be detected. However, each electrode would still be appropriate for use at high temperatures when analysing redox peaks within this range. The unpolished electrodes would offer the most versatility as these electrodes have the widest electrochemical windows, so allow the detection of the widest range of ions.
Conclusions
This work has been an investigation into how the electrochemical window of BDD electrodes is affected by temperature and how the method used to determine the electrochemical window from experimental data can affect the apparent result. The experiment was run at 5 bar pressure to avoid complications due to bubble formation. For every electrode, the electrochemical windows became narrower as the temperature increased from 21 to 125 °C, which is to be expected since the redox reaction is thermally activated. The widest electrochemical windows were reported for the unpolished electrodes, which have a lower proportion of sp2 carbon at their surfaces in comparison to the polished electrodes. The influence on the ratio between sp2 and sp3 carbon at the electrode surfaces was shown to have a much more significant impact on the electrochemical window value reported than the hydrogen or oxygen termination of the diamonds.
A reliable standard procedure for determining electrochemical windows has been sought that could be used to make accurate comparisons across the published literature, unlike the commonly used Jcut-off method. The linear fit method proposed by Olson and Bühlmann is less affected by the assumptions required in the Jcut-off method; the application of this method to the results reported across the published literature would therefore enable a more accurate comparison of the variation in the value of the electrochemical window due to varying measurement conditions. This is valuable to select one type of electrode versus another. However, it is the electrochemical window determined when using the Jcut-off method, with a carefully selected Jcut-off value, that will give the effective electrochemical window that will be relevant for a given experimental arrangement where sensitivity to a given current level is required.
References
Macpherson, J. V. A practical guide to using boron doped diamond in electrochemical research. Phys. Chem. Chem. Phys. 17, 2935–2949 (2015).
Svítková, J., Ignat, T., Švorc, L., Labuda, J. & Barek, J. Diamond sensors for applications to biosensors and biosensing. Crit. Rev. Anal. Chem. 46, 248–256 (2016).
Shadravan, A. & Amani, M. HPHT 101: What every engineer or geoscientist should know about high pressure high temperature wells. Soc. Pet. Eng. J. SPE-163376-MS, 917–943 (2012).
Mousavi, M. P. S. et al. Unbiased quantification of the electrochemical stability limits of electrolytes and ionic liquids. J. Electrochem. Soc. 162, A2250–A2258 (2015).
O’Mahony, A. M., Silvester, D. S., Aldous, L., Hardacre, C. & Compton, R. G. Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J. Chem. Eng. Data 53, 2884–2891 (2008).
Xu, K., Ding, S. P. & Jow, T. R. Towards reliable values of electrochemical stability limits for electrolytes. J. Electrochem. Soc. 146, 4172–4178 (1999).
De Vos, N., Maton, C. & Stevens, C. V. Electrochemical stability of ionic liquids: General influences and degradation mechanisms. ChemElectroChem 1, 1258–1270 (2014).
Olson, E. J. & Bühlmann, P. Unbiased assessment of electrochemical windows: Minimizing mass transport effects on the evaluation of anodic and cathodic limits. J. Electrochem. Soc. 160, A320–A323 (2013).
Lindner, E. & Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro- and microfabricated ion-selective electrodes (IUPAC technical report). Pure Appl. Chem. 80, 85–104 (2008).
Tryk, D., Tsunozaki, K., Rao, T. N. & Fujishima, A. Relationship between surface character and electrochemical processes on diamond electrodes: Dual roles of surface termination and near-surface hydrogen. Diam. Relat. Mater. 10, 1804–1809 (2001).
McLaughlin, M. H. S., Pakpour-Tabrizi, A. C. & Jackman, R. B. Diamond electrodes for high sensitivity mercury detection in the aquatic environment: Influence of surface preparation and gold nanoparticle activity. Electroanalysis 31, 1775–1782 (2019).
Baral, B., Chan, S. S. M. & Jackman, R. B. Cleaning thin-film diamond surfaces for device fabrication: An Auger electron spectroscopic study. J. Vac. Sci. Technol. A 14, 2303–2307 (1996).
Bormett, R. W. et al. Ultraviolet Raman spectroscopy characterizes chemical vapor deposition during diamond film growth and oxidation. J. Appl. Phys. 77, 5916–5923 (1995).
Coffman, F. L. et al. Near-edge X-ray absorption of carbon materials for determining bond hybridization in mixed sp2/sp3 bonded materials. Appl. Phys. Lett. 69, 568–570 (1996).
Huang, L. J. et al. Synchrotron radiation X-ray absorption of ion bombardment induced defects on diamond (100). J. Appl. Phys. 76, 7483–7486 (1994).
Prawer, S. & Nemanich, R. J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. A 362, 2537–2565 (2004).
Read, T. L., Bitziou, E., Joseph, M. B. & Macpherson, J. V. In situ control of local pH using a boron doped diamond ring disk electrode: Optimizing heavy metal (mercury) detection. Anal. Chem. 86, 367–371 (2014).
Zielinski, A. et al. Multifrequency nanoscale impedance microscopy (m-NIM): A novel approach towards detection of selective and subtle modifications on the surface of polycrystalline boron-doped diamond electrodes. Ultramicroscopy 199, 34–45 (2019).
Mertens, M. et al. Patterned hydrophobic and hydrophilic surfaces of ultra-smooth nanocrystalline diamond layers. Appl. Surf. Sci. 390, 526–530 (2016).
Williams, O. A. & Jackman, R. B. Surface conductivity on hydrogen terminated diamond. Semicond. Sci. Technol. 18, S34–S40 (2003).
Einaga, Y., Foord, J. S. & Swain, G. M. Diamond electrodes diversity and maturity. Mater. Res. Bull. 39, 525–532 (2014).
Garcia-Segura, S., Dos Santos, E. V. & Martínez-Huitle, C. A. Role of sp3/sp2 ratio on the electrocatalytic properties of boron-doped diamond electrodes: A mini review. Electrochem. Commun. 59, 52–55 (2015).
Acknowledgements
MHSM thanks the UKs Engineering and Physical Sciences Research Council (EPSRC) and Schlumberger Cambridge Research Ltd. (SCR) for the award of a CASE PhD studentship (EP/N509577/1).
Author information
Authors and Affiliations
Contributions
The work was designed and construed by R.B.J., M.H.S.M., A.P.-T. and D.C.F., and carried out by M.H.S.M. and E.C., under the supervision of R.B.J., A.P.-T. and D.C.F. Data analysis was driven by M.H.S.M., but involved all authors. All authors contributed to the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McLaughlin, M.H.S., Corcoran, E., Pakpour-Tabrizi, A.C. et al. Influence of temperature on the electrochemical window of boron doped diamond: a comparison of commercially available electrodes. Sci Rep 10, 15707 (2020). https://doi.org/10.1038/s41598-020-72910-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72910-x
This article is cited by
-
Electrified water treatment: fundamentals and roles of electrode materials
Nature Reviews Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.