Abstract
When individuals breed more than once, parents are faced with the choice of whether to re-mate with their old partner or divorce and select a new mate. Evolutionary theory predicts that, following successful reproduction with a given partner, that partner should be retained for future reproduction. However, recent work in a polygamous bird, has instead indicated that successful parents divorced more often than failed breeders (Halimubieke et al. in Ecol Evol 9:10734–10745, 2019), because one parent can benefit by mating with a new partner and reproducing shortly after divorce. Here we investigate whether successful breeding predicts divorce using data from 14 well-monitored populations of plovers (Charadrius spp.). We show that successful nesting leads to divorce, whereas nest failure leads to retention of the mate for follow-up breeding. Plovers that divorced their partners and simultaneously deserted their broods produced more offspring within a season than parents that retained their mate. Our work provides a counterpoint to theoretical expectations that divorce is triggered by low reproductive success, and supports adaptive explanations of divorce as a strategy to improve individual reproductive success. In addition, we show that temperature may modulate these costs and benefits, and contribute to dynamic variation in patterns of divorce across plover breeding systems.
Similar content being viewed by others
Introduction
The decision to retain or divorce a mate between successive breeding events is an important aspect of mating systems2, with direct implications for reproductive success and subsequent survival of the parents3,4,5,6,7. Mate fidelity, defined as retaining the same mate for subsequent breeding attempt(s), is commonly observed in a variety of taxa8,9. Mate fidelity varies widely among species in terms of duration, with some exhibiting short-term mate fidelity within a single season, in which an individual remains faithful to a mate throughout one breeding season and initiates another breeding season with a new mate while the old partner is still alive, whereas other species show long-term (i.e. between seasons) or even life-time mate fidelity8,9,10,11,12,13. Understanding the drivers of interspecific variation in mate fidelity is thus crucial to understand the evolutionary diversity of animal mating systems.
Various factors have been proposed to explain variation in mate fidelity across taxa. The abiotic environment (such as temperature and precipitation) often shapes mating decisions. Variation the in abiotic environment may affect resource availability and the duration of suitable breeding periods, creating different ecological constraints that may limit or promote mate fidelity14,15,16. For example, arctic bird species have typically short breeding seasons due to the harsh and stochastic environmental conditions, and tend to exhibit high fidelity to a mate, which is likely to improve offspring survival17,18. In contrast, mild environments in temperate and tropical regions tend to provide a more prolonged breeding season so that an individual might initiate multiple clutches with the same or different mates1,19,20. The influence of environmental conditions on mating decisions has been observed in a variety of taxa including flies, fish, frogs and birds21,22,23. Aspects of the social environment, such as adult sex ratio (ASR) are also known to influence mating decisions24. In species or populations with a biased ASR, the rare sex is more likely to initiate divorce since the rare sex has higher mate availability than the common sex (e.g. frogs24, birds25,26). Life history traits may also influence rates of mate fidelity; for instance, species with a high divorce rate have a high mortality rate, whereas species with high adult survival rate (or long-lived species) tend to retain the same mates from year to year27,28, suggesting that survival rate or longevity predict mating decisions. Although life history theory predicts that large body size is usually related to high survival rate and longevity10, there is sparse evidence to show whether larger species exhibit stronger mate fidelity than smaller ones10,28. Sexual size dimorphism (SSD) may also relate to patterns of mate fidelity, since more exaggerated SSD may reflect more intense sexual selection and polygamy29. Other life history traits (e.g. age of first reproduction, life span) have also been proposed to be linked to mate fidelity30,31.
Several hypotheses have been put forward to explain variation in mate fidelity, emphasizing the costs and benefits of individual mating decisions, and their relationship with breeding success or breeding time. The “fast-track hypothesis” suggests that individuals retain a mate to reduce the time and energy costs of searching for a new mate32,33. The “mate familiarity hypothesis” highlights that retaining a mate could improve breeding performance by enhancing coordination between parents and thereby improving reproductive success13,34,35. In contrast, changing a mate may be beneficial in some long-lived species, as individuals may divorce their current partner to mate with relatively higher quality or more compatible partners in order to improve breeding success (“incompatibility hypothesis”36; also see37); as a corollary, successful breeding pairs are more likely to stay together for future breeding attempts11,38,39. It has also been suggested that divorcing and rapidly changing a mate may be favoured by some species in order to make the most out of a restricted time budget (e.g. short life span or short breeding season)1,40.
Mating decisions are also associated with breeding dispersal, i.e. the movement of an adult from one breeding location to another between consecutive breeding attempts within a breeding year41,42. Breeding dispersal may exhibit sex differences as males and females can adopt different mating strategies, for instance, the more polygamous sex is more likely to disperse farther to find new mating partners than the less polygamous sex41,43,44,45. Studies also suggest that mate fidelity can be a by-product of site fidelity28,46, and conversely, mate change can be a result of changing nest sites47,48. Nonetheless, studies of mate fidelity mostly centre around socially monogamous bird species between years28,49, and studies that investigate mate fidelity in multiple species and populations that exhibit variable duration of pair-bonds within a single breeding season are scarce1.
Here we focus on Charadrius plovers—small ground-nesting shorebirds—for four reasons. First, they exhibit intra- and interspecific variation in several behavioural, ecological, demographic and life history traits16,45, making them an excellent model system for addressing mate choice decisions. Second, plovers are globally distributed, breeding on all continents except Antarctica, providing an excellent opportunity to conduct a geographically large-scale study16,45. Third, they display flexible mating systems including short-term within-year pair-bonds. In some plover populations, both males and females may have up to four breeding attempts with the same or different mate sequentially within a single season45,50,51. Finally, their breeding biology is well characterised: plovers typically lay two to four eggs (depending on the species) in poorly insulated nest scrapes with both parents typically providing care during the incubation stage51. Plover chicks are precocial and nidifugous, and although in most species post-hatch care is provided by both parents, in others, either parent (usually females) may desert their mate during brood care to become polygamous50,51. Furthermore, plovers show low extra-pair paternity rates (less than 5%), indicating that social mates are a good proxy for genetic mates and thus the reproductive success of social pairs accurately reflects Darwinian fitness52.
In a recent study, Halimubieke et al.1 reported that snowy plovers (Charadrius nivosus), especially females, are more likely to divorce after successful nesting, simultaneously deserting their current brood, and initiate a new breeding attempt with a different mate, whereas, pairs tend to stay together after failed breeding attempts and initiate a second nesting attempt with the same mate. Divorcing individuals reared more offspring than those that retained their mates. This difference in mating strategy between male and female snowy plovers led to female-biased breeding dispersal, as females divorced their mates more often than males and subsequently dispersed to pursue additional mating opportunities.
Here we use data from 8 plover species across 14 populations (see Table 1 and Fig. 1 for study sites, species and study periods) to investigate four issues. First, we explore the variation in mate fidelity in both males and females across populations within breeding years, and the abiotic environmental and life history correlates of variation in mate fidelity across populations. We characterise the mean ambient temperature and temperature variation (see Methods for details), and expect that colder ambient temperature and greater temperature variation promote mate fidelity. We also investigate body weights and the sexual size dimorphism (SSD, see Methods) as life history traits, and expect that populations with heavier plovers (or less extensive SSD) show higher mate fidelity rate than populations with small plovers and more extensive SSD—based on studies that show sexual selection is associated with the extent of SSD53,54. Second, we evaluate the generality of the previous study in snowy plover1, and expect that successful breeding leads to divorce whereas failed breeding leads to mate fidelity. Third, we investigate the fitness consequences of mating decisions, and expect that birds that divorce and desert their broods have higher reproductive success than individuals that retained their mates within breeding seasons. Finally, we investigate whether mating decisions are related to breeding dispersal, as we expect that individuals that divorce their previous mate disperse greater distances to initiate another breeding attempt with a different mate than those who retain their existing mate for their next breeding attempt1.
Results
Mate fidelity rate
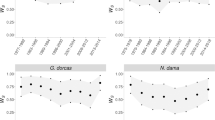
Mate fidelity rates (proportion of retained individuals in given breeding season; see Methods for further details) of both males and females were different between plover populations in both sexes (see Fig. 2; male: F = 8.33, df = 13, P < 0.001; female: F = 6.34, df = 13, P < 0.001; one-way ANOVA).
Mate fidelity rates in plover populations. Annual mate fidelity rate of each population is shown in different colours. Means of annual mate fidelity rate of each population, lower and upper 95% confidence intervals are shown. See Table 1 for details of the population codes.
Environmental and life history predictors of mate fidelity
Mate fidelity rate decreased with ambient temperature (i.e., mean temperature over breeding season, see Methods; Table 2). Males (but not females) in warmer climates had lower mate fidelity than individuals that breed in colder environments (Fig. 3a). However, mate fidelity was unrelated to temperature variation (i.e., between-year fluctuations in ambient temperature, see Methods). Within populations, mate fidelity was unrelated to daily temperature in both males (P = 0.41, N = 788 observations) and females (P = 0.69, N = 776 observations; see Table S1 in the Supporting information).
Mate fidelity rate in relation to (a) ambient temperature and (b) nesting success rate in male and female plovers (see Table 2). Annual mate fidelity rate of each population is shown in different colours. Linear regression lines are shown in black with lower and upper 95% confidence intervals (grey).
Mate fidelity rates were unrelated to body weight or SSD between populations (Table 2; see Methods), nor within populations (male: P = 0.34, N = 136 observations; female: P = 0.12, N = 193 observations; GLMM; Table S1).
Mate fidelity in relation to nesting success and egg-laying date
Mate fidelity rate declined with nesting success rate (i.e., proportion of successfully hatched nests) since populations with high nesting success rates showed lower mate fidelity rate (Table 2, Fig. 3b). Consistently, within populations mate fidelity was related to nesting success, as divorce was more likely when the nest hatched successfully, whereas mate retention was more likely if the nest failed (Fig. 4; Table 3). However, egg-laying date was not significantly related to mate fidelity (Table 3).
Mate fidelity in relation to nesting success in male and female plovers (see Table 3). Predicted probabilities of divorce, lower and upper 95% confidence intervals are shown.
Implications of mate fidelity
Divorced plovers (both males and females) produced significantly more hatchlings within years than those that retained their mate, although reproductive success (defined as the total number of hatchlings produced within a breeding year) was not different between divorced males and divorced females (Fig. 5a; Table 4). Divorced females dispersed greater distances than divorced males (breeding dispersal was defined as the straight‐line distance in meters between an individual's successive nests within a breeding season); however, divorced males did not disperse farther than retained pairs (Fig. 5b, Table 5).
Discussion
Three major insights have emerged from our global study. First, our results indicate that mate fidelity rates within both sexes differ among populations, consistent with previous studies of plovers4,16,45. For instance, Kentish plover Charadrius alexandrinus populations in Europe and China commonly display serial polygamy with mostly females divorcing their mate soon after the chicks hatched55,56,57; whereas the island population of Kentish plovers in Cape Verde is exclusively monogamous58. The social mating system of all other plover species included in our study is monogamy except for the snowy plover which exhibit serial polygamy59,60. Variation in mate fidelity between closely related species and populations is also common in primates, ungulates and fishes61,62,63.
Our study revealed that mate fidelity variation among plover populations is predicted by the ambient temperature, since populations in colder climates had higher mate fidelity rates than populations in warmer climates. We suggest that ambient temperature may largely influence the mate fidelity rate by its association with an increase in the time available for breeding, and by increasing the chance that at least one breeding attempt will be successful14,15,16. For example, cold environments with short breeding seasons may limit the opportunity of multiple breeding with a new mate given that mate-search and courtship are time consuming, therefore, the best strategy is to re-mate immediately with same mate if there is a breeding failure17,32,33,64. In contrast, mild environments with a prolonged breeding season enable a single parent to rear the offspring, and thus provide an opportunity for multiple breeding attempts for the other parent1,19,20. However, it is also possible that temperature may influence mate fidelity rate by directly influencing other related behaviours or physiological processes14. Ambient temperature appears to exert a weaker influence on mate fidelity of females than on males across populations, as males of populations from colder environments exhibit significantly higher mate fidelity rates than those from warmer environments. Further research is needed to clarify whether the different responses of males and females to environmental conditions are directly influenced by abiotic factors65,66,67, or indirectly influenced by social environment (e.g. ASR)60.
Our results also showed that mate fidelity rate exhibits no relationship with temperature variation between years, although studies suggest that annual fluctuations in temperature affect mating decisions in insect, reptile and mammalian species68,69,70. We argue that temperature variation is probably a crude proxy of ambient environment fluctuation, since fluctuations in other abiotic environmental factors, for example precipitation and habitat quality, may also influence mate fidelity14,21,22,23.
While we are unable to measure variation in the social environment across populations in this study, we also propose that aspects of the social environment, such as ASR, may also be strong contributor to mate fidelity variation. Recent studies show that ASR may deviate from 1:1 in a variety of organisms26,71,72,73. Variation in the ASR can alter the mating opportunities of breeding males and females, thus, influence divorcing and re-mating strategies25,60,72. The role of ASR influencing mating system variation in plovers and beyond will need to be revisited in the near future, although provisional studies of 4 populations suggest ASR does relate to parental care19,60,71.
The second major insight of our study is that breeding success is an important predictor of divorce. At the population level, populations with high nesting success rates have lower mate fidelity rates compared to populations with low nesting success rates. Consistently, individuals were more likely to divorce after clutches hatched rather than when they did not hatch, and failed breeders typically re-nested with the same partner in each population with the possible exception of white-fronted plovers, Charadrius marginatus in which divorce was not observed. As a consequence, divorced individuals, counterintuitively, rear more offspring compared to faithful individuals (Fig. 5a). This finding does not support the “incompatibility hypothesis”37,38, which predicts that breeding pairs with low breeding success should be more likely to divorce32,74. We posit that by divorcing and rapidly changing partners, while simultaneously deserting their current brood, individuals can produce more offspring within a limited season to maximize their reproductive success. Why would divorce be beneficial? (i) Offspring mortality is generally high and stochastic in shorebirds, thus individuals may need to reproduce several times within a breeding season to produce at least some offspring75. (ii) Chicks are precocial and only require modest care19, which provides the opportunity for one parent to terminate care and initiate a new clutch with another mate76,77,78. In contrast, mate retention was more likely after breeding failure. We suggest re-mating with the previous partner is the fastest way for both pair members to breed again (“fast-track hypothesis”79; reviewed by Fowler80; also see32,33); Breeding failure is related to partner compatibility in insects and mammalian species81,82, although we suspect that nest failure in shorebirds is majorly driven by predation83,84,85, thus we presume the role of partner compatibility in divorce is weaker compared to extrinsic forces like predation. Therefore, re-mating seems more important than changing partners and risking not finding a new mate.
Third, we found that mate fidelity is related to breeding dispersal. After divorce, female plovers disperse significantly farther than males. Sex‐biased dispersal has been well-documented in invertebrates, reptiles, birds, and mammals, and it is proposed to be related to mating system41,42,43. For example, many mammals are socially polygynous, males do not participate in parental care, females rely on home ranges with resources to successfully rear offspring, therefore, male-biased dispersal is expected. Whereas in birds, which are typically socially monogamous, males demonstrate territorial defence behaviour because high quality breeding sites provide good resources and opportunities for successful breeding, thus reducing male’s tendency to move large distance between breeding attempts86,87,88,89. Our result follows the general pattern of female-biased breeding dispersal observed in most bird species including shorebirds90,91. Additionally, we propose that for polygamous populations there is an additional reason: females have the opportunity to desert the brood and seek a new mate from within a wide geographical area44.
Taken together, our results illustrate that (i) mating decisions are associated with the abiotic environmental conditions; (ii) birds that divorce and desert their broods generally attain higher breeding success than individuals that retained their mates; and (iii) the asymmetric mating opportunities of males and females result in different spatial dispersal patterns. Our results support the proposition that divorce is a strategy employed to improve reproductive success1,55,92. We suggest that divorce is an adaptive response to environmental constraints (e.g. limited breeding time), life history traits (e.g. low survival rate of the young, uniparental care) and population demography (e.g. biased ASR). We call for further studies to build upon our research framework by augmenting these analyses with other more environmental variables (e.g. precipitation) and incorporating information on the social environment (e.g. ASR) and broader scope of life history traits (e.g. survival rate, longevity). In addition, we encourage the development of theoretical models investigating the influence of ecological/ social environment and life history on the evolution of breeding systems.
Methods
Study site and fieldwork
Fieldwork was carried out in 14 breeding populations of 8 plover species and ranged from 1 to 9 breeding seasons per population (see Table1 and Fig. 1 for study sites and study periods). Egg-laying date of nests was either known (for nests that were found during egg-laying) or estimated by floating eggs or measuring egg mass relative to egg size26,93. Breeding pairs were captured on their nest while incubating eggs, using funnel traps, noose mats, box traps or bownet traps. Morphological data of each individual were collected: body weight was collected from eight populations (body weight data from six populations were not collected in the field, see below for details); sex was determined by morphological features, for monomorphic species, molecular sexing was applied to identify the sex of the individual. Finally, each individual was banded with a unique combination of colour rings/flags and a metal ring (see Appendix S2 in Supplementary Material and further references in93). Nests were monitored until hatching to obtain nesting success data (see below for details).
Data collection
Quantification of mate fidelity and mate fidelity rate within breeding years
Plovers that were included in this study could freely retain or divorce their mates in natural conditions without manipulation. The mating decision of each individual was recorded as either mate retention or divorce with respect to their previous breeding attempt within each breeding year. The mating decision of males and females were not independent from each other, therefore, we assessed mating decisions separately for banded males and females in each population. We used the same criteria, following Halimubieke et al.1, for individuals that were included in the analyses: (a) the identities of the individuals and their mate(s) were known, (b) they were observed in at least two reproductive attempts within a breeding year, and (c) if there was a mate change, only those who changed their mates while the previous mate was known to be alive were included. In total, 1927 breeding events (Table 1, 124 divorces in males, 205 divorces in females, and 799 retentions in both sex) fitted the criteria for the mate fidelity analysis in the 14 populations. Mate fidelity rate represents the proportion of retained individuals in given breeding year(s) in each population.
Abiotic environment, body weight and sexual size dimorphism (SSD)
In this study, the ambient temperature and temperature variation of each population over the study period are used as the proxies of abiotic environmental conditions. We extracted high resolution historical daily temperature data collected by the nearest weather stations for each study site from the National Oceanic and Atmospheric Administration (NOAA) database and University of East Anglia Climate Research Unit database (CRU; https://www.cru.uea.ac.uk/, version 3.10.01), using the R package “rnoaa”94. The average distance between weather stations and study sites is 60.17 km (see Table S2 for more details). If the weather record was incomplete for any study site, we used the R package “GSODR”95 to extract weather data from the USA National Center for Environmental Information (NCEI) database. Since our study focused on breeding behaviour, we only extracted daily temperatures from each month of the breeding season(s) when capture data were collected in a given population. and we calculated the average temperature of the breeding season(s); we refer to this variable as ambient temperature. The temperature variation refers to average between-year fluctuations in ambient temperature, and it was calculated as the standard deviation of the average temperature of all breeding years for a given population.
The average body weight of birds from each population was calculated from individual body weight data collected from the fieldwork over the study period. We also searched for average body weight data in Handbook of the Bird of the World51 and CRC Handbook of Avian Body Masses96 for the following populations for which we did not have field data: the Kentish plover population from Italy, two piping plover populations from USA, the red-capped plover population, the white-fronted plover population and the Malaysian plover population. To quantify sexual size dimorphism, we divided the male average body weight by that of the female and log-transformed this ratio, and assigned positive signs when males were the larger sex and negative ones when females were larger.
Nesting success and egg-laying date
Nesting success was determined based on the fate of the nest(s) of each individual included in our study. The fate of a nest was recorded as either successful (at least one chick hatched) or failed (no chicks hatched due to predation, destruction, abandonment, eggs disappeared < 15 days after estimated laying date, eggs did not hatch, or the nest was flooded). The nesting success rate represents the proportion of nests with at least one successfully hatched egg in each population over the study period. The egg-laying date was used to quantify breeding phenology. We controlled for breeding phenological differences between years by converting egg‐laying dates into Julian dates (“lubridate” package in R97), and standardised egg-laying date using the z‐transformation (mean = 0, SD = 1).
Reproductive success and breeding dispersal
Reproductive success was quantified as the cumulative number of hatchlings each individual produced in all breeding attempts within each breeding year. We did not use the number of fledglings as the proxy for reproductive success as the fates of fledglings are difficult to estimate in precocial species like plovers due to the high mobility and camouflage of broods16. Breeding dispersal was defined as the straight‐line distance (in meters) between an individual's successive nests within a year for those populations with nest location data.
Statistical analyses
To investigate variation in mate fidelity rate across populations, first, we used analysis of variance ANOVA to compare the mate fidelity rates of both sexes across 14 populations. We then constructed two generalised linear mixed models (GLMM) via Template Model Builder (TMB) with binomial error structure to test environmental and life history predictors of mate fidelity rate in both sexes. In these models, the mate fidelity rate (male or female) of each population over the study period was the dependent variable. Ambient temperature, temperature variation and nesting success rate of each population over the study period, alongside average body weight (male or female) and SSD of the populations were used as explanatory variables. Species and population were included as random effects.
To investigate individual mating decisions, we first constructed a GLMM for each sex with a binomial error structure, and examined how mate fidelity relates to nesting success and relative egg-laying date. A similar model for each sex was developed to explore mate fidelity in relation to daily temperature and individual body size. In these models, species, population, individual identity and year were used as random effect variables.
Next, we used a GLMM to investigate if reproductive success is related to mate fidelity by comparing the total number of hatchlings from all clutches among divorced males, divorced females and retained pairs. A Poisson error structure was used because: (i) Gaussian version of the model suggested normality assumptions were violated; (ii) reproductive success is a count and thus an integer variable. Species, population and individual identity were used as random effects.
To investigate the relationship between breeding dispersal and mate fidelity, we use same methods as Halimubieke et al.1. We built a linear mixed-effects model (LMM) using log-transformed (ln) breeding dispersal as the dependent variable, and mate fidelity groups (divorced males, divorced females and retained pairs) as the explanatory variable. LMM via REML was fitted and included population and individual identity as random effect variables. The goodness-of-fit test showed that the residuals of the model show equal variances and follow normal distribution, supporting the validation of the model we used.
Estimated marginal means (emmeans from package “emmeans” in R98) were calculated for each group in the latter two models, and post-hoc pairwise comparisons adjusted by Tukey were applied to test group differences. There was no model simplification and all terms were retained in all the models above.
To test whether phylogenetic relatedness influenced our results, we followed the same method as Vincze et al.99, the above models were repeated using Bayesian Markov chain Monte Carlo GLMM (MCMCglmm100), including a correlational structure based on the species-level phylogenetic tree of the 8 Charadrius species studied here. The phylogenetic signal of the investigated trait in these models was low (model description and calculation of the phylogenetic signal are given in Appendix S1). All statistical analyses were performed using R version 3.5.1101.
Ethical statement
This study did not involve any manipulation experiments, and all methods were carried out in accordance with relevant guidelines and regulations of each country in which it was performed. Fieldwork and bird-ringing procedures were authorized by relevant authorities: Hungary (Environmental Ministry and Kiskunság National Park); Australia (Deakin University Animal Welfare Committee Permits B02-2012, B20-2014 and B10-2016, State Government Permits 10006205, 10007918 and 10007241 and Australian Bird and Bat Banding Scheme (ABBBS) Authorities 1763, 3271 and 3033); Mexico (#SGPA/ DGVS/01717/10, #SGPA/DGVS/01367/11), Spain (Ministry of Environment #660117); California (U.S. Fish and Wildlife (USFWS) #TE807078 and U. S. Geological Survey (USGS) #09316); Great Plain, USA (the U.S. Geological Survey Bird Banding Laboratory with Federal Master Bander permit #21446 with threatened and endangered species endorsements, Federal Threatened and Endangered Species handling permit#TE103272-3, and IACUC protocol #14-003.); China (Hebei Forestry Bureau); South Africa (Cape Nature and SAFRING); Cape Verde (Directorate Geral Ambiente); Turkey (Turkish Ministry of National Parks, Tuzla Municipality and Governor of Karatas, Mr. E. Karakaya).
Data availability
All data will be archived at Dryad once the manuscript is accepted for publication. Relevant data is available from GitHub (https://github.com/narhulan29/charadriusplovers).
Change history
07 January 2021
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Halimubieke, N. et al. Mate fidelity in a polygamous shorebird, the snowy plover (Charadrius nivosus). Ecol. Evol. 9, 10734–10745. https://doi.org/10.1002/ece3.5591 (2019).
Reynolds, J. D. Animal breeding systems. Trends Ecol. Evol. 11, 68–72. https://doi.org/10.1016/0169-5347(96)81045-7 (1996).
Neff, B. D. & Pitcher, T. E. Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. https://doi.org/10.1111/j.1365-294X.2004.02395.x (2005).
Székely, T., Thomas, G. H. & Cuthill, I. C. Sexual conflict, ecology, and breeding systems in shorebirds. Bioscience 56, 801–808. https://doi.org/10.1641/0006-3568(2006)56[801:SCEABS]2.0.CO;2 (2006).
Culina, A., Radersma, R. & Sheldon, B. C. Trading up: The fitness consequences of divorce in monogamous birds. Biol. Rev. Camb. Philos. Soc. 90, 1015–1034. https://doi.org/10.1111/brv.12143 (2014).
Székely, T., Weissing, F. J. & Komdeur, J. Adult sex ratio variation: Implications for breeding system evolution. J. Evol. Biol. 27, 1500–1512. https://doi.org/10.1111/jeb.12415 (2014).
Culina, A., Lachish, S., Pradel, R., Choquet, R. & Sheldon, B. C. A multievent approach to estimating pair fidelity and heterogeneity in state transitions. Ecol. Evol. 3, 4326–4338. https://doi.org/10.1002/ece3.729 (2013).
Møller, A. P. The evolution of monogamy: Mating relationships, parental care and sexual selection. In Monogamy Mating Strategies and Partnerships in Birds, Humans and Other Mammals (eds Reichard, U. H. & Boesch, C.) 29–41 (Cambridge University Press, Cambridge, 2003).
Lukas, D. & Clutton-Brock, T. H. The evolution of social monogamy in mammals. Science 314, 526–530. https://doi.org/10.1126/science.1238677 (2013).
Black, J. M. Partnerships in birds (Oxford University Press, Oxford, 1996).
Black, J. M. Fitness consequences of long-term pair bonds in barnacle geese: Monogamy in the extreme. Behav. Ecol. 12, 640–645. https://doi.org/10.1093/beheco/12.5.640 (2001).
Reichard, U. H. & Boesch, C. Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals (Cambridge University Press, Cambridge, 2003).
Sánchez-Macouzet, O., Rodríguez, C. & Drummond, H. Better stay together: Pair bond duration increases individual fitness independent of age-related variation. Proc. R. Soc. B Biol. Sci. 281, 20132843. https://doi.org/10.1098/rspb.2013.2843 (2014).
Botero, C. A. & Rubenstein, D. R. Fluctuating environments, sexual selection and the evolution of flexible mate choice in birds. PLoS ONE 7, e32311. https://doi.org/10.1371/journal.pone.0032311 (2012).
Blomqvist, D., Wallander, J. & Andersson, M. Successive clutches and parental roles in waders: The importance of timing in multiple clutch systems. Biol. J. Linn. Soc. 74, 549–555. https://doi.org/10.1111/j.1095-8312.2001.tb01412.x (2001).
Eberhart-Phillips, L. J. Plover breeding systems: Diversity and evolutionary origins. In The Population Ecology and Conservation of Charadrius Plovers (eds Colwell, M. A. & Haig, S. M.) 65–88 (CRC Press, Boca Raton, 2019).
Green, G. H., Greenwood, J. J. D. & Lloyd, C. S. The influence of snow conditions on the date of breeding of wading birds in north-east Greenland. J. Zool. 183, 311–328. https://doi.org/10.1111/j.1469-7998.1977.tb04190.x (1977).
Saalfeld, S. T. & Lanctot, R. B. Conservative and opportunistic settlement strategies in Arctic-breeding shorebirds. Auk 132, 212–234. https://doi.org/10.1642/AUK-13-193.1 (2015).
Székely, T., Cuthill, I. C. & Kis, J. Brood desertion in Kentish plover sex differences in remating opportunities. Behav. Ecol. 10, 185–190. https://doi.org/10.1093/beheco/10.2.185 (1999).
Yasué, M. & Dearden, P. Replacement nesting and double-brooding in Malaysian plovers Charadrius peronii: Effects of season and food availability. Ardea 96, 59–72. https://doi.org/10.5253/078.096.0107 (2008).
Gilburn, A. S. & Day, T. H. Evolution of female choice in seaweed flies: Fisherian and good genes mechanisms operate in different populations. Proc. R. Soc. B Biol. Sci. 255, 159–165. https://doi.org/10.1098/rspb.1994.0023 (1994).
Candolin, U., Salesto, T. & Evers, M. Changed environmental conditions weaken sexual selection in sticklebacks. J. Evol. Biol. 20, 233–239. https://doi.org/10.1111/j.1420-9101.2006.01207.x (2007).
Welch, A. M. Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution 57, 883–893. https://doi.org/10.1111/j.0014-3820.2003.tb00299.x (2003).
Lode, T., Holveck, M. J., Lesbarreres, D. & Pagano, A. Sex-biased predation by polecats influences the mating system of frogs. Proc. R. Soc. B Biol. Sci. 271, 399–401. https://doi.org/10.1098/rsbl.2004.0195 (2004).
Liker, A., Freckleton, R. P. & Székely, T. Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr. Biol. 24, 880–884. https://doi.org/10.1016/j.cub.2014.02.059 (2014).
Parra, J. E., Beltrán, M., Zefania, S., dos Remedios, N. & Székely, T. Experimental assessment of mating opportunities in three shorebird species. Anim. Behav. 90, 83–90. https://doi.org/10.1016/j.anbehav.2013.12.030 (2014).
Jeschke, J. M. & Kokko, H. Mortality and other determinants of bird divorce rate. Behav. Ecol. Sociobiol. 63, 1–9. https://doi.org/10.1007/s00265-008-0646-9 (2008).
Bried, J., Pontier, D. & Jouventin, P. Mate fidelity in monogamous birds: A re-examination of the Procellariiformes. Anim. Behav. 65, 235–246. https://doi.org/10.1006/anbe.2002.2045 (2003).
Andersson, M. Sexual selection (Princeton University Press, Princeton, 1994).
Choudhury, S. Divorce in birds: A review of the hypotheses. Anim. Behav. 50, 413–429. https://doi.org/10.1006/anbe.1995.0256 (1995).
Wheelwright, N. T. & Teplitsky, C. Divorce in Savannah sparrows: Causes, consequences and lack of inheritance. Am. Nat. 190, 557–569. https://doi.org/10.1086/693387 (2017).
Adkins-Regan, E. & Tomaszycki, M. Monogamy on the fast track. Biol. Lett. 3, 617–619. https://doi.org/10.1098/rsbl.2007.0388 (2007).
Perfito, N., Zann, R. A., Bentley, G. E. & Hau, M. Opportunism at work: Habitat predictability affects reproductive readiness in free-living zebra finches. Funct. Ecol. 21, 291–301. https://doi.org/10.1111/j.1365-2435.2006.01237.x (2007).
Ens, B. J., Choudhury, S. & Black, J. M. Mate fidelity and divorce in monogamous birds. In Partnerships in Birds: The Study of Monogamy (ed. Black, J. M.) 344–401 (Oxford University Press, Oxford, 1996).
Gabriel, P. O., Black, J. M. & Foster, S. Correlates and consequences of the pair bond in Steller’s Jays. Ethology 119, 178–187. https://doi.org/10.1111/eth.12051 (2013).
Coulson, J. C. The influence of the pair-bond and age on the breeding biology of the kittiwake gull Rissa tridactyla. J. Anim. Ecol. 35, 269–279. https://doi.org/10.2307/2394 (1966).
Kempenaers, B., Adriaensen, F. & Dhondt, A. A. Inbreeding and divorce in blue and great tits. Anim. Behav. 56, 737–740. https://doi.org/10.1006/anbe.1998.0800 (1998).
Pyle, P., Sydeman, W. J. & Hester, M. Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in declining populations of Cassin’s auklets. J. Anim. Ecol. 70, 1088–1097. https://doi.org/10.1046/j.0021-8790.2001.00567.x (2001).
Flodin, L. A. & Blomqvist, D. Divorce and breeding dispersal in the dunlin Calidris alpina: Support for the better option hypothesis?. Behaviour 149, 67–80. https://doi.org/10.1163/156853912X626295 (2012).
Arnqvist, G. & Nilsson, T. The evolution of polyandry: Multiple mating and female fitness in insects. Anim. Behav. 60, 145–164. https://doi.org/10.1006/anbe.2000.1446 (2000).
Greenwood, P. J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. https://doi.org/10.1016/S0003-3472(80)80103-5 (1980).
Clobert, J., Danchin, E., Dhondt, A. & Nichols, J. D. Dispersal (Oxford University Press, Oxford, 2001).
Trochet, A. et al. Evolution of sex-biased dispersal. Q. Rev. Biol. 91, 297–320. https://doi.org/10.1086/688097 (2016).
D’Urban Jackson, J. et al. Polygamy slows down population divergence in shorebirds. Evolution 71, 1313–1326. https://doi.org/10.1111/evo.13212 (2017).
Székely, T. Why study plovers? The significance of non-model organisms in avian ecology, behaviour and evolution. J. Ornithol. 160, 923–933. https://doi.org/10.1007/s10336-019-01669-4 (2019).
Morse, D. H. & Kress, S. W. The effect of burrow loss on mate choice in the Leach’s Storm-Petrel. Auk 101, 158–160 (1984).
Pietz, P. J. & Parmelee, D. F. Survival, site and mate fidelity in south polar skuas Catharacta maccormicki at Anvers Island, Antarctica. Ibis 136, 32–38. https://doi.org/10.1111/j.1474-919X.1994.tb08128.x (2014).
Thibault, J.-C. Nest-site tenacity and mate fidelity in relation to breeding success in Cory’s Shearwater Calonectris diomedea. Bird Study 41, 25–28. https://doi.org/10.1080/00063659409477193 (1994).
Dubois, F. & Cézilly, F. Breeding success and mate retention in birds: A meta-analysis. Behav. Ecol. Sociobiol. 52, 357–364. https://doi.org/10.1007/s00265-002-0521-z (2002).
Kosztolányi, A., Székely, T., Cuthill, I. C., Yilmaz, K. T. & Berberoǧlu, S. Ecological constraints on breeding system evolution: The influence of habitat on brood desertion in Kentish plover. J. Anim. Ecol. 75, 257–265. https://doi.org/10.1111/j.1365-2656.2006.01049.x (2006).
del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. A. & de Juana, E. Handbook of the Birds of the World Alive (Lynx Edicions, 2018). (retrieved from https://www.hbw.com/ on 30 October 2019).
Maher, K. H. et al. High fidelity: Extra-pair fertilisations in eight Charadrius plover species are not associated with parental relatedness or social mating system. J. Avian. Biol. 48, 910–920. https://doi.org/10.1111/jav.01263 (2017).
Székely, T., Freckleton, R. P. & Reynolds, J. D. Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proc. Natl. Acad. Sci. USA 101, 12224–12227. https://doi.org/10.1073/pnas.0404503101 (2004).
Székely, T., Lislevand, T. & Figuerola, J. Sexual size dimorphism in birds. In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism (eds Fairbairn, D. J. et al.) (Oxford University Press, Oxford, 2007). https://doi.org/10.1093/acprof:oso/9780199208784.003.0004
Lessells, C. M. The mating system of Kentish plovers Charadrius alexandrinus. Ibis 126, 474–483. https://doi.org/10.1111/j.1474-919X.1984.tb02074.x (1984).
Székely, T. & Lessells, C. M. Mate change by Kentish plovers Charadrius alexandrinus. Ornis. Scand. 24, 317–322 (1993).
Amat, J. A., Fraga, R. M. & Arroyo, G. M. Brood desertion and polygamous breeding in the Kentish plover Charadrius alexandrinus. Ibis 141, 596–607. https://doi.org/10.1111/j.1474-919X.1999.tb07367.x (1999).
Carmona-Isunza, M. C., Küpper, C., Serrano-Meneses, M. A. & Székely, T. Courtship behavior differs between monogamous and polygamous plovers. Behav. Ecol. Sociobiol. 69, 2035–2042. https://doi.org/10.1007/s00265-015-2014-x (2015).
Warriner, J. S., Warriner, J. C., Page, G. W. & Stenzel, L. E. Mating system and reproductive success of a small population of polygamous snowy plover. Wilson Bull. 98, 15–37 (1986).
Eberhart-Phillips, L. J. et al. Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat. Commun. 9, 1651. https://doi.org/10.1038/s41467-018-03833-5 (2018).
Kappeler, P. M. & van Schaik, C. P. Evolution of primate social systems. Int. J. Primatol. 23, 707–740. https://doi.org/10.1023/A:1015520830318 (2002).
Avise, J. C. et al. Genetic mating systems and reproductive natural histories of fishes: Lessons for ecology and evolution. Annu. Rev. Genet. 36, 19–45. https://doi.org/10.1146/annurev.genet.36.030602.090831 (2002).
Bowyer, R. T., McCullough, D. R., Rachlow, J. L., Ciuti, S. & Whiting, J. C. Evolution of ungulate mating systems: Integrating social and environmental factors. Ecol. Evol. 10, 5160–5178. https://doi.org/10.1002/ece3.62 (2020).
Johnson, M. & Walters, J. R. Effects of mate and site fidelity on nest survival of western sandpipers (Calidris mauri). Auk 125, 76–86. https://doi.org/10.1525/auk.2008.125.1.76 (2008).
Brandt, E. E., Kelley, J. P. & Elias, D. O. Temperature alters multimodal signaling and mating success in an ectotherm. Behav. Ecol. Sociobiol. 72, 191. https://doi.org/10.1007/s00265-018-2620-5 (2018).
Conrad, T., Stöcker, C. & Ayasse, M. The effect of temperature on male mating signals and female choice in the red mason bee, Osmia bicornis (L.). Ecol. Evol. 7, 8966–8975. https://doi.org/10.1002/ece3.3331 (2017).
Silva, K., Vieira, M. N., Almada, V. C. & Monteiro, N. M. The effect of temperature on mate preferences and female–female interactions in Syngnathus abaster. Anim. Behav. 74, 1525–1533. https://doi.org/10.1016/j.anbehav.2007.03.008 (2007).
Twiss, S. D., Thomas, C., Poland, V., Graves, J. A. & Pomeroy, P. The impact of climatic variation on the opportunity for sexual selection. Biol. Lett. 3, 12–15. https://doi.org/10.1098/rsbl.2006.0559 (2007).
Olsson, M. et al. In hot pursuit: Fluctuating mating system and sexual selection in sand lizards. Evolution 65, 574–583. https://doi.org/10.1111/j.1558-5646.2010.01152.x (2011).
Suzaki, Y. et al. Temperature variations affect postcopulatory but not precopulatory sexual selection in the cigarette beetle. Anim. Behav. 144, 115–123. https://doi.org/10.1016/j.anbehav.2018.08.010 (2018).
Eberhart-Phillips, L. J. et al. Sex-specific early survival drives adult sex ratio bias in snowy plovers and impacts mating system and population growth. Proc. Natl. Acad. Sci. USA 114, E5474–E5481. https://doi.org/10.1073/pnas.1620043114 (2017).
Liker, A., Freckleton, R. P. F. & Székely, T. The evolution of sex roles in birds is related to adult sex ratio. Nat. Commun. 4, 1587 (2013).
Kosztolányi, A., Barta, Z., Küpper, C. & Székely, T. Persistence of an extreme male-biased adult sex ratio in a natural population of polyandrous bird. J. Evol. Biol. 24, 1842–1846. https://doi.org/10.1111/j.1420-9101.2011.02305.x (2011).
Handel, C. M. & Gill, R. E. Mate fidelity and breeding site tenacity in a monogamous sandpiper, the black turnstone. Anim. Behav. 60, 471–481. https://doi.org/10.1006/anbe.2000.1505 (2000).
Cruz-López, M. et al. The plight of a plover: Viability of an important snowy plover population with flexible brood care in Mexico. Biol. Conserv. 209, 440–448. https://doi.org/10.1016/j.biocon.2017.03.009 (2017).
Székely, T., Webb, J. N., Houston, A. I. & McNamara, J. M. An evolutionary approach to offspring desertion in birds. In Current Ornithology (eds Nolan, V. & Ketterson, E. D.) 271–330 (Springer, Berlin, 1996).
McNamara, J. M., Forslund, P. & Lang, A. An ESS model for divorce strategies in birds. Philos. Trans. R. Soc. B 354, 223–236. https://doi.org/10.1098/rstb.1999.0374 (1999).
Houston, A. I., Székely, T. & McNamara, J. M. The parental investment models of Maynard Smith: A retrospective and prospective view. Anim. Behav. 86, 667–674. https://doi.org/10.1016/j.anbehav.2013.08.001 (2013).
Zann, R. A. Reproduction in a zebra finch colony in south-eastern Australia: The significance of monogamy, precocial breeding and multiple broods in a highly mobile species. Emu 94, 285–299. https://doi.org/10.1071/MU9940285 (1994).
Fowler, G. S. Stages of age-related reproductive success in birds: Simultaneous effects of age, pair-bond duration and reproductive experience. Am. Zool. 35, 318–328. https://doi.org/10.1093/icb/35.4.318 (1995).
Champion de Crespigny, F. E., Hurst, L. D. & Wedell, N. Do Wolbachia-associated incompatibilities promote polyandry?. Evolution 62, 107–122. https://doi.org/10.1111/j.1558-5646.2007.00274.x (2007).
Schwensow, N., Eberle, M. & Sommer, S. Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proc. R. Soc. B Biol. Sci. 275, 555–564. https://doi.org/10.1098/rspb.2007.1433 (2008).
Fraga, R. M. & Amat, J. A. Breeding biology of a Kentish plover (Charadrius alexandrinus) population in an inland saline lake. Ardeola 43, 69–85 (1996).
Ferreira-Rodríguez, N. & Pombal, M. A. Predation pressure on the hatching of the Kentish plover (Charadrius alexandrinus) in clutch protection projects: A case study in north Portugal. Wildl. Res. 45, 55–63. https://doi.org/10.1071/WR17122 (2018).
Kubelka, V. et al. Global pattern of nest predation is disrupted by climate change in shorebirds. Science 362, 680–683. https://doi.org/10.1126/science.aat8695 (2018).
Greenwood, P. J. & Harvey, P. H. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Evol. Syst. 13, 1–21. https://doi.org/10.1146/annurev.es.13.110182.000245 (1982).
Sandercock, B. K., Lank, D. B., Lanctot, R. B., Kempenaers, B. & Cooke, F. Ecological correlates of mate fidelity in two Arctic-breeding sandpipers. Can. J. Zool. 78, 1948–1958. https://doi.org/10.1139/z00-146 (2000).
Liu, Y. & Zhang, Z. Research progress in avian dispersal behavior. Front. Biol. 3, 375. https://doi.org/10.1007/s11515-008-0066-2 (2008).
Végvári, Z. et al. Sex-biased breeding dispersal is predicted by social environment in birds. Ecol. Evol. 8, 6483–6491. https://doi.org/10.1002/ece3.4095 (2018).
Pearson, W. J. & Colwell, M. A. Effects of nest success and mate fidelity on breeding dispersal in a population of snowy plovers Charadrius nivosus. Bird Conserv. Int. 24, 342–353. https://doi.org/10.1017/S0959270913000403 (2013).
Lloyd, P. Adult survival, dispersal and mate fidelity in the white-fronted plover Charadrius marginatus. Ibis 150, 182–187. https://doi.org/10.1111/j.1474-919X.2007.00739.x (2008).
McNamara, J. M. & Forslund, P. Divorce rates in birds: Predictions from an optimization model. Am. Nat. 147, 609–640 (1996).
Székely, T., Kosztolányi, A. & Küpper, C. Practical guide for investigating breeding ecology of Kentish plover Charadrius alexandrinus. https://www.pennuti.net/wp-content/uploads/2010/08/KP_Field_Guide_v3.pdf (University of Bath, 2008).
Chamberlain, S. et al. rnoaa: “NOAA” Weather data from R. R package version 0.7. 0. 2017. https://cran.r-project. org/web/packages/rnoaa/ (2017).
Sparks, A. H., Hengl, T. & Nelson, A. GSODR: Global summary daily weather 800 data in R. J. Open Source Softw. https://doi.org/10.21105/joss.00177 (2017).
Dunning, J. B. CRC Handbook of Avian Body Masses (CRC Press, Boca Raton, 2008).
Grolemund, G. & Wickham, H. Dates and times made easy with lubridate. J. Stat. Softw. 40, 25. https://doi.org/10.18637/jss.v040.i03 (2011).
Searle, S. R., Speed, F. M. & Milliken, G. A. Population marginal means in the linear model: An alternative to least squares means. Am. Stat. 34, 216–221. https://doi.org/10.1080/00031305.1980.10483031 (1980).
Vincze, O. et al. Parental cooperation in a changing climate: Fluctuating environments predict shifts in care division. Global Ecol. Biogeogr. 26, 347–358. https://doi.org/10.1111/geb.12540 (2017).
Hadfield, J. D. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. J. Stat. Softw. 33, 1–22. https://doi.org/10.18637/jss.v033.i02 (2010).
R Core Team. R: A language and environment for statistical computing in R Foundation for Statistical Computing. https://www.R-project.org (2018).
Acknowledgements
This work was supported by the Chinese Scholarship Council (to NH). Royal Society Wolfson Merit Award WM170050, APEX APX\R1\191045 and Leverhulme Trust (RF/2/RFG/2005/0279, ID200660763) (to TS). the National Research, Development and Innovation Office of Hungary (ÉLVONAL KKP-126949, K-116310 (to TS, JOV, VK and NH); NN 125642 (to AK and GCM)). NBAF-Sheffield supported by grants (NBAF547, NBAF933, NBAF441). Other funding sources of fieldworks are provided in the Supplementary acknowledgements. We thank all fieldwork volunteers and people who have helped with data collection. Thanks to András Liker, Philippa Harding, Zitan Song, Claire Tanner and Kees Wanders for their advice on previous versions of the manuscript. We also thank Siyu Ding for providing original plover illustrations and the map in Fig. 1.
Author information
Authors and Affiliations
Contributions
N.H. and T.S. conceived the project and designed methodology; K.K., M.C.C.I., D.B.R., D.C., J.J.H.S.C., J.C., J.F., M.Y., M.J., M.M., M.C.L., M.S., M.A.W., P.L., P.L., P.Q., T.M., U.B., Y.L., A.K. provided the data; N.H., J.O.V., V.K. carried out the statistical analyses. N.H., T.S. and G.C.M. led the writing of the manuscript. All authors contributed critically to the drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halimubieke, N., Kupán, K., Valdebenito, J.O. et al. Successful breeding predicts divorce in plovers. Sci Rep 10, 15576 (2020). https://doi.org/10.1038/s41598-020-72521-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72521-6
This article is cited by
-
Breeding ecology of a high-altitude shorebird in the Qinghai–Tibetan Plateau
Journal of Ornithology (2024)
-
OpenBioMaps – self-hosted data management platform and distributed service for biodiversity related data
Earth Science Informatics (2022)
-
Dispersal distance is driven by habitat availability and reproductive success in Northern Great Plains piping plovers
Movement Ecology (2021)
-
The allocation between egg size and clutch size depends on local nest survival rate in a mean of bet-hedging in a shorebird
Avian Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.