Abstract
An association between appendectomy and subsequent gastrointestinal (GI) cancer development has been postulated, although the evidence is limited and inconsistent. To provide clarification, we investigated the link between appendectomy and GI cancers in a large nationwide appendectomy cohort. This cohort was derived from the claims database of the National Health Insurance Service in South Korea and comprised 158,101 patients who had undergone appendectomy between 2007 and 2014. A comparison cohort of 474,303 subjects without appendectomy was selected after 1:3 matching by age and sex. The incidence of GI cancers after appendectomy was observed, and risk factors for GI cancers were determined by using a multivariable-adjusted proportional hazards model. Appendectomy did not significantly increase the incidence of GI cancers in the overall population (1.529 and 1.557 per 1000 person-years in the non-appendectomy and appendectomy cohorts, respectively). However, appendectomy significantly increased the incidence of GI cancers in subgroups consisting of elderly (≥ 60 years) patients (adjusted HR, 1.102; 95% confidence interval, 1.011–1.201; p = 0.028) or women (adjusted HR, 1.180; 95% confidence interval, 1.066–1.306; p = 0.001).
Similar content being viewed by others
Introduction
Appendectomy is one of the most frequently performed surgical procedures. The belief that the appendix is a vestigial structure whose removal merely affects clinical outcome has been challenged over decades1. As a gut-associated lymphoid tissue, the appendix mediates host immune functions and hence might defend against enteric pathogens initiating malignant changes1,2.
The appendix also serves as a so-called “safe house” for biofilms, enabling preservation of the colonic microbiome2,3,4. Given the recently suggested link between the gut microbiome and gastrointestinal (GI) cancer, appendectomy might result in dysbiosis and consequent cancer development5,6,7,8,9,10,11,12,13,14. However, the association between appendectomy and GI cancer is controversial1,15,16,17,18, and epidemiologic reports using large databases to address this issue are limited in number15,17.
Therefore, we aimed to investigate the link between appendectomy and GI cancer development and to identify subgroups with a high risk of GI cancer by analysing a large-scale national appendectomy cohort in South Korea.
Methods
Study subjects and data collection
This study used the claims database of the National Health Insurance Service (NHIS), which was established for reimbursement of South Koreans covered by the NHIS or the medical aid program. The study protocol was approved by the official review committee of the National Health Insurance Corporation. Informed consent was waived by the Institutional Review Board of Uijeongbu St. Mary’s Hospital, Catholic University of Korea in South Korea (No. UC19ZESI0003).
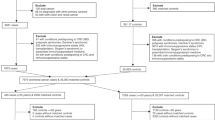
Using the NHIS claims database, we identified 807,275 patients who underwent appendectomy (NHIS procedure codes Q2861, Q2862, and Q2863) between 2007 and 2014 (Fig. 1). Among these patients, 164,112 had participated in the NHIS screening program. Demographic data retrieved from the program participants included age, sex, body mass index (BMI), smoking status, alcohol consumption, and history of hypertension, diabetes mellitus (DM), dyslipidaemia, and colorectal cancer. To avoid enrolling patients with pre-existing GI cancers, we excluded program participants with any GI cancers before appendectomy or who developed any GI cancers or died within 1 year after appendectomy. Finally, we identified an appendectomy cohort of 158,101 patients and a non-appendectomy cohort of 474,303 patients without appendectomy matched by age and sex (1:3).
The primary outcome was the number of newly developed GI cancers, which were defined in accordance with the International Classification of Diseases, 10th revision. The codes for oesophageal, gastric, small bowel, and colorectal cancer were C15, C16, C17, and C18–C20, respectively.
Obesity was defined as a BMI ≥ 25 kg/m2, and hypertension as a claim for antihypertensive agents owing to a diagnosis of I10–I15 or a systolic/diastolic blood pressure ≥ 140/90 mmHg. DM was defined as a claim for antidiabetic agents owing to a diagnosis of E10–14 or a fasting glucose level ≥ 7 mmol/L according to the health screening program database. Dyslipidaemia was defined as a claim for antihyperlipidemic agents owing to a diagnosis of E78 or a total cholesterol level ≥ 6.21 mmol/L according to the health screening program database19.
Statistical analyses
Student's t-test and the chi-square test were used to analyse continuous and categorical variables, respectively. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of GI cancers in the appendectomy vs. non-appendectomy cohort were determined using Cox proportional hazards regression models. For estimation of adjusted HRs (aHRs), a model adjusted for age, sex, BMI, smoking status, alcohol consumption, hypertension, DM, dyslipidaemia, and appendectomy was used. Kaplan–Meier curves for incidence probability were constructed and were compared using the log–rank test. A p value < 0.05 was considered significant. Statistical analyses were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Demographic characteristics of the study population
The mean age of the patients in our study was 44.06 ± 14.09 years, and the median follow-up duration was 5.6 (3.78–7.59) years. The demographic characteristics of the appendectomy and non-appendectomy cohorts are shown in Table 1. Percentagewise, more subjects were current smokers or obese, consumed alcohol, or had hypertension or DM in the non-appendectomy vs. appendectomy cohort; however, the absolute differences were small. The proportion of the subjects diagnosed with GI cancers did not differ between the cohorts.
Incidence of GI cancers in the overall population
The incidence of all GI cancers did not differ significantly in the non-appendectomy and appendectomy cohorts (1.529 and 1.557 per 1,000 person-years [PY], respectively) (Table 2). Risk analyses identified age, sex, obesity, current smoking, alcohol consumption, hypertension, DM, and dyslipidaemia as risk factors for GI cancers in both crude and multiple regression models.
Risk analysis was also performed for each type of GI cancers. In the non-appendectomy and appendectomy cohorts, the incidence ratios per 1,000 PY were 0.068 and 0.077 for oesophageal cancer, 0.781 and 0.760 for gastric cancer, 0.025 and 0.028 for small bowel cancer, and 0.738 and 0.774 for colorectal cancer, respectively. There was no significant association between appendectomy and any type of GI cancers in analyses adjusted for age, sex, obesity, current smoking, alcohol consumption, hypertension, DM, and dyslipidaemia (Table 3). Kaplan–Meier curves showed no significant difference in the cumulative proportional incidence of GI cancers, either as a whole (Fig. 2) or per type, between the non-appendectomy and appendectomy cohorts.
Subgroup analyses for the incidence of GI cancers
We also analysed patients stratified by three risk factors for GI cancers: age (< 60 years vs. ≥ 60 years), sex (male vs. female), and DM (non-DM vs. DM). The incidence of all GI cancers was significantly higher in the appendectomy cohort (5.342 per 1,000 PY) than in the non-appendectomy (4.910 per 1,000 PY) cohort, showing aHR, 1.102 (95% CI of 1.011–1.201, p = 0.028) in elderly (≥ 60 years) subgroup. It was also significantly higher in the appendectomy cohort (1.346 per 1,000 PY) than in the non-appendectomy cohort (1.147 per 1,000 PY), showing aHR of 1.180 (95% CI of 1.066–1.306, p = 0.001) in female subgroup (Table 4).
Table 5 presents the subgroup analyses for each type of GI cancers. Appendectomy did not significantly correlate with gastric or small bowel cancer in any of the subgroups. However, it was significantly associated with oesophageal cancer in the female subgroup (aHR, 2.742; 95% CI, 1.289–5.835; p = 0.009) and with colorectal cancer in the elderly (aHR, 1.145; 95% CI, 1.012–1.295; p = 0.032) and the female (aHR, 1.243; 95% CI, 1.09–1.418; p = 0.001) subgroups.
We further stratified the subjects by sex and age into 4 subgroups (males under 60 years, females under 60 years, males ≥ 60 years and females ≥ 60 years) to minimize possible confounding effect which might arose from disproportion of age distribution within the female subgroup and disproportion of sex distribution within the elderly subgroups. Appendectomy did not increase the risk of overall GI cancers in the 3 subgroups except the elderly female subgroup (aHR 1.229, 95% CI of 1.066–1.418). As regards to each type of GI cancers, the appendectomy cohort showed higher aHR for oesophageal cancer (aHR, 4.432; 95% CI of 1.576–12.462) and colorectal cancer (aHR 1.349; 95% CI of 1.119–1.626) compared to the non-appendectomy cohort within the elderly female subgroup. However, appendectomy did not increase risk of small bowel cancer and gastric cancer within the elderly female subgroup (Supplementary Table S1 online).
Discussion
This large-scale population-based cohort study showed no significant association between appendectomy and GI cancer in the overall population. However, there was a significant association between appendectomy and GI cancers within elderly patients ≥ 60 years and within female patients. In analyses adjusted for BMI, smoking status, alcohol consumption, hypertension, DM, and dyslipidaemia, the appendectomized female subgroup was susceptible to oesophageal cancer and colorectal cancer, whereas the appendectomized elderly subgroup was susceptible to colorectal cancer.
The appendix houses a diverse colonic microbiome and, as a gut-associated lymphoid tissue, is part of the immune system. Therefore, its removal might decrease microbial diversity and hamper host immune function. Appendectomized subjects might be susceptible to dysbiosis and unable to mount a microbial response to counteract or adapt to the dysbiosis owing to loss of the microbiome. By altering metabolite levels and impairing host immune responses, persistent dysbiosis can promote the development of various diseases such as inflammatory bowel disease, celiac disease, cardiovascular disease, DM, rheumatoid arthritis, osteoarthritis, and even neurologic disorders via the brain–gut axis20,21,22,23,24,25,26,27. A significant association between appendectomy and these diseases has been observed in some large cohort studies28,29,30,31,32,33,34.
Dysbiosis has also been linked to lung, breast, and GI cancers5,6,7,8,12,13,14. Because interactions between the microbiome and the host are more direct in the GI tract than in other organs, we can postulate that appendectomy critically affects the pathogenesis of malignancy by interrupting the microbial ecosystem in the GI tract. However, only a few studies have examined the risk of GI cancers after appendectomy, and the results are conflicting15,17,35.
Although appendectomy did not increase the risk of GI cancers in our entire study cohort, it did increase risk in the elderly and female subgroups. Along with intestinal physiologic and nutritional changes, intestinal microbe diversity decreases in the elderly36,37. Furthermore, age-related impairment of immune function could enable the evolution of the bacterial strains responsible for dysbiosis and elderly-specific infectious conditions 36,37,38. Biofilms are most abundant in the appendix, followed in order by the cecum and ascending colon3,4. Taken together, the microbial ecosystem would not be resilient after appendectomy owing to its depletion; moreover, the underlying diminished microbial diversity caused by aging could accelerate and perpetuate dysbiosis.
Differences in microbial composition between men and women have been reported by the Human Microbiome Project consortium, the Belgian Flemish Gut Flora Project, and the Dutch LifeLines-DEEP study; all found greater α-diversity in women39,40. Although the incidence of GI cancers is lower in women than in men, microbial adaptation or resilience and consequent eubiosis might be more challenging in appendectomized women than in women with an intact appendix in post-infectious conditions.
This study has some limitations. First, incidental appendectomy was not differentiated from appendectomy due to appendicitis; therefore, we could not assess the impact of incidental appendectomy in the non-inflammatory context. Second, irritable bowel disease and pre-existing adenomas were not taken into account in the risk analysis. Considering the fact that irritable bowel disease might be suppressed by appendectomy and colonic adenomas are premalignant lesions, they are potential hidden confounding factors28,29. Third, the follow-up period in our study was shorter than the conventional follow-up period of 10 years, which is based on the time frame (7–10 years on average) of the adenoma to carcinoma transition15,41,42. However, in the large cohort study by Wu et al.15, the overall colorectal cancer incidence was highest during the first 1.5–3.5 years after appendectomy; therefore, a median follow-up duration of 5.6 (3.78–7.59) years is acceptable. Further, we applied one year of lag time for wash-out to exclude patients with pre-existing but undetected cancers at the index date.
In conclusion, this large-scale population-based study showed that appendectomy did not increase the risk of GI cancers in the overall population. However, it identified elderly patients and female patients as at-risk subpopulations owing to their higher rates of GI cancers after appendectomy.
Change history
17 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-85952-6
References
James, R. & McVay, J. The appendix in relation to neoplastic disease. Cancer 17, 929–937. https://doi.org/10.1002/1097-0142 (1964).
Rhee, K. J., Sethupathi, P., Driks, A., Lanning, D. K. & Knight, K. L. Role of commensal bacteria in development of gut-associated lymphoid tissues and preimmune antibody repertoire. J. Immunol. 172, 1118–1124. https://doi.org/10.4049/jimmunol.172.2.1118 (2004).
Bollinger, R. R. et al. Biofilms in the normal human large bowel: fact rather than fiction. Gut 56, 1481–1482 (2007).
Bollinger, R. R., Barbas, A. S., Bush, E. L., Lin, S. S. & Parker, W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J. Theor. Biol. 249, 826–831. https://doi.org/10.1016/j.jtbi.2007.08.032 (2007).
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V. & Wargo, J. A. The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388. https://doi.org/10.1038/s41591-019-0377-7 (2019).
Wong, S. H. & Yu, J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat. Rev. Gastroenterol Hepatol. 16, 690–704. https://doi.org/10.1038/s41575-019-0209-8 (2019).
Gao, Z., Guo, B., Gao, R., Zhu, Q. & Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 6, 20. https://doi.org/10.3389/fmicb.2015.00020 (2015).
Abdulamir, A. S., Hafidh, R. R. & Bakar, F. A. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol. Cancer 9, 249. https://doi.org/10.1186/1476-4598-9-249 (2010).
Kostic, A. D. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298. https://doi.org/10.1101/gr.126573.111 (2012).
Kasai, C. et al. Comparison of human gut microbiota in control subjects and patients with colorectal carcinoma in adenoma: Terminal restriction fragment length polymorphism and next-generation sequencing analyses. Oncol. Rep. 35, 325–333. https://doi.org/10.3892/or.2015.4398 (2016).
Shanan, S., Gumaa, S. A., Sandstrom, G. & Abd, H. Significant association of streptococcus bovis with malignant gastrointestinal diseases. Int. J. Microbiol. 2011, 792019. https://doi.org/10.1155/2011/792019 (2011).
Peters, B. A. et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome 4, 69. https://doi.org/10.1186/s40168-016-0218-6 (2016).
Wang, T. et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 6, 320–329. https://doi.org/10.1038/ismej.2011.109 (2012).
Alhinai, E. A., Walton, G. E. & Commane, D. M. The role of the gut microbiota in colorectal cancer causation. Int. J. Mol. Sci. 20, 5295. https://doi.org/10.3390/ijms20215295 (2019).
Wu, S. C. et al. Association between appendectomy and subsequent colorectal cancer development: an Asian population study. PLoS ONE 10, e0118411. https://doi.org/10.1371/journal.pone.0118411 (2015).
Friedman, G. D. & Fireman, B. H. Appendectomy, appendicitis, and large bowel cancer. Cancer Res 50, 7549–7551 (1990).
Song, H., Abnet, C. C., Andren-Sandberg, A., Chaturvedi, A. K. & Ye, W. Risk of gastrointestinal cancers among patients with appendectomy: a large-scale swedish register-based cohort study during 1970–2009. PLoS ONE 11, e0151262. https://doi.org/10.1371/journal.pone.0151262 (2016).
Hyams, L. & Wynder, E. L. Appendectomy and cancer risk. J. Chronic Dis. 21, 391–415. https://doi.org/10.1016/0021-9681(68)90003-9 (1968).
Yang, H. et al. Obesity, metabolic health, and mortality in adults: a nationwide population-based study in Korea. Sci. Rep. 6, 30329. https://doi.org/10.1038/srep30329 (2016).
Nishida, A. et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11, 1–10. https://doi.org/10.1007/s12328-017-0813-5 (2018).
Girbovan, A., Sur, G., Samasca, G. & Lupan, I. Dysbiosis a risk factor for celiac disease. Med. Microbiol. Immunol. 206, 83–91. https://doi.org/10.1007/s00430-017-0496-z (2017).
Yoshida, N., Yamashita, T. & Hirata, K. I. Gut microbiome and cardiovascular diseases. Diseases 6, 56. https://doi.org/10.3390/diseases6030056 (2018).
Sircana, A. et al. Altered gut microbiota in type 2 diabetes: just a coincidence?. Curr. Diab. Rep. 18, 98. https://doi.org/10.1007/s11892-018-1057-6 (2018).
Horta-Baas, G. et al. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. J. Immunol. Res. 2017, 4835189. https://doi.org/10.1155/2017/4835189 (2017).
Szychlinska, M. A., Di Rosa, M., Castorina, A., Mobasheri, A. & Musumeci, G. A correlation between intestinal microbiota dysbiosis and osteoarthritis. Heliyon 5, e01134. https://doi.org/10.1016/j.heliyon.2019.e01134 (2019).
Griffiths, J. A. & Mazmanian, S. K. Emerging evidence linking the gut microbiome to neurologic disorders. Genome Med. 10, 98. https://doi.org/10.1186/s13073-018-0609-3 (2018).
Maguire, M. & Maguire, G. Gut dysbiosis, leaky gut, and intestinal epithelial proliferation in neurological disorders: towards the development of a new therapeutic using amino acids, prebiotics, probiotics, and postbiotics. Rev. Neurosci. 30, 179–201. https://doi.org/10.1515/revneuro-2018-0024 (2019).
Parian, A. et al. Appendectomy does not decrease the risk of future colectomy in UC: results from a large cohort and meta-analysis. Gut 66, 1390–1397. https://doi.org/10.1136/gutjnl-2016-311550 (2017).
Stellingwerf, M. E. et al. The risk of colectomy and colorectal cancer after appendectomy in patients with ulcerative colitis: a systematic review and meta-analysis. J. Crohns Colitis 13, 309–318. https://doi.org/10.1093/ecco-jcc/jjy163 (2019).
Tzeng, Y. M. et al. An appendectomy increases the risk of rheumatoid arthritis: a five-year follow-up study. PLoS ONE 10, e0126816. https://doi.org/10.1371/journal.pone.0126816 (2015).
Lee, Y. M. et al. Impact of age at appendectomy on development of type 2 diabetes: A population-based cohort study. PLoS ONE 13, e0205502. https://doi.org/10.1371/journal.pone.0205502 (2018).
Kaplan, G. G. et al. The risk of developing Crohn’s disease after an appendectomy: a population-based cohort study in Sweden and Denmark. Gut 56, 1387–1392. https://doi.org/10.1136/gut.2007.121467 (2007).
Janszky, I., Mukamal, K. J., Dalman, C., Hammar, N. & Ahnve, S. Childhood appendectomy, tonsillectomy, and risk for premature acute myocardial infarction–a nationwide population-based cohort study. Eur. Heart J. 32, 2290–2296. https://doi.org/10.1093/eurheartj/ehr137 (2011).
Lai, S. W., Lin, C. L., Liao, K. F. & Tsai, S. M. Increased risk of pulmonary tuberculosis among patients with appendectomy in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1573–1577. https://doi.org/10.1007/s10096-014-2112-0 (2014).
Mellemkjær, L. et al. Cancer risk following appendectomy for acute appendicitis (Denmark). Cancer Causes Control 9, 183–187 (1998).
Salazar, N., Valdes-Varela, L., Gonzalez, S., Gueimonde, M. & de Los Reyes-Gavilan, C. G. Nutrition and the gut microbiome in the elderly. Gut Microbes 8, 82–97. https://doi.org/10.1080/19490976.2016.1256525 (2017).
Aleman, F. D. D. & Valenzano, D. R. Microbiome evolution during host aging. PLoS Pathog. 15, e1007727. https://doi.org/10.1371/journal.ppat.1007727 (2019).
Singh, P. & Manning, S. D. Impact of age and sex on the composition and abundance of the intestinal microbiota in individuals with and without enteric infections. Ann. Epidemiol. 26, 380–385. https://doi.org/10.1016/j.annepidem.2016.03.007 (2016).
Human Microbiome Project. C. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. https://doi.org/10.1038/nature11234 (2012).
Falony, G. et al. Population-level analysis of gut microbiome variation. Science 352, 560–564. https://doi.org/10.1126/science.aad3503 (2016).
Brenner, H., Kloor, M. & Pox, C. P. Colorectal cancer. The Lancet 383, 1490–1502. https://doi.org/10.1016/s0140-6736(13)61649-9 (2014).
Nakao, S. K., Fassler, S., Sucandy, I., Kim, S. & Zebley, D. M. Colorectal cancer following negative colonoscopy: is 5-year screening the correct interval to recommend?. Surg. Endosc. 27, 768–773. https://doi.org/10.1007/s00464-012-2543-6 (2013).
Acknowledgements
The authors wish to acknowledge the financial support of The Catholic University of Korea Uijeongbu St. Mary’s Hospital Clinical Research Laboratory Foundation made in the program year of 2019.
Author information
Authors and Affiliations
Contributions
Y.Y.P. and J.I.L. conceived the study, analysed and interpreted the data, and primarily wrote the manuscript. Y.Y.P., K.D.H., and S.H.P. collected and analysed the data. Y.Y.P., J.I.L., S.T.O., and K.-Y.L. interpreted the data. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Data source
This study used NHIS data (NHIS-2019-1-089) provided by the NHIS in South Korea.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, Y.Y., Lee, Ky., Oh, S.T. et al. A link between appendectomy and gastrointestinal cancers: a large-scale population-based cohort study in Korea. Sci Rep 10, 15670 (2020). https://doi.org/10.1038/s41598-020-72770-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72770-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.