Abstract
Palaeoenvironmental reconstructions of the interior of South Africa show a wetter environment than today and a non-analogous vegetation structure in the Early Pleistocene. This includes the presence of grasses following both C3 and C4 photosynthetic pathways, whereas C3 grasses decline after the mid-Pleistocene transition (MPT, c. 1.2–0.8 Ma). However, the local terrestrial proxy record cannot distinguish between the potential drivers of these vegetation changes. In this study we show that low glacial CO2 levels, similar to those at the MPT, lead to the local decline of C3 grasses under conditions of decreased water availability, using a vegetation model (LPX) driven by Atmosphere–Ocean coupled General Climate Model climate reconstructions. We modelled vegetation for glacial climates under different levels of CO2 and fire regimes and find evidence that a combination of low CO2 and changed seasonality is driving the changes in grass cover, whereas fire has little influence on the ratio of C3:C4 grasses. Our results suggest the prevalence of a less vegetated landscape with limited, seasonal water availability, which could potentially explain the much sparser mid-Pleistocene archaeological record in the southern Kalahari.
Similar content being viewed by others
Introduction
South Africa can be divided into three seasonal rainfall zones (winter, summer and year-round rainfall), which in turn strongly influence the distribution of vegetation1 (Supplementary Fig. S3). Palaeoenvironmental studies hypothesized that areas that are in the summer rainfall zone of the savanna biome might have been under the influence of an expanding winter rainfall zone during Pleistocene glacial conditions2,3,4. This could have led to shifts in the vegetation structure of the savanna biome, which today consists broadly of tropical and subtropical grasses following the C4 photosynthetic pathway with scattered small trees and bushes following the C3 photosynthetic pathway1. Rainfall seasonality, in turn, is a major control of fire5, and regular wildfire is common in African savannas, enabling turnover in the grass layer. C4 grasses, on the whole, tend to recover faster after fires than C3 grasses. This may be due to rapid regrowth due to generally higher photosynthetic rate, especially with the removal of shading from higher canopies post-fire6. The importance of fire in maintaining modern ecosystems has been shown in modelling studies7. Fire intensity and regularity is challenging to reconstruct for the Pleistocene, particularly when microcharcoal records are absent. However, there can be considerable overlap between C3 and C4 species of grass8. Atmospheric CO2 levels are another major control of plant photosynthesis. C3 plants, which can also include temperate grasses, thrive under high CO2 levels, whereas C4 grasses are more efficient under low CO2 levels (Fig. 1). In laboratory studies, for example, glacial CO2 has been found to increase the stomatal conductance of C4 grasses to greater effect than C3 grasses, while maintaining its high water-use efficiency9. This difference in water-use efficiency is a likely mechanism for C4 grasses to outcompete C3 grasses in arid regions. The vegetation response to changing CO2 is therefore heavily dependent on local subsurface conditions10.
Prediction of atmospheric CO2 and daytime growing season temperature conditions that favour C3 or C4 grasses. Black box covers CO2 levels and potential temperatures during glacials in the central interior of South Africa before the Mid-Pleistocene transition (‘40 k world’) and during the Mid-Pleistocene transition (‘100 k world’) (adapted after11 and12).
Approximately 900,000 years ago, ice ages switched from occurring every 41 kyr to every 100 kyr, called the mid-Pleistocene transition (MPT), which in turn had consequences for the global CO2 record. Boron isotope reconstructions of the global CO2 records during the MPT (c. 1.2–0.8 Ma) show glacial (c. 160–200 ppm) CO2 levels to be particularly low; lower than in the preceding Early Pleistocene (c. 185–250 ppm). At the same time interglacial CO2 levels are similar before and after the MPT (c. 250–320 ppm), but on average lower during the MPT (c. 200–300 ppm)13. CO2 reconstructions from the oldest ice in Antarctica confirm lower glacial CO2 concentrations during the MPT in comparison to the 40 kyr world before and the 100 kyr world afterwards14. In total, the glacial-interglacial span of CO2 increased substantially. In accordance with their physiology, going from, on average, glacial 185–250 ppm to 160–200 ppm has a much larger effect on photosynthesis in C4 plants than on C3 plants (Fig. 1).
Recent studies suggested early human evolution during the Pliocene and Pleistocene in non-analogous, productive C3 plant dominated environments in parts of Africa15,16. In the central interior of South Africa, Wonderwerk Cave is the site with the longest and most fine-grained record of palaeoenvironmental change, covering the last two million years17. This site and other local multi-proxy records showed an environment during the Early Pleistocene (c. 1.96–0.78 Ma) that included the presence of persistent standing bodies of water and grasses following both C3 and C4 photosynthetic pathways18,19,20 (Supplementary Table S1). After c. 800 kyr, C3 grasses show a decline in abundance at Wonderwerk Cave and are not a part of the local vegetation during the Holocene, as is common in African savannas. Grazer enamel isotopes and short cell grass phytoliths records show changing proportions of C3 and C4 grasses but cannot distinguish between the drivers and their interactions.
Dynamic Global Vegetation Models (DGVMs), which simulate climate-driven vegetation processes, can generate spatially-continuous reconstructions of past environments to complement discrete and dispersed proxy records. They also allow insight into past biosphere–atmosphere interactions using the body of ecophysiological theory embedded in DGVMs. However, it is critical that model output be regarded in the context of independent palaeoecological records to build confidence in their findings. After knowing the state of past environments, it is imperative that we identify and firmly understand the processes that led to their formation. In this study, we use the LPX-DGVM21 driven by Atmosphere–Ocean coupled General Climate Model (AOGCM) climate output22, to test three possible drivers forcing vegetation change that have been suggested in the Wonderwerk Cave palaeoenvironmental study18: (1) atmospheric CO2 levels, (2) rainfall seasonality, and (3) disturbance through fire. Under identical glacial climate reconstructions from four different AOGCM outputs, we modulated CO2 levels to determine its effect on vegetation cover, particularly the expansion or contraction of C3 and C4 grasses. We test if the proposed drivers were influencing the changes that have been reconstructed from proxy records for the Early- and mid-Pleistocene in the southern Kalahari at c. 27° southern latitude. Model runs were performed under 150 ppm (post-MPT scenario) and 250 ppm (pre-MPT scenario) glacial CO2 conditions, with and without the possibility of natural fires occurring (Supplementary Fig. S1 and S2). We used extreme values on both ends for the CO2 values to explore the maximum sensitivity of the system. From the results, we estimate the effect of CO2 concentration on C3/C4 grass cover, determine the climatic niches of C3/C4 grasses and elucidate ecophysiological mechanisms to explain low CO2-mediated C4 grass expansion.
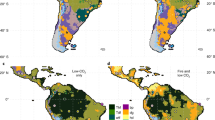
Consistent with our hypothesis, model reconstructions suggest that CO2 levels have a clear influence on C4 grass cover over Africa. However, C4 grass cover is most significantly impacted over East Africa and to a smaller degree over Southern Africa (Fig. 2). For all four LGM climate reconstructions, the low CO2 (150 ppm) scenario, tends to expand and increase the density of C4 grass cover. This trend is consistent for runs with and without fire, suggesting that fire has little influence on the competition between grass types within the model in this context (Supplementary Fig. S1 and S2). The large difference in C4 grass cover particularly around the central African regions is a result of reduced tree cover instead of direct competition with C3 grass, as tree cover is sensitive to fire regime changes. There is significant inter-climate model variation in Southern Africa, where the FGOALS-g1.0 reconstructions show clearest an increase in the western half of Southern Africa in C3 grass cover and decrease in the eastern half of Southern Africa in the pre-MPT scenario compared to the post-MPT scenario (Fig. 2). In contrast, the other models show a more uniform increase in C3 grass cover due to low CO2, except for the eastern coastal areas (Fig. 2). These differences can be traced back to details in rainfall and temperature differences in the models, as shown in Supplementary Fig. S3. Figure 3 shows grass cover in the climate space of mean annual temperature (MAT) and mean annual precipitation (MAP), showing that C4 cover tends to increase when MAT is above 15 °C and MAP is above 500 mm/year in areas dominated by concentrated summer rainfall. At low CO2, this border becomes more prominent, while at the same time, less aridity is needed for a shift to occur.
Foliage projective cover of (a) C3 and (b) C4 grasses at 150 ppm and 250 ppm, and their difference derived from vegetation reconstructions simulated by LPX-DGVM21. In the difference plot, green indicates increases and purple indicates decreases. All models are shown with the impact of fire on in the model. The figure was constructed using raster2.8–19 (https://CRAN.R-project.org/package=raster) and mapproj1.2.6 (https://CRAN.R-project.org/package=mapproj) in R 3.5.2 (https://www.R-project.org/). The present-day coastline was obtained from mapsv3.3.0 (https://CRAN.R-project.org/package=maps), while LGM coastlines are based on data provided by PMIP222.
C4 grass cover, with fire on, in climate space as simulated by LPX-DGVM21. Left is 150 ppm runs, right 250 ppm runs. (a–d) MAP (x-axis) versus MAT (y-axis), (e–f) seasonal concentration (distance from centre) and phase (direction) based on23. Higher concentration means shorter rain season. Colours are: (a,b) C4 grass coverage as % of land (c–f) % of grass that is C4.
The modelling results match well with the palaeoenvironmental changes seen in interior South Africa18. In the early Pleistocene, dry and wet phases with both C3 and C4 grasses exist. After the MPT, evidence for increased seasonality focussed on summer rainfall18 and significant groundwater changes in the Kuruman hills area19,20 indicate increasing aridity. In these arid, low glacial CO2 conditions C3 grasses, as with all C3 plants, were more stressed than C4 plants. On long timescales, C4 grasses may have outcompeted C3 grasses, which do not return in significant numbers during the Pleistocene24,25. Low levels of CO2 during glacials alone may not have led to major changes in vegetation in the interior of South Africa during the Pleistocene. However, the combined effects of low CO2 in addition to changes in the growing season could have pushed vegetation through certain bioclimatic thresholds (Fig. 3). C4 plants require on average over c. 22 °C growing season (warmest month) temperature to effectively compete against C3 plants, limiting their distribution to warm tropical and subtropical areas11,26,27. Below this ‘crossover temperature’, C3 photosynthesis has a higher quantum yield11. In glacial periods during the Pleistocene, when temperatures were lower in all seasons, the growing season in South Africa might have shifted when winter rainfall influences extended east2,3,4. This would increase water availability at Wonderwerk Cave year-round, which could then support a mix of C3 and C4 grasses, as exists today in a very small area in the southern Karoo, close to the year-round rainfall zone28. In addition, lower temperatures mean less evapotranspirative demands on plants, resulting in lower water stress. Changes in rainfall seasonality or amount alone without CO2 changes cannot explain the long-term change to C4 grass savannas as there is no return of a substantial amount of C3 grasses in the humid late Pleistocene at Wonderwerk Cave24. Our results have implications for hominin responses during the later phase of the Early Stone Age as seen in the rich Acheulean cultural record in the southern Kalahari, which contrasts with a much sparser mid- and late Pleistocene record24,29. The much sparser archaeological record could be a reaction to the less vegetated landscape (less C3 plants) and limited, seasonal water availability. Major changes in the cultural record of the region, for example, the transition from the Early to the Middle Stone Age (c. 500–200 kyr), occur not at the start but during and after this ongoing environmental change.
Our study is an example of testing local terrestrial proxy reconstructions, as other areas of Africa clearly follow different trajectories (Fig. 2). Eastern parts of South Africa30,31 and East Africa32,33, have a dominant C4 grass component before the MPT. The impact of low CO2 on the already existing C4 grass cover here is shown as substantial in our modelling results. Our study shows how vegetation models are able to test hypotheses generated from local palaeoecological research targeting specific drivers and their interactions. However, any model output is always a reflection of larger patterns rather than a site-specific environmental reconstruction. It is noteworthy that in all reconstructions, C4 grasses tend not to appear south of ~ 18°S, where C3 grasses dominate regardless of CO2 level (Fig. 2), even though C4 grasses dominate the landscape there today26. One reason is possibly the different winter rainfall expansion between the models, and C3 grasses being a result of winter rainfall influence. However, the models only simulate winter rainfall for the far south-west of the continent (Fig. 4, Supplementary Fig. S3). Another could be the models lower temperature limits for C4 photosynthesis (see “Methods”) interacting with possible local temparure biases in climate model simulation. More attention has to be paid in future work on such discrepancies between vegetation models and modern environmental data. In turn, this can be used to refine the models for use in past as well as in future climate change predictions.
Mean annual precipitation, mean annual temperature and temperature during peak rainfall. Peak rainfall is equivalent to the growing season. Present day climate conditions taken from CRU TS 4.0134. Peak rainfall is based on the month of the phase (Supplementary Fig. S3). Contours at mean annual temperatures of 15.5 °C and peak rainfall temperatures of 20 °C. The figure was constructed using raster 2.8–19 (https://CRAN.R-project.org/package=raster) and mapproj1.2.6 (https://CRAN.R-project.org/package=mapproj) in R 3.5.2 (https://www.R-project.org/). The present-day coastline was obtained from mapsv3.3.0 (https://CRAN.R-project.org/package=maps), while LGM coastlines are based on data provided by PMIP222.
Methods
Model description The Land surface Processes and eXchanges (LPX) is a coupled, process-based fire-enabled dynamic global vegetation model (DGVM). Full details of the dynamic vegetation component can be found in Sitch et al. (2003)35, while the fire model is described in Thonicke et al. (2010)36 and Prentice et al.21. LPX simulates atmosphere-to-biosphere interactions and ecosystem structure and function; computing spatially and temporally resolved estimates of potential vegetation cover and height, biomass, soil carbon and water and energy fluxes. LPX uses nine Plant Functional Types (PFT) to represent potential vegetation. PFTs compete within grid cells, where their differential ecophysiological responses to driving climate data, background turnover (or mortality) and resistance to fire disturbance determine their relative abundances. PFTs can be either tree or grasses. Trees are split by climate range (tropical, temperate, boreal), leaf type (broadleaf, evergreen), and phenological response (evergreen, raingreen, summergreen) and grasses are split between by C3 and C4 photosynthetic pathways.
To represent CO2 fertilization, LPX explicitly couples CO2 assimilation with transpiration using the photosynthesis-water balance scheme37,38 adapted from the Farquhar model39,40. Available CO2 reduces potential water stress on a plant by lowering the required stomatal conductance (gc) for a given photosynthetic rate. Maximum potential stomatal conductance (gcMax) for when water is not limiting depends on the maximum potential day-time pathway-specific photosynthetic assimilation rate (Amax) and minimum canopy conductance parameters, and atmospheric CO2 concentration. If gcMax requires a transpiration rate (D) that is greater than the available water supply (S, which is a function of soil water content and soil properties), then gc (and therefore photosynthesis and production) is reduced in such a way as to be consistent with an empirical formulation derived from Monteith (1995)41. At higher atmospheric CO2 concentrations, gcMax decreases while Amax remains the same, and the value of S that induces water stress is, therefore, lower and maximum production rates can occur at lower moisture availability. Furthermore, when S is less than D, gc (and therefore production) requires less down-regulation. Increased CO2 thus leads to a fertilization effect, with increases production in drier conditions.
The distribution of C3 versus C4 plants is dynamically determined by light-use competition between the two photosynthetic pathways35. The ratio of internal to ambient CO2 concentrations optimal for photosynthesis is lower in C4 compared to C3, thereby reducing photorespiration and making photosynthesis more efficient. It also means that C4 productivity is less sensitive to the lowering of atmospheric CO2 concentrations. Leaf respiration as a fraction of Rubisco capacity is, however, higher for C4 photosynthesis.
Under plentiful water supply and present-day CO2, C4 plants tend to have higher Gross Primary Production (GPP) than C3 plants at temperatures above ~ 20 °C36. Increased water stress, induced by dry conditions or reduced CO2 concentrations, tends to reduce this crossover temperature. Under cooler temperatures, the simulated higher metabolic costs of C4 photosynthesis outway advantages in photorespiration, and C3 gains a competitive edge, Additionally, C3 has no lower temperature limit for survival—through reduced productivity will effectively prevent establishment at very low temperatures, this is well those considered in this study. C4 grasses, however, do not survive temperatures of less than 15.5 °C.
Fires occur from lightning ignitions, and the probability of an ignition event causing a fire depends on local fuel and atmospheric moisture content. Fire spread, flame height and residence time are based on weather conditions and fuel moisture, calculated using the Rothermel equations42. The area affected by fires is the product of the number of fires and the average spread of fire. LPX simulates fire mortality through two pathways: leaf and crown scorching, affecting all PFTs and cambial damage, affecting just tree PFTs. The amount of crown scorching depends on the height and intensity of the fire in relation to the height of the local vegetation. The probability of mortality from crown scorch increases as flame height increases beyond the canopy height of each PFT. While the fire mortality in this scheme will kill the shorter grass PFTS more than tree PFTs, grass PFTs tend to recover faster. Depending on other environmental conditions, water supply and CO2 concentration, differing growth rates will preferentially select either C3 and C4 grass PFTs.
Modelling Protocol We follow the same modelling protocol as43. Four distinct reconstructions of LGM climate (MIROC3.2, HadCM3M2, FGOALS-g1.0, CNRM-CM33)44, generated by four atmosphere–ocean general circulation models (AOGCM) derived from the paleoclimate model intercomparison project 2 (PMIP2), were used to drive the Land surface Processes and eXchanges (LPX) DGVM21. Vegetation was ‘spun-up’ from bare ground for 4000 years to reach equilibrium from which model runs were set for an additional 1380 years. Raw model output was then post-processed to show distributions of vegetation and climate-vegetation relationships as per43.
Seasonal comparison We use season phase and concentration metrics from23. Each month, m, was represented by a vector with direction (m) corresponding to the time of year and the length corresponding to the magnitude of the variable for that month. A mean vector was calculated the average of x and y vectors:
The ratio of the mean vector length to annual average described the seasonal concentration (C) of the variable, timing is described by the mean direction (P):
C is equal to 1 when the variable is 0 in all but 1 month (i.e., there is only 1 month of rainfall when assessing rainfall seasonality) and P is equal to that month. C is 0 and P is undefined when the variable is evenly distributed throughout the year.
Data availability
Available from the authors on request.
Code availability
Available from the authors on request.
References
Mucina, L. & Rutherford, M. C. The Vegetation of South Africa, Lesotho and Swaziland (South African National Biodiversity Institute, Pretoria, 2006).
van Zinderen Bakker, E. M. The evolution of late Quaternary paleoclimates of Southern Africa. Palaeoecol. Afr. 9, 160–202 (1976).
Cockcroft, M. J., Wilkinson, M. J. & Tyson, P. D. The application of a present-day climatic model to the late Quaternary in southern Africa. Clim. Change 10, 161–181 (1987).
Chase, B. M. & Meadows, M. E. Late Quaternary dynamics of southern Africa’s winter rainfall zone. Earth Sci. Rev. 84(3), 103–138 (2007).
Bistinas, I., Harrison, S. P., Prentice, I. C. & Pereira, J. M. C. Causal relationships vs. emergent patterns in the global controls of fire frequency. Biogeosciences 11, 5087–5101 (2014).
Hoetzel, S., Dupont, L., Schefuß, E., Rommerskirchen, F. & Wefer, G. The role of fire in Miocene to Pliocene C 4 grassland and ecosystem evolution. Nat. Geosci. 6(12), 1027–1030 (2013).
Bond, W. J., Woodward, F. I. & Midgley, G. F. The global distribution of ecosystems in a world without fire. New Phytol. 165(2), 525–538 (2005).
Ripley, B. et al. Fire ecology of C3 and C4 grasses depends on evolutionary history and frequency of burning but not photosynthetic type. Ecology 96(10), 2679–2691 (2015).
Pinto, H., Sharwood, R. E., Tissue, D. T. & Ghannoum, O. Photosynthesis of C3, C3–C4, and C4 grasses at glacial CO2. J. Exp. Bot. 65(13), 3669–3681 (2014).
Roth-Nebelsick, A. & Konrad, W. Habitat responses of fossil plant species to palaeoclimate—possible interference with CO2?. Palaeogeogr. Palaeoclimatol. Palaeoecol. 467, 277–286 (2017).
Ehleringer, J. R., Cerling, T. E. & Helliker, B. R. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112(3), 285–299 (1997).
Edwards, E. J., Osborne, C. P., Strömberg, C. A., Smith, S. A. & C4 Grasses Consortium. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328(5978), 587–591 (2010).
Hönisch, B., Hemming, N. G., Archer, D., Siddall, M. & McManus, J. F. Atmospheric carbondioxide concentration across the mid-Pleistocene transition. Science 324(5934), 1551–1554 (2009).
Yan, Y. et al. Two-million-year-old snapshots of atmospheric gases from Antarctic ice. Nature 574(7780), 663–666 (2019).
Faith, J. T., Rowan, J. & Du, A. Early hominins evolved within non-analog ecosystems. Proc. Natl. Acad. Sci. 116(43), 21478–21483 (2019).
Sealy, J., Naidoo, N., Hare, V. J., Brunton, S. & Faith, J. T. Climate and ecology of the palaeo-Agulhas Plain from stable carbon and oxygen isotopes in bovid tooth enamel from Nelson Bay Cave, South Africa. Quat. Sci. Rev. 235, 105974 (2019).
Horwitz, L. K. & Chazan, M. Past and present at Wonderwerk Cave (Northern Cape Province, South Africa). Afr. Archaeol. Rev. 32(4), 595–612 (2015).
Ecker, M. et al. The palaeoecological context of the Oldowan-Acheulean in southern Africa. Nat. Ecol. Evol. 2(7), 1080–1086 (2018).
Matmon, A. et al. New chronology for the southern Kalahari Group sediments with implications for sediment-cycle dynamics and early hominin occupation. Quat. Res. 84(1), 118–132 (2015).
Vainer, S., Erel, Y. & Matmon, A. Provenance and depositional environments of Quaternary sediments in the southern Kalahari Basin. Chem. Geol. 476, 352–369 (2018).
Prentice, I. C. et al. Modeling fire and the terrestrial carbon balance. Glob. Biogeochem. Cycles 25(3), 2–13 (2011).
Braconnot, P. et al. Results of PMIP2 coupled simulations of the Mid-Holocene and Last Glacial Maximum-Part 1: experiments and large-scale features. Clim. Past 3(2), 261–277 (2007).
Kelley, D. I. et al. A comprehensive benchmarking system for evaluating global vegetation models. Biogeosciences 10(5), 3313–3340 (2013).
Chazan, M. et al. Archaeology, paleoenvironment and chronology of the early middle stone age component of Wonderwerk cave in the interior of southern Africa. J. Palaeolithic Archaeol. https://doi.org/10.1007/s41982-020-00051-8 (2020).
Lee-Thorp, J. A. & Beaumont, P. B. Vegetation and seasonality shifts during the late Quaternary deduced from 13C/12C ratios of grazers at Equus Cave, South Africa. Quat. Res. 43, 426–432 (1995).
Vogel, J. C. The geographical distribution of Kranz species in southern Africa. South Afr. J. Sci. 75, 209–215 (1978).
Zhou, H., Helliker, B. R., Huber, M., Dicks, A. & Akçay, E. C4 photosynthesis and climate through the lens of optimality. Proc. Natl. Acad. Sci. 115(47), 12057–12062 (2018).
Rubin, F., Palmer, A. R. & Tyson, C. Patterns of endemism within the Karoo National Park, South Africa. Bothalia 31(1), 117–133 (2001).
Walker, S. J., Lukich, V. & Chazan, M. Kathu townlands: a high density earlier stone age locality in the interior of South Africa. PLoS ONE 9(7), e103436 (2014).
Lee-Thorp, J. A., Sponheimer, M. & Luyt, J. Tracking changing environments using stable carbon isotopes in fossil tooth enamel: an example from the South African hominin sites. J. Hum. Evol. 53(5), 595–601 (2007).
Codron, D., Brink, J. S., Rossouw, L. & Clauss, M. The evolution of ecological specialization in southern African ungulates: competition- or physical environmental turnover?. Oikos 117, 344–353 (2008).
Plummer, T. W. et al. The environmental context of Oldowan hominin activities at Kanjera South, Kenya. In Interdisciplinary approaches to the Oldowan (eds Hovers, E. & Braun, D. R.) 149–160 (Springer, Berlin, 2009).
Cerling, T. E. et al. Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 Ma. Proc. Natl. Acad. Sci. 112(37), 11467–11472 (2015).
Harris, I., Osborn, T. J., Jones, P. & Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data. https://doi.org/10.1038/s41597-020-0453-3 (2020).
Sitch, S. et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Change Biol. 9(2), 161–185 (2003).
Thonicke, K. et al. The influence of vegetation, fire spread and fire behaviour on biomass burning and trace gas emissions: results from a process-based model. Biogeosciences 7(6), 1991–2011 (2010).
Haxeltine, A. & Prentice, I. C. BIOME3: an equilibrium terrestrial biosphere model based on ecophysiological constraints, resource availability, and competition among plant functional types. Glob. Biogeochem. Cycles 10(4), 693–709 (1996).
Haxeltine, A. & Prentice, I. C. A general model for the light-use efficiency of primary production. Funct. Ecol. 10, 551–561 (1996).
Farquhar, G. D., Von Caemmerer, S. & Berry, J. A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 plants. Planta 149, 78–90 (1980).
Farquhar, G. D. & Von Caemmerer, S. Modelling of photosynthetic response to environmental conditions. In Physiological Plant Ecology II: Water Relations and Carbon Assimilation (eds Nobel, P. S. et al.) 549–587 (Springer, Berlin, 1982).
Monteith, J. L. A reinterpretation of stomatal responses to humidity. Plant Cell Environ. 18, 357–364 (1995).
Rothermel, R. C. A Mathematical Model for Predicting Fire Spread in Wildland Fuels (Vol. 115). Intermountain Forest and Range Experiment Station, Forest Service, US Department of Agriculture (1972).
Sato, H., Kelley, D. I., Mayor, S. J., Cowling S. A., Calvo, M. M. & Prentice, I. C. Fire and low CO2 opened dry corridors in South America during the Last Glacial Maximum. Under Review for Nature Geosciences: NGS-2019–07–01558B (2020).
Prentice, I. C., Harrison, S. P. & Bartlein, P. J. Global vegetation and terrestrial carbon cycle changes after the last ice age. New Phytol. 189(4), 988–998 (2011).
Acknowledgements
M.E. has received funding from the European Union's Framework Programme for Research and Innovation Horizon 2020 (2014–2020) under the Marie Skłodowska-Curie Grant Agreement No. 837730. The contribution by D.K. was supported by the UK Natural Environment Research Council through The UK Earth System Modelling Project (UKESM, Grant No. NE/N017951/1).
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.E. and H.S. designed research. H.S. and D.K. performed research. M.E., H.S. and D.K. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ecker, M., Kelley, D. & Sato, H. Modelling the effects of CO2 on C3 and C4 grass competition during the mid-Pleistocene transition in South Africa. Sci Rep 10, 16234 (2020). https://doi.org/10.1038/s41598-020-72614-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72614-2
This article is cited by
-
Niche-dependent forest and savanna fragmentation in Tropical South America during the Last Glacial Maximum
npj Biodiversity (2024)
-
Eolian chronology reveals causal links between tectonics, climate, and erg generation
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.