Abstract
The combination of pyrimethamine and sulfadiazine is the standard care in cases of congenital toxoplasmosis. However, therapy with these drugs is associated with severe and sometimes life-threatening side effects. The investigation of phytotherapeutic alternatives to treat parasitic diseases without acute toxicity is essential for the advancement of current therapeutic practices. The present study investigates the antiparasitic effects of oleoresins from different species of Copaifera genus against T. gondii. Oleoresins from C. reticulata, C. duckei, C. paupera, and C. pubiflora were used to treat human trophoblastic cells (BeWo cells) and human villous explants infected with T. gondii. Our results demonstrated that oleoresins were able to reduce T. gondii intracellular proliferation, adhesion, and invasion. We observed an irreversible concentration-dependent antiparasitic action in infected BeWo cells, as well as parasite cell cycle arrest in the S/M phase. The oleoresins altered the host cell environment by modulation of ROS, IL-6, and MIF production in BeWo cells. Also, Copaifera oleoresins reduced parasite replication and TNF-α release in villous explants. Anti-T. gondii effects triggered by the oleoresins are associated with immunomodulation of the host cells, as well as, direct action on parasites.
Similar content being viewed by others
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite belonging to the Apicomplexa phylum1. T. gondii is the etiologic agent of toxoplasmosis, a zoonotic food-borne infection, which is a significant public health issue worldwide with a broad range of clinical syndromes in humans2. Epidemiological surveys show that this intracellular parasite chronically infects 30 to 90% of the global population with substantive differences between countries3,4,5,6,7.
Infection with T. gondii is usually asymptomatic in healthy individuals, but it can cause severe symptoms in infected children, newborns, and immunocompromised individuals7. Infection during or just before pregnancy can result in the vertical transmission of T. gondii tachyzoites, which may cross the placenta and invade fetal tissues8. The congenital infection may be systemic and can be particularly serious, resulting in miscarriage, stillbirth, fetal death, fetal abnormalities, encephalitis, chorioretinitis, and child disability8,9.
The rate of congenital transmission during the first and second trimesters of pregnancy is less than 10 to 30%, respectively, and increases to nearly 90% during of third trimester10,11,12. In contrast, the severity of fetal damage decreases with the gestational progression13,14. The placental barrier is more efficient in inhibiting vertical transmission of T. gondii tachyzoites at the beginning of gestation but becomes more susceptible at the end of pregnancy15.
Pregnant women infected by T. gondii require early diagnosis, and anti-parasitic treatment in order to improve both mother and child health12. The current literature shows that early treatment of the infected mother could prevent or reduce vertical transmission and, consequently, the fetal damage12,16,17,18. When maternal infection by T. gondii is detected, and there is no evidence of fetal infection, the common therapeutic practice indicates the use of spiramycin, a macrolide antibiotic that prevents the congenital transmission8,19,20. However, this macrolide does not cross the placenta and is not suitable for treatment when a fetal infection is confirmed21.
In cases of congenital toxoplasmosis, a combination of pyrimethamine and sulfadiazine is the first choice for treatment. When combined, the drugs act in synergism to inhibit crucial enzymes involved in the biosynthesis of T. gondii pyrimidines, which are essentials for both parasite survival and replication22,23,24. Despite the importance of these drugs to control infection by T. gondii, their use is linked to serious toxicity, such as gradual dose-related bone marrow depression, gastrointestinal disorders, and teratogenic effects on the fetus during the first trimester of pregnancy3,25,26,27.
During the last decade, our research group has been making efforts to find more effective and less toxic therapeutic alternatives for the treatment of congenital toxoplasmosis. In this context, our group showed that azithromycin treatment reduced the vertical transmission of T. gondii tachyzoites in pregnant Calomys callosus rodents and was able to control parasite infection in human trophoblastic cells (BeWo cells)28,29. Moreover, we demonstrated that azithromycin treatment promoted inhibition of proliferation of T. gondii Brazilian strains in human villous explants from the third trimester of pregnancy30,31. Also, our work with other compounds showed that both enrofloxacin and toltrazuril impairs T. gondii infection in vitro, ex vivo, and in vivo experimental models32,33.
In summary, conventional therapy for congenital toxoplasmosis suppresses the active infection; however, it does not cure the latent infection34,35. Moreover, treatment options include the use of drugs, which can cause serious side effects in both mother and child, leading to discontinuation of therapy in up to 40% of patients34,35. Thus, current treatment for congenital toxoplasmosis is still limited, affecting mortality and quality of life on pregnancy and neonatal health7.
In this scenario, it is relevant to consider plant-derived compounds as the source of new bioactive substances for the treatment of congenital toxoplasmosis36. The search for alternative therapeutic tools gathered great interest in the past few decades, where plants with medicinal properties are systematically screened for their potential to treat parasitic diseases37,38,39,40,41. Several studies have evaluated the anti-Toxoplasma effects of many plant-based products, and promising results have been published39,40,41,42,43,44,45,46,47,48.
The Copaifera genus belongs to the Fabaceae family (Leguminosae) and is present throughout the American and African continents. Their oleoresins are obtained by tapping the trunk of trees and have been extensively studied because of its medicinal properties49. These oleoresins exhibit remarkable biological properties such as antimicrobial, anti-inflammatory, and antiparasitic activity49,50,51,52,53. However, no current studies investigated the impact of oleoresins from Copaifera genus in T. gondii infection. The present work investigates the antiparasitic effects of oleoresins from different species of Copaifera genus against T. gondii. We used two distinct experimental models to assess the effect of Copaifera oleoresins: an in vitro model using human trophoblastic cells (BeWo cells) as host cells and an ex vivo model using human villous explants from the third trimester of pregnancy.
Results
Oleoresin treatments altered viability in BeWo cells at higher concentrations
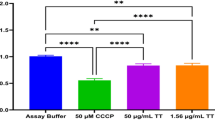
Evaluation of the oleoresin impact in cell viability, human trophoblastic cells (BeWo lineage) were treated with four oleoresins extracted from different species from Copaifera spp., as follows: Copaifera reticulata, Copaifera duckei, Copaifera paupera and Copaifera pubiflora (Fig. 1). BeWo cells exposed to oleoresins in different concentration only showed loss of viability at 24 h after treatment, and only at high doses. The minimal dose for toxic effect, as measured by loss of viability, was 256 µg/mL for oleoresins of C. reticulata (**P = 0.0063), C. paupera (*P = 0.0138) (Fig. 1A,C), and 128 µg/mL for C. duckei (*P = 0.032), and C. pubiflora (**P = 0.0015) (Fig. 1B,D). All treated experiments were compared to cells incubated only with culture medium (control group). As vehicle control, BeWo cells treated with DMSO 1.2% did not present loss of cell viability.
Host cell viability by MTT assay. BeWo cells were treated for 24 h in twofold serial dilutions (ranging from 256 to 4 µg/mL) with oleoresins from (A) Copaifera reticulata, (B) Copaifera duckei, (C) Copaifera paupera and (D) Copaifera pubiflora. BeWo cells were treated with culture medium alone (control group) and DMSO 1.2% (vehicle of oleoresins in the concentration of 256 µg/mL). Cell viability was expressed in percentages (cell viability %), with the absorbance of cells incubated only with culture medium considered to be 100% viability. Data are expressed as means ± standard deviation from experiments performed in eight replicates. Significant differences detected by the Kruskal–Wallis test and Dunn’s multiple comparison post-test are labeled (statistically significant when P < 0.05).
Copaifera oleoresins significantly reduced T. gondii intracellular proliferation in BeWo cells
In our host cell viability assay, we established concentrations of each oleoresin that did not alter the viability of BeWo cells for further set up of experiments. We performed the assays using twofold serial dilutions of oleoresins from C. reticulata, C. paupera (ranging from 128 to 4 µg/mL), and C. duckei, C. pubiflora (ranging from 64 to 4 µg/mL). Quantification of T. gondii growth inhibition was performed by incubating these selected oleoresin concentrations with infected BeWo cells for 24 h. Parasite growth (% of T. gondii proliferation) was quantified by measuring the β-galactosidase activity of viable parasites. All tested oleoresins inhibited parasite proliferation, as follow: C. reticulata (128, 64 and 32 µg/mL; ****P < 0.0001) (Fig. 2A), C. duckei (64 and 32 µg/mL; ****P < 0.0001|16 µg/mL; *P = 0.0372) (Fig. 2B), C. paupera (128, 64 and 32 µg/mL; ****P < 0.0001) (Fig. 2C), C. pubiflora (64 and 32 µg/mL; ****P < 0.0001|16, 8 and 4 µg/mL; ***P = 0.0009, *P = 0,0,361 and ***P = 0.0001, respectively) (Fig. 2D), when compared to untreated and infected BeWo cells (control group).
β-galactosidase activity-based screen. T. gondii-infected BeWo cells were treated for 24 h with non-toxic concentrations in twofold serial dilutions of oleoresins from (A) Copaifera reticulata (ranging from 128 to 4 µg/mL), (B) Copaifera duckei (ranging from 64 to 4 µg/mL), (C) Copaifera paupera (ranging from 128 to 4 µg/mL) and (D) Copaifera pubiflora (ranging from 64 to 4 µg/mL). We treated BeWo cells with culture medium only (control group—considered as 100% parasite proliferation), or a combination of 200 µg/mL of sulfadiazine and 8 µg/mL of pyrimethamine (SDZ + PYR). T. gondii intracellular proliferation was analyzed using a colorimetric β-galactosidase assay and expressed in percentage change compared to control (% of T. gondii proliferation). Our results are shown as means ± standard deviation from experiments performed in eight replicates. *Comparison between infected/untreated cells and infected/treated cells; &Comparison to SDZ + PYR-infected/treated cells; $Comparison between both treatment concentrations of each Copaifera oleoresin. We used One-Way ANOVA and Turkey’s multiple comparisons post-test to evaluate significant differences (P < 0.05).
Doses of 128, 64, and 32 µg/mL of all Copaifera oleoresins tested displayed a significant reduction of T. gondii intracellular proliferation while staying safely below the toxic dose for the cells. The concentration of 64 µg/mL was more efficient to inhibit parasite replication (~ 85–100%) compared to the concentration of 32 µg/mL (~ 40–80%), as follow: C. reticulata, C. duckei, C. paupera ($P < 0.0001) and C. pubiflora ($P = 0.0060) (Fig. 2). The combination of sulfadiazine and pyrimethamine (SDZ + PYR) (200 + 8 μg/mL, respectively) was also able to reduce parasite replication (~ 70%) in comparison with untreated and infected cells (****P < 0.0001) (Fig. 2). In comparison with classical drugs commonly used to treat congenital toxoplasmosis (SDZ + PYR), treatment with 128 and 64 µg/mL of oleoresins from C. reticulata (&P < 0.0001 and &P = 0.0007, respectively), and 64 µg/mL C. duckei (&P < 0.0001) were significantly more effective to control parasite growth (~ 90–100%) (Fig. 2A,B). In addition, both 128 µg/mL C. paupera (&P < 0.0007) and 64 µg/mL C. pubiflora (&P < 0.0001) oleoresins were also significantly more effective to inhibit parasite proliferation (~ 95–100%) in relation to SDZ + PYR treatment (~ 70%) (Fig. 2C,D).
Pre-treatment of T. gondii tachyzoites with oleoresins impairs adhesion, invasion and subsequent proliferation
The success in the establishment of infection by T. gondii requires the ability of the parasites to attach and invade host cells54,55. To evaluate whether the oleoresin treatments target the host cell or the parasite, we performed a variety of assays. In the first set of experiments, we assessed whether oleoresin treatments would affect the parasite adhesion to host cells. T. gondii tachyzoites were pre-incubated with different oleoresins and then incubated with previously fixed BeWo cells. We observed that the pre-treatment of parasites with 64 and 32 µg/mL oleoresins from C. reticulata (****P < 0.0001), C. duckei (****P < 0.0001), C. paupera (64 µg/mL; ****P < 0.0001) and C. pubiflora (***P < 0.0006 and ****P < 0.0001, respectively) reduced their cellular adherent capacity compared to untreated parasites (control group) (Fig. 3A). Interestingly, the pre-treatment of parasites with 64 and 32 µg/mL of oleoresins significantly reduced the number of BeWo cells with parasites attached to them, in comparison to parasites treated with SDZ + PYR. Data from C. reticulata (&P = 0.0003 and &P = 0.0039, respectively), C. duckei (&P = 0.0031 and &P = 0.0001, respectively), C. paupera (64 µg/mL; &P < 0.0143) and C. pubiflora (&P < 0.0267 and &P < 0.0010, respectively) are shown in Fig. 3A. Also, the pre-treatment of parasites with 64 and 32 µg/mL oleoresins from C. reticulata (****P < 0.0001), C. duckei (****P < 0.0001), C. paupera (64 µg/mL; ****P < 0.0001) and C. pubiflora (****P < 0.0001) promoted a reduction in the number of adhered parasites to BeWo cells (Fig. 3B). The oleoresin treatments with 64 and 32 µg/mL showed a greater reduction in the number of adhered parasites when compared to the standard treatment with SDZ + PYR. Data from C. reticulata (&P = 0.0007 and &P = 0.0196, respectively), C. duckei (&P = 0.0342 and &P = 0.0003, respectively), C. paupera (64 µg/mL; &P = 0.0342) and C. pubiflora (&P = 0.0143 and &P = 0.0023, respectively) is presented in Fig. 3B. On the other hand, pre-treatment of parasites with SDZ + PYR did not interfere with the number of cells with parasites attached to them, nor with the number of parasites adhered to cells in comparison to controls (Fig. 3A,B). Representative images highlighting the impact of oleoresin treatments (64 µg/mL) in the tachyzoites-host cell interaction are in Fig. 3C–H.
Adhesion assay of T. gondii tachyzoites to BeWo cells. T. gondii tachyzoites were pre-incubated for one h with 64 and 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, C. pubiflora, SDZ + PYR (200 + 8 μg/mL) and culture medium only (control group) and then allowed to interact for three h with previously fixed BeWo cells. (A) The number of cells with adhered parasites and (B) the total number of adhered parasites to BeWo cells in a total of approximately 20 fields examined randomly. Representative images highlighting the tachyzoites-host cell interaction are presented according to the conditions analyzed: (C) control group (untreated parasites), (D) SDZ + PYR, (E) C. reticulata, (F) C. duckei, (G) C. paupera and (H) C. pubiflora. Representative images of oleoresin treatments showed only the concentration of 64 µg/mL. Data are expressed as means ± standard deviation. The experiment was performed with four replicates. *Comparison between untreated parasites and treated parasites; &Comparison to SDZ + PYR-treated parasites; $Comparison between either treatment concentrations of each Copaifera oleoresin. Significant differences were determined by using one-way ANOVA and Tukey’s multiple comparisons post-test. Differences were considered significant when P < 0.05. White arrows indicate tachyzoites adhered to BeWo cells. The cell nucleus is labeled with DAPI (blue). T. gondii tachyzoites labeled with Alexa Fluor 488-conjugated anti-mouse IgG are shown green. F-actin labeled with Phalloidin-TRITC is shown red. Scale bar: 50 µm.
In the second set of experiments, we assessed whether oleoresins were able to affect parasite invasion and subsequent intracellular proliferation. Concerning parasite invasion, our data suggest that the pre-treatment of T. gondii tachyzoites for one hour with 64 and 32 µg/mL oleoresins from C. reticulata, C. duckei, C. pubiflora (****P < 0.0001) and C. paupera (64 µg/mL; ****P < 0.0001) reduced significantly parasite invasion (3 h) compared to control group (Fig. 4A). Also, the pre-treatment with both concentrations of oleoresins was significantly more effective in inhibiting parasite invasion (3 h) when compared to parasites treated with SDZ + PYR. Data from C. reticulata, C. duckei, (&P < 0.0001), C. paupera (64 µg/mL; &P < 0.0001) and C. pubiflora (64 and 32 µg/mL; &P < 0.0001 and &P = 0.0090, respectively) are shown in Fig. 4A. Moreover, oleoresins from C. duckei, C. pubiflora ($P < 0.0001), and C. paupera ($P = 0.0062) in the concentration of 64 µg/mL were more efficient to reduce parasite invasion, in comparison with 32 µg/mL concentration. The pre-treatment with SDZ + PYR did not alter the parasite invasion (Fig. 4A).
Invasion and intracellular proliferation of pre-treated T. gondii tachyzoites in BeWo cells. T. gondii tachyzoites were pre-incubated for one h with 64 or 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, C. pubiflora, SDZ + PYR (200 + 8 μg/mL) or culture medium alone (control group/untreated group). (A) % of T. gondii invasion and (B) % of T. gondii intracellular proliferation after 24 h were determined using the β-galactosidase assay. Untreated parasites (control group) were considered as 100% of invasion and proliferation, respectively. Data are expressed as means ± standard deviation from experiments performed in eight replicates. *Comparison between untreated parasites and treated parasites; &Comparison to SDZ + PYR-treated parasites; $Comparison between both treatment concentrations of each Copaifera oleoresin. Significant differences were analyzed using one-way ANOVA and Tukey’s multiple comparisons post-test. Differences were considered significant when P < 0.05.
Addressing the impact of oleoresins in the intracellular proliferation of T. gondii (24 h), the concentrations 64 and 32 µg/mL of oleoresins reduced the replication of pre-treated parasites compared to untreated parasites, as follows: C. reticulata, C. duckei, C. pubiflora (****P < 0.0001) and C. paupera (64 and 32 µg/mL; ****P < 0.0001 and ***P = 0.0002, respectively) (Fig. 4B). Related to the action of each concentration from selected oleoresin in the inhibition of parasite replication, the pre-treatment with 64 µg/mL oleoresins from C. reticulata ($P < 0.0038), C. duckei, C. paupera and C. pubiflora ($P < 0.0001) was able of blocking parasite proliferation when compared to the concentration of 32 µg/mL (Fig. 4B). Similarly, all oleoresins, except C. paupera at 32 µg/mL, showed inhibition of T. gondii proliferation when compared to parasites treated with SDZ + PYR. Data from C. reticulata, C. duckei, C. paupera, and C. pubiflora (&P < 0.0001) (Fig. 4B).
Oleoresins exhibited an irreversible concentration-dependent antiparasitic action
To determine whether the antiparasitic effects promoted by Copaifera oleoresins were reversible, we exposed parasite-infected BeWo cells with all four oleoresins for 24 h; rinsed the cell monolayers, and then incubated the cells in the treatment-free medium for a further 24 h. In parallel, we quantified the parasite proliferation at 24 h of treatment with oleoresins; the results served as the baseline for comparison. In agreement with our previous results from the β-galactosidase activity-based screen, all four oleoresins tested in the concentrations of 64 and 32 µg/mL were able to control parasite proliferation after 24 h of treatment, compared to the untreated group (control) (Fig. 5A). Interestingly, the treatment with 64 and 32 µg/mL oleoresins reduced the rate of parasite proliferation even after 24 h of treatment removal in contrast with the untreated group (considered as 100% of reversibility); C. duckei, C. paupera, C. pubiflora (****P < 0.0001), C. reticulata (64 and 32 µg/mL; ****P < 0.0001 and *P = 0.0099, respectively) and SDZ + PYR (****P < 0.0001). It is noteworthy that the treatment with 64 µg/mL oleoresins from C. duckei (&P < 0.0231) and C. pubiflora (&P < 0.0134) were more efficient in the inhibition of parasite growth in comparison with SDZ + PYR treatment. Additionally, the oleoresin concentration of 64 µg/mL from C. reticulata, C. duckei, C. paupera, and C. pubiflora ($P < 0.0001) demonstrated to be more efficient than the concentration of 32 µg/mL (Fig. 5A). Our experiments suggest that treatment with C. duckei, C. paupera, C. pubiflora, and SDZ + PYR did not show significant differences in antiparasitic effect, even after 24 h of treatment removal. Only the treatment with C. reticulata (64 and 32 µg/mL; #P < 0.0001) tended to be reversible after 24 h. These results suggest that the oleoresins, as well as a combination of SDZ + PYR, maintained their anti-proliferative effect in a concentration-dependent manner, even treatment removal (Fig. 5A).
Evaluation of the maintenance of antiparasitic effects of oleoresins by reversibility assay. T. gondii-infected BeWo cells were exposed to 64 and 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, C. pubiflora, SDZ + PYR (200 + 8 μg/mL) and culture medium alone (control group/untreated group) for 24 h, followed by treatment removal for an additional 24 h. Parasite proliferation at 24 h of treatment with oleoresins was considered the baseline for comparison. (A) The reversibility ratio measures the ability of the parasites to recover from treatment and regain infectiveness. The ratio is shown in percentage change (% reversibility of treatment) in comparison with the control group, which was considered as 100% reversibility. The β-galactosidase assay was used as an indicator of parasite activity. (B) Parasites obtained from oleoresin-treated BeWo cells were harvested and used to infect new host cells for 24 h. The number of tachyzoites was determined by β-galactosidase assay and expressed as % of T. gondii proliferation. Data are shown as means ± standard deviation from experiments performed in eight replicates. *Comparison between infected/untreated cells and infected/treated cells; &Comparison to SDZ + PYR-infected/treated cells; $Comparison between either treatment concentrations of each species of Copaifera oleoresin. #Comparison with the respective treatment in the condition of 24 h of treatment. Significant differences were analyzed using one-way ANOVA and Tukey’s multiple comparisons post-test. Differences were considered significant when P < 0.05.
In order to gain insights into the effect triggered by oleoresins in T. gondii tachyzoites, we assessed whether intracellular parasites obtained from oleoresin-treated BeWo cells could sustain their ability to infect new host cells. BeWo cells were infected and treated with Copaifera oleoresins for 24 h. Next, the parasites were harvested and used to infect new host cells. We demonstrated that the treatment with 64 and 32 µg/mL oleoresin from C. reticulata (***P = 0.0001) C. duckei (64 and 32 µg/mL; ****P < 0.0001 and ***P = 0.0008, respectively), C. paupera, C. pubiflora (****P < 0.0001) and SDZ + PYR (***P < 0.0001) were able to impair T. gondii infection, compared to parasites from untreated cells (control group) (Fig. 5B). These findings indicate that the inhibitory effects promoted by oleoresins from C. reticulata, C. duckei, C. paupera, and C. pubiflora, as well as SDZ + PYR treatment, are irreversible.
Oleoresin treatments cause cell cycle arrest in parasites in the S/M phase
Copaifera oleoresins have an efficient antiparasitic activity by inhibition of parasite proliferation. In this sense, to confirm the anti-proliferative effect showed by these oleoresins, we proposed to evaluate whether progression through the parasite cell cycle was blocked or altered by the oleoresin treatments. First, intracellular parasites were obtained from infected BeWo cells treated for 24 h with oleoresins. Subsequently, we determined the percentages of parasites in each cell cycle phase (G1 and S/M) through the quantification of DNA content by flow cytometry. We observed that the treatment with 64 and 32 µg/mL of oleoresins caused a significant decrease in the 2 N cell number (G1 phase) in the parasite cell cycle compared to untreated parasites in the control group (Fig. 6A). Data from C. reticulata (****P < 0.0001) (Fig. 6C,D), C. duckei (64 and 32 µg/mL; ****P < 0.0001 and **P = 0.0014, respectively) (Fig. 6E,F), C. paupera (64 and 32 µg/mL; ****P < 0.0001 and **P = 0.0023, respectively) (Fig. 6G,H), C. pubiflora (****P < 0.0001) (Fig. 6I,J) are summarized in the Fig. 6. Oleoresin treatment resulted in significant accumulation in the number of cells in the S/M phase in the parasite cell cycle. Data from 64 and 32 µg/mL of oleoresins from C. reticulata (****P < 0.0001) (Fig. 6C,D), C. duckei (64 and 32 µg/mL; ****P < 0.0001 and *P = 0.0210, respectively) (Fig. 6E,F), C. paupera (64 and 32 µg/mL; ****P < 0.0001 and **P = 0.0023, respectively) (Fig. 6G,H), C. pubiflora (****P < 0.0001) (Fig. 6I,J) related to untreated parasites (control group) (Fig. 6A), thus suggesting parasite cell cycle arrest in the S/M phase. To determine the specificity of oleoresin effects on the parasite cell cycle, we assessed the effects of classical antiparasitic drugs (SDZ + PYR). Similarly to oleoresins effects on parasite cell cycle, our results showed that SDZ + PYR treatment caused a decrease in G1 (****P < 0.0001) and a prominent accumulation of cells in the S/M phase (****P < 0.0001) in comparison with the control group (Fig. 6B).
Effects of oleoresins on T. gondii cell cycle. Intracellular parasites were exposed for 24 h to either (A) culture medium only (control group), (B) SDZ + PYR (200 + 8 µg/mL), oleoresins from (C) C. reticulata 64 µg/mL, (D) 32 µg/mL, (E) C. duckei 64 µg/mL, (F) 32 µg/mL, (G) C. paupera 64 µg/mL, (H) 32 µg/mL, (I) C. pubiflora 64 µg/mL and (J) 32 µg/mL. Each representative histogram shows 20.000 total events. Percentage ± standard deviations of parasites in G1 or S/M phases determined by gating is indicated in the accompanying histogram. The experiment was carried out in quadruplicates. *Comparison between infected and untreated cells (control group). Significant differences were analyzed using one-way ANOVA and Tukey’s multiple comparisons post-test. Differences were considered significant when P < 0.05.
Oleoresin treatments promoted ultra-structural alterations in intracellular parasite morphology
We performed the ultrastructural analysis of intracellular T. gondii tachyzoites treated with oleoresins to investigate the direct effect of oleoresins in the parasite’s morphology. BeWo cells were infected and treated with 64 µg/mL Copaifera oleoresins and SDZ + PYR for 24 h. Next, the infected cells were fixed and processed for transmission electron microscopy (TEM). Untreated cells harbor a parasitophorous vacuole (Pv) containing tachyzoites with a characteristic arc-like shape, a well-defined tubulovesicular network, duple membrane (arrows), rhoptries (Rp), nucleus (Nu), dense granule (Dg) and mitochondria (M), suggesting a normal endogenic process of replication (Fig. 7A). SDZ + PYR-treated infected cells resulted in the disruption of parasite organelles, ruffled duple membranes, as well as parasites united by their basal ends (arrowheads) (Fig. 7B).
Parasite ultrastructure after exposure to treatments. Transmission electron micrographs of intracellular T. gondii after 24 h exposure to either (A) culture medium only (control group), (B) SDZ + PYR (200 + 8 µg/mL), 64 µg/mL oleoresins from (C) C. reticulata, (D) C. duckei, (E) C. paupera and (F) C. pubiflora. Pv, parasitophorous vacuole; Rp, rhoptries; Nu, nucleus; Vls, vacuole-like structure; M, mitochondria; and Dg, dense granule of T. gondii tachyzoites. Arrow: parasite duple membrane; arrowhead: tethered parasites (basal ends) or single membrane of parasites. Bars scale (bottom right): 1 µm or 2 µm.
Similarly to standard treatment, oleoresin promoted notable alteration in parasite morphology. Infected cells treated with all four oleoresins frequently showed PV containing parasites with multiply intracellular vacuole-like structures (Vls), and tethered tachyzoites with probable difficulty in completing cytokinesis, since the treatment resulted in growth arrest. Also, we visualized the disruption of parasite organelles, as they were poorly defined and reduced to a single membrane structure in some areas, suggesting damage to the formation of the duple membrane (arrowheads) (Fig. 7C–F).
Copaifera oleoresins modulate cytokine release and ROS production by BeWo cells
While our previous results described above indicate that Copaifera oleoresins have a direct effect on parasites, we could not exclude the possibility that these oleoresins may affect intracellular proliferation by altering the host cell environment. In this context, firstly, we investigated the potential immunomodulatory effects of Copaifera oleoresins by measuring the levels of IL-6, MIF, TNF-α, TGF-β, and IL-10 present in the supernatants from BeWo cells infected or not by T. gondii. Interestingly, our results showed that in the absence of infection, except for C. paupera oleoresin in the concentration of 32 µg/mL, the treatment with 64 and 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, and C. pubiflora (****P < 0.0001; &P < 0.0001) induced an increase in the levels of IL-6 in comparison to controls. Additionally, SDZ + PYR treatment did not interfere in the IL-6 production (Fig. 8A). Similarly, even after T. gondii infection, except for C. paupera oleoresin in the concentration of 32 µg/mL, infected BeWo cells treated with 64 and 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera and C. pubiflora (****P < 0.0001; &P < 0.0001) continued producing high levels of IL-6, compared to infected and untreated cells (control group) and SDZ + PYR-treated cells. Moreover, T. gondii infection (control T.g.) and the SDZ + PYR standard treatment did not alter the IL-6 production in comparison with uninfected and untreated cells (control) (Fig. 8B). Addressing MIF production, we observed that only the treatment with 64 µg/mL C. duckei oleoresin (****P < 0.0001; &P < 0.0001), 64 and 32 µg/mL C. pubiflora oleoresin (****P < 0.0001; &P < 0.0001) caused an augmentation of MIF levels by uninfected BeWo cells in comparison with uninfected and untreated cells (control group) and SDZ + PYR-treated cells (Fig. 8C). Curiously, T. gondii infection increased MIF production by BeWo cells related to uninfected and untreated cells (#P = 0.0030). Our results showed that the treatment of infected cells only with oleoresins from 32 µg/mL C. reticulata (****P < 0.0001) and 64 µg/mL C. pubiflora (*P = 0.0270) up-regulated the MIF levels in comparison to infected and untreated cells (Fig. 8D). In contrast, oleoresins from 32 µg/mL C. duckei (**P = 0.0032), 64 and 32 µg/mL C. paupera (****P < 0.0001 and **P = 0.0014, respectively), 32 µg/mL C. pubiflora (*P = 0.0139) and SDZ + PYR treatment (***P = 0.0009) down-modulated the MIF production, when compared to infected and untreated cells (control T.g.) (Fig. 8D). Besides, the treatment with oleoresins from 32 µg/mL C. reticulata (&P < 0.0001), 64 µg/mL C. duckei (&P = 0.0069) and 64 µg/mL C. pubiflora (&P < 0.0001) led to the infected cells to produce more MIF that the cells treated with SDZ + PYR (Fig. 8D). TNF-α, TGF-β, and IL-10 cytokines were not detected in supernatants from BeWo cells under any experimental conditions (data not shown).
Cytokine and ROS production by T. gondii-infected BeWo cells after treatments. BeWo cells were infected or not with T. gondii tachyzoites, followed by treatment for 24 h with 64 and 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, C. pubiflora, SDZ + PYR (200 + 8 μg/mL) and culture medium only (control group/untreated group). Supernatants were collected for measurement of (A,B) IL-6 and (C,D) MIF. (E,F) BeWo cells were incubated with the probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) and the ROS production was measured by flow cytometry and expressed as median fluorescence intensity (MFI). Data are expressed as means ± standard deviation from experiments performed in five replicates. *Comparison between uninfected/untreated cells (control) and uninfected/treated cells (A,C,E); *Comparison between infected/untreated cells (control T.g.) and infected/treated cells (B,D,F); #Comparison between uninfected/untreated cells (control) and infected/untreated cells (control T.g.); &Comparison in relation to the SDZ + PYR treatment. Significant differences were determined by using one-way ANOVA and Tukey’s multiple comparisons post-test. Differences were considered significant when P < 0.05.
Moreover, we also investigated the effects of Copaifera oleoresins in ROS production by BeWo cells. Our data indicates that in the absence of T. gondii infection, the treatment with 64 µg/mL of oleoresins from C. reticulata (***P = 0.0002; &P < 0.0001), C. duckei (**P = 0.0012; &P = 0.0004), C. paupera (*P = 0.0133; &P = 0.0047) and C. pubiflora (**P = 0.0013; &P = 0.0004) caused a downregulation in ROS production in relation to uninfected and untreated cells (control group) and SDZ + PYR-treated cells (Fig. 8E). Also, T. gondii infection (control T.g.) promoted an increase in ROS levels related to uninfected and untreated cells (control group) (#P = 0.0007). Interestingly, except for C. duckei oleoresin in the concentration of 32 µg/mL, the treatment with 64 and 32 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera and C. pubiflora (****P < 0.0001) was able to reduce the ROS levels caused by T. gondii infection (Fig. 8F). Furthermore, except for C. duckei oleoresin in the concentration of 32 µg/mL, the reduction in ROS production was significantly higher in the treatments with oleoresins from C. reticulata (64 and 32 µg/mL; &P < 0.0001 and &P = 0.0173, respectively), C. duckei (64 µg/mL; &P < 0.0001), C. paupera (64 and 32 µg/mL; &P < 0.0001 and &P = 0.0125, respectively) and C. pubiflora (64 and 32 µg/mL; &P < 0.0001) in comparison with cells infected and treated with SDZ + PYR (Fig. 8F). Finally, SDZ + PYR treatment did not affect the ROS production in both the absence and presence of infection (Fig. 8E,F).
Human chorionic villous explants treated with oleoresins altered viability only at the highest concentration
In addition to investigating the effects of Copaifera oleoresins in T. gondii-infected BeWo cells, we also evaluated the action of these oleoresins against parasite infection in human placental villous explants. Firstly, to determine a non-toxic concentration to use in the further set up of experiments, the viability of villous explants after oleoresin treatments was performed by measuring LDH release and MTT assay. Our data demonstrated that the treatment only in the concentration of 256 µg/mL oleoresins from C. reticulata (***P = 0.0004), C. duckei (*P = 0.0136), and C. pubiflora (*P = 0.0226) caused a significant increase in the release of LDH (U/L) by villous explants when compared to untreated villous explants (control group);. In contrast, C. paupera oleoresins did not alter the LDH levels at all concentrations tested (Fig. 9A). Also, the treatment with SDZ + PYR and DMSO 1.2% did not cause significant cytotoxicity in villous explants compared to untreated villous explants (control group) (Fig. 9A). Similar to LDH measurements, our results from MTT incorporation showed a significant reduction in the viability of villous explants only at 256 µg/mL of oleoresins from C. reticulata, C. duckei (****P < 0.0001), and C. pubiflora (**P = 0.0073) compared to untreated controls; while all others experimental conditions did not alter tissue integrity (Fig. 9B). Based on these results, we established the use of 128 µg/mL as the highest concentration that did not change the tissue integrity at all four oleoresins for further experiments. We analyzed the morphology of the villus explants to corroborate with the data from the viability assay. Our results demonstrated that the oleoresin treatments did not alter the tissue morphology, which was highlighted by the typical morphology of the syncytiotrophoblast cells (black arrows) and mesenchyme (M) when compared to untreated villous explants (Fig. 9C–H).
Viability of human villous explant after treatments. Villous explants were treated for 24 h with high oleoresin concentrations of C. reticulata, C. duckei, C. paupera and C. pubiflora (256, 128 and 64 µg/mL), SDZ + PYR (150 and 200 µg/mL), DMSO (1.2%) and culture medium alone (control group/untreated group). (A) Supernatants were collected and used to measure LDH (U/L) levels. (B) MTT assays with villous explants and the tissue viability are shown in percentages (viability % by MTT incorporation). Representative photomicrographs of villi incubated with (C) culture medium alone (control group), (D) SDZ + PYR, (E) C. reticulata, (F) C. duckei, (G) C. paupera and (H) C. pubiflora are shown in this figure. Representative images of oleoresin treatments are shown only at the concentration of 128 µg/mL. Data are expressed as means ± standard deviation from experiments performed in eight replicates. *Comparison between untreated cells and treated cells. Significant differences were analyzed by the Kruskal–Wallis test and Dunn’s multiple comparison post-test (statistically significant when P < 0.05). Hematoxylin–eosin (HE) stained histological sections show Syncytiotrophoblast cells (black arrows) and mesenchyme (M). Scale bar: 50 µm.
Copaifera oleoresins can control T. gondii intracellular proliferation in human villous explants
We evaluated the effects of oleoresins from C. reticulata, C. duckei, C. paupera, and C. pubiflora (128 µg/mL) in the control of T. gondii intracellular proliferation in villous explants using the β-galactosidase assay. We observed that the treatment with all four oleoresins in the concentration of 128 µg/mL significantly reduced the percentage of intracellular parasites, as follow: C. reticulata (~ 30%; *P = 0.0339), C. duckei (~ 60%; ****P < 0.0001), C. paupera (~ 30%; *P = 0.0404) and C. pubiflora (~ 65%; ****P < 0.0001) compared to untreated villous explants (control group—considered as 100% of parasite proliferation). Interestingly, the treatment with 128 µg/mL C. pubiflora oleoresin (&P = 0.0056) was more efficient in the control of parasite replication than the SDZ + PYR treatment (Fig. 10). As expected, the treatment with SDZ + PYR (150 and 200 µg/mL, respectively) also inhibited parasite growth (~ 30%; **P = 0.0093) compared to untreated villous explants (Fig. 10). Additionally, we also tested whether the concentration of 64 µg/mL (non-toxic concentration) of all oleoresins used in this work would be able to inhibit parasite growth in villous explants. However, we observed that only the C. pubiflora oleoresin was capable of reducing T. gondii tachyzoites proliferation in comparison with untreated villous explants (data not shown).
T. gondii intracellular proliferation in infected-villous explants. Human villous explants were infected with T. gondii tachyzoites for 24 h, followed by treatment for additional 24 h with 128 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, C. pubiflora, SDZ + PYR (150 + 200 μg/mL) and culture medium alone (control group/untreated group). T. gondii intracellular proliferation in villous explants was measured by β-galactosidase assay, and the number of tachyzoites was expressed in percentage (% of T. gondii proliferation), which the untreated/infected (control group) was considered as 100% of parasite proliferation. Data are expressed as means ± standard deviation from experiments performed in five replicates. *Comparison between infected/untreated cells and infected/treated cells; &Comparison in relation to SDZ + PYR-infected/treated cells. Significant differences were determined by using One-way ANOVA and Sidak’s multiple comparisons post-test. Differences were considered significant when P < 0.05.
Oleoresin treatments down-modulated the TNF-α production in T. gondii-infected villous explants
In order to gain insights into the action of oleoresins on the physiology of villous explants, we evaluated whether oleoresin treatments modulate cytokine production profile by measuring the levels of IL-6, MIF, TNF-α, TGF-β and IL-10 present in the supernatants from villous explants infected or not by T. gondii. In the absence of infection, the treatment with all four oleoresins and SDZ + PYR did not alter IL-6 and MIF production in comparison to uninfected and untreated villous explants (control group) (Fig. 11A,C). T. gondii infection (control T.g. group) did not alter the IL-6 production but promoted a reduction of MIF levels compared to uninfected and untreated villous (control group) (#P < 0.0001). Oleoresins and SDZ + PYR treatment did not change the release of IL-6 or MIF when compared with untreated and infected villous explants (control group) (Fig. 11B,D). Moreover, TNF-α production was not detected in supernatants from uninfected villous explants under any experimental conditions (data not shown). Toxoplasma gondii infection (control T.g.) caused a sharp increase in the TNF-α levels related to uninfected and untreated villous (control group) (#P < 0.0001). Interestingly, parasite-infected villous explants treated with 128 µg/mL oleoresins from C. reticulata (**P = 0.0091), C. duckei, C. paupera (****P < 0.0001), C. pubiflora (**P = 0.0022) and SDZ + PYR treatment (****P < 0.0001) showed a significant reduction in TNF-α release, when compared to untreated and infected villous explants (control group) (Fig. 11E). TGF-β and IL-10 cytokines were not detected in supernatants from villous explants under any experimental conditions (data not shown).
Cytokine production by T. gondii-infected human villous explants after treatments. Human villous explants were infected or not with T. gondii tachyzoites for 24 h, followed by treatment for additional 24 h with 128 µg/mL of oleoresins from C. reticulata, C. duckei, C. paupera, C. pubiflora, SDZ + PYR (150 + 200 μg/mL) and culture medium only (control group/untreated group). Supernatants were collected for measurement of (A,B) IL-6; (C,D) MIF and (E) TNF-α. Cytokine levels were expressed in pg/mg of tissue. Data are shown as means ± standard deviation from experiments performed in five replicates. *Comparison between uninfected/untreated cells (control) and uninfected/treated cells (A,C); *Comparison between infected/untreated cells (control T.g.) and infected/treated cells (B,D,E); #Comparison between uninfected/untreated cells (control) and infected/untreated cells (control T.g.); Significant differences were analyzed using one-way ANOVA and Tukey’s multiple comparisons post-test. Differences were considered significant when P < 0.05.
Proposed model of the effects triggered by Copaifera spp. oleoresins during T. gondii infection in both BeWo cells and human villous explants
Based on our results, a proposed model of the mechanism of action of oleoresins against T. gondii in both BeWo cells and human villous explants is shown (Fig. 12). Our data showed that previously pre-treatment of T. gondii tachyzoites with Copaifera oleoresins were able to reduce T. gondii adhesion, invasion, and intracellular proliferation in BeWo cells. Also, oleoresin treatments exhibited an irreversible concentration-dependent antiparasitic action and caused a parasite cell cycle arrest in the S/M phase, as well as promoted ultra-structural alterations in the morphology of intracellular parasites. Interestingly, the oleoresin treatments were capable of modulating the cytokine production on uninfected BeWo cells by an augmentation of IL-6 and MIF levels, and a reduction in the ROS production. Moreover, after T. gondii infection, BeWo cells responded with increasing the levels of IL-6, MIF, and ROS. In contrast, oleoresins-treated infected BeWo cells culminated in control of parasite proliferation with a downmodulation of MIF (oleoresins from C. duckei, C. paupera and C. pubiflora) and ROS, and an upregulation of MIF (oleoresins from C. reticulata and C. pubiflora) and IL-6 (Fig. 12A). As shown in BeWo cells, T. gondii is capable of infecting and proliferating in human villous explants, inducing an increase in TNF-α concentration. On the other hand, oleoresin treatments were able to control intracellular parasite proliferation with a reduction of the TNF-α level (Fig. 12B). Taken together, we suggest that the anti-T. gondii effects triggered by these oleoresins are probably related to the immunomodulation of the host cells and mainly by a direct action on parasites, highlighted by the block of parasite cell cycle progression and ultra-structural alterations, which may compromise the parasite viability.
Scheme summarizing the effects of Copaifera spp. oleoresins against T. gondii infection in both human trophoblastic cells (BeWo cells) and human villous explants. (A) The previously pre-treatment of T. gondii tachyzoites with Copaifera oleoresins (C. reticulata, C. duckei, C. paupera, and C. pubiflora) promoted a reduction in the adhesion, invasion and the subsequent proliferation of parasites in BeWo cells. Oleoresin treatments were capable of modulating the cytokine on uninfected BeWo cells by increasing levels of IL-6 and MIF, and a reduction in the ROS production. Moreover, after T. gondii infection, BeWo cells responded with increasing the levels of IL-6, MIF, and ROS. In contrast, the oleoresin treatments of infected BeWo cells culminated in control of parasite proliferation with a downmodulation of MIF (oleoresins from C. duckei, C. paupera and C. pubiflora) and ROS, and an upregulation of MIF (oleoresins from C. reticulata and C. pubiflora) and IL-6. Besides, the impairment in the proliferation of intracellular parasites treated by oleoresins was confirmed by the parasite cell cycle arrest (reduction in G1 phase and an increase in S/M phase), which was evidenced by parasites bound by their basal ends, with multiples vacuolar-like structures and a single membrane in some areas. (B) T. gondii is capable of infecting and proliferating in human villous explants (crossing the placental barrier: syncytiotrophoblast and cytotrophoblasts), promoting an increase in the concentrations of TNF-α. On the other hand, the oleoresin treatments were able to control intracellular parasite proliferation with a reduction of the TNF-α level.
Discussion
Toxoplasma gondii is the causative agent of toxoplasmosis, one of the most important and challenging diseases in public health1. In the context of congenital transmission, when a woman gets the infection during pregnancy, recommended drugs for treatment are limited to a combination of sulfadiazine plus pyrimethamine (SDZ + PYR) and spiramycin8,19,20,24. Unfortunately, the use of these drugs has been associated with severe side effects and uncommon reactions in both mother and child3,27,56. Additionally, several independent studies have shown that T. gondii has an exceptional adaptive potential, which has led to the development of drug-resistant parasites56,57,58. Therefore, it is clear the urgency of studies that focus on finding compounds that are both efficacious and non-toxic for the treatment of congenital toxoplasmosis.
Plant-based compounds are promising alternative treatments for various parasitic diseases, and some have shown antiparasitic effects against T. gondii in vitro and in vivo. These compounds may also have fewer side effects when compared to standard drug treatment for congenital toxoplasmosis47,59. In this sense, the present study investigated the effects of four oleoresins from different species of Copaifera genus (C. reticulata, C. duckei, C. paupera, and C. pubiflora) in T. gondii infection of human trophoblastic cells (BeWo cells; in vitro model) and human villous explants from the third trimester of pregnancy (ex vivo model). While several studies have demonstrated the antiparasitic effects of Copaifera oleoresins60,61,62,63,64, this is the first study showing the activity of these oleoresins against T. gondii.
In order to investigate the impact of Copaifera oleoresins in the control of parasite proliferation, we firstly verified the viability of BeWo cells treated with different concentrations of oleoresins. Our results showed that oleoresins-treated BeWo cells exhibited altered cell viability only at the highest concentrations (256 and 128 µg/mL). Following, we treated T. gondii-infected BeWo cells for 24 h with different non-toxic concentrations of each oleoresin. Interestingly, we observed that Copaifera oleoresins were as effective as SDZ + PYR treatment in reducing the intracellular proliferation of a highly virulent strain (RH) of T. gondii in BeWo cells. Interestingly, all oleoresins tested were able to control parasite proliferation, at the highest concentrations, more efficiently than SDZ + PYR treatment. Curiously, C. pubiflora oleoresin was the only compound that inhibited parasite growth at all concentrations used.
Toxoplasma gondii is an obligate intracellular protozoan parasite capable of infecting a broad range of nucleated cells. Thus, successful infection the parasites to attach, invade, and proliferate inside host cell36,55. Hence, the next question was: would the treatment of T. gondii tachyzoites with Copaifera oleoresins be able to interfere in essential processes for the perpetuation of the parasite infection? Thus, we set out to assess the impact of oleoresin treatments in adhesion, invasion, and proliferation of the parasites. The pre-treatment of tachyzoites for 1 h with all four oleoresins inhibited parasite attachment (with a decrease in the number of BeWo cells containing adhered parasites and in the number of parasites attached to the cells). Oleoresins also inhibit invasion (observation at 3 h after infection showed a decrease in the percentage of invading parasites) and subsequent proliferation (quantification at 24 h after infection showed a decrease in the percentage of intracellular parasites). In addition, our results showed that the pre-treatment of the parasites with SDZ + PYR did not alter adhesion, invasion, and proliferation processes in vitro. Curiously, Castro-Filice et al. (2014)30 demonstrated that the pre-treatment of the parasites with a combination of PYR, SDZ, and folinic acid for 1 h reduced T. gondii proliferation after 72 h in human villous explants. In comparison with the literature, we conclude in our findings that pre-treatment of parasites with the standard drugs is insufficient to reduce parasitism, at least, during the early stages of infection.
Taken together, we observed that oleoresins and SDZ + PYR exposure seem to affect the parasites in the model of T. gondii-infected BeWo cells and the pre-treatment. Thus, we suggest that oleoresin treatments are likely targeting the parasites directly, which may be interfering in essential parasite functions. This hypothesis is only in part, supported by the reversibility assay. Here, we had demonstrated that, after 24 h of treatment, when the oleoresin treatments were removed, the parasites did not recover their proliferation capacity, evidencing that oleoresin treatments were able to promote a powerful blockage in the parasite physiology. Moreover, SDZ + PYR treatment also promoted an irreversible effect in parasite proliferation. Interestingly, oleoresins from both C. duckei and C. pubiflora (64 µg/mL) showed almost complete ablation of parasite proliferation with 24 h of treatment, and their effects persisted after treatment removal. This data suggests that oleoresins are more efficient to block the recovery of the parasite growth than SDZ + PYR treatment. To corroborate these findings, we exposed parasites to the oleoresin treatments after infection of BeWo cells; the recovered parasites were evaluated for the ability to reinfect new host cells. We showed that the treatment with all four oleoresins at all concentrations impaired T. gondii reinfection. In addition, SDZ + PYR treatment promoted similar results when compared to oleoresin treatments.
In summary, we hypothesized that Copaifera oleoresins could bind directly in extracellular parasites and are also able to cross the host cell membrane and reach the intracellular parasites. The binding of oleoresins to the parasite membrane may be leading to the morphological alteration in parasite’s structures, and that relates to the impairment of infection by T. gondii. Izumi and colleagues partially corroborate the findings shown in this study (2013)49; the authors report the effect of eight different species of Copaifera oleoresins in the growth inhibition of all life cycle forms of Trypanosoma cruzi; showing that the intracellular forms were the most susceptible to treatment. Moreover, these authors also demonstrated that the trypanocidal activity of these oleoresins is mediated by lipid peroxidation and permeability changes in the parasite’s cellular and mitochondrial membranes, leading to high production of reactive oxygen species that can cause parasite damage and culminate in apoptosis65,66. Another study showed that C. paupera oleoresin was also able to eliminate the two evolutive forms of Leishmania amazonensis similarly, as shown against T. cruzi60. Furthermore, there is one report of C. reticulata oleoresin showing antimicrobial activity against Plasmodium falciparum, reducing parasitemia levels of infected animals, and showing low cytotoxicity effects50. Thus, multiple reports in literature reinforce the concept that Copaifera oleoresins are a promising source of compounds with notable antiparasitic effects67.
Based on the current literature, we investigated potential mechanisms by which Copaifera oleoresins affect T. gondii proliferation directly. We tested whether oleoresin treatments blocked or altered parasite cell cycle progression by the quantification of DNA content using flow cytometry techniques. We observed that all four oleoresin treatments at all concentrations caused a significant decrease in the number of parasites in G1 and accumulation in the S/M phase of the parasite cell cycle. Similarly, SDZ + PYR treatment also promoted a reduction in the G1 phase and a strong arrest in S/M phase. These data are in agreement with Lavine and Arrizabalaga (2011)68, who demonstrated that the treatment with a low dose of pyrimethamine altered the cell cycle of T. gondii by decreasing the number of parasites in G1 phase and promoting a loss of late S/M phase peak, potentially as a result of degradation of DNA. In contrast with the literature, our results suggest disruption of the parasite’s cell cycle (G1 reduction and arrest in S/M phase) in the SDZ + PYR group; which can be explained by the fact that we used the combination of PYR and SDZ and a concentration of PYR about four-fold higher than used by the authors mentioned above. Our data and literature reports suggest that a possible mechanism of action for oleoresins is to arrest parasites in the S/M phase, as has been shown with other compounds that inhibit T. gondii growth68,69,70.
Lavine and Arrizabalaga (2011)68 showed that monensin, a polyether ionophore antibiotic that inhibits T. gondii replication, increases the number of parasites in the S/M phase of the cell cycle, maybe as a consequence of DNA damage or cellular stress. Furthermore, another study suggested that the cell cycle arrest in S/M caused by monensin in T. gondii would serve as a signal to initiate an autophagy-like cell death70,71. Our results suggest that oleoresin treatments blocked progression trough the parasite cell cycle by promoting arrest in S/M phase. To corroborate this evidence, we showed that the Copaifera oleoresin treatments were able to promote apparent ultra-structural parasite modifications. In general, we observed a possible compromising of endodyogeny, which was evidenced by the parasites bound by their basal ends. Moreover, oleoresin treatments promoted the formation of the multiple intracellular vacuole-like structures, as well as the presence of a single membrane in some areas, raising the possibility that the activity of these oleoresins can be related to some kind of DNA damage or replicative stress in the parasite. This hypothesis is corroborated by the results showing irreversible oleoresin inhibition of parasite proliferation. Besides, parasites from oleoresins-treated BeWo cells also had an impairment in their ability to infect new host cells, thus highlighting that the oleoresin treatment is probably causing some damage to parasites, which could be compromising the parasite physiology.
After evaluating the effects of Copaifera oleoresins against T. gondii infection in BeWo cells, we also studied the action of these same oleoresins using human villous explants from the third trimester of pregnancy. The concentration used for all oleoresins (128 µg/mL) was selected based on our results of LDH and MTT assays showing no significant toxicity to villous explants. As expected, we saw no morphological alterations of the villous exposed to oleoresins, at the set concentration. In order to corroborate with our in vitro findings, we demonstrated that the treatment with all four oleoresins (128 µg/mL) was able to inhibit T. gondii intracellular proliferation in villous explants. Interestingly, C. pubiflora oleoresin appeared to be more efficient in the inhibition of parasite growth than classical drugs (SDZ + PYR). As expected, SDZ + PYR treatment did not show cytotoxicity in villous explants and inhibited the intracellular proliferation of the parasites33.
Even though the mechanism of action of oleoresins appears to target the parasites, an additional and non-exclusive hypothesis to explain the impact of oleoresins against T. gondii could involve the modulation of the host cell environment. In order to support this hypothesis, we demonstrated that the oleoresins were capable of altering the cytokine production by both BeWo cells and villous explants infected or not by T. gondii. In general, we observed that the oleoresin treatments promoted a strong up-regulation of IL-6, regardless of the presence or absence of parasites in BeWo cells. Moreover, we showed that oleoresins (C. duckei and C. pubiflora) increase the MIF levels by uninfected cells. In agreement with the literature, T. gondii infection increased MIF production33. However, the treatment with oleoresins from C. duckei (32 µg/mL), C. paupera (64 and 32 µg/mL), and C. pubiflora (32 µg/mL) reduced MIF production by parasite-infected BeWo cells. Additionally, oleoresins treatments in specific concentrations down-modulated ROS production by uninfected and infected BeWo cells. Kian and colleagues corroborate these findings (2018)53 demonstrating that the treatment with Copaifera martii oleoresin in highest concentration reduced ROS production in T. cruzi-infected macrophages.
We reported that both uninfected and infected villous treated with all four oleoresins exhibited no significant alteration in the IL-6 and MIF production. However, TNF-α production was not detected in supernatants from the uninfected group, but the treatment with all four oleoresins was able to reduce the TNF-α release, primarily caused by T. gondii infection. In the context of the maternal–fetal interface, previous independent studies have reported that IL-6 and MIF are cytokines essentials to impair T. gondii infection. It was demonstrated by our research group that IL-6 is a multifaceted cytokine involved in the control of T. gondii infection in human monocytes and human trophoblastic cells72,73. Besides, this cytokine was also associated with the parasite control in infected-villous explants30,31,33. Several studies have demonstrated that human trophoblastic cells and human villous explants produce MIF and, this cytokine release has an important role during infection by different parasites, including T. gondii. Besides that, this cytokine has a critical function to reduce T. gondii proliferation in maternal–fetal interface29,31,33,74,75,76,77.
Thus, based on the literature and our data, we proposed that the modulation of cytokine production in BeWo cells during T. gondii infection by oleoresins is dependent on both the Copaifera species and the concentration of oleoresin used. Even though the IL-6 production appears to be a key cytokine related to parasite growth, our data suggest an alternative mechanism for the oleoresins to alter the host cell environment and therefore impair parasite growth. In this study, we demonstrated no significant differences in the production of IL-6 and MIF by uninfected and infected villous explants treated with oleoresins, but the oleoresin treatments significantly decreased TNF-α production in villous explants infected by T. gondii.
Taken together and despite the increase in IL-6 and MIF production by BeWo cells during parasite infection and under some oleoresin treatment conditions, in general, we observed that the oleoresin induced an anti-inflammatory profile to control parasite infection. This observation is in agreement with the literature, in which several publications have demonstrated that Copaifera oleoresins, including C. cearensis, C. duckei, C. langsdorffii, C. multijuga, C. officinalis, and C. reticulata have shown anti-inflammatory and antiparasitic properties67. In this line of discussion, we demonstrated that the treatment with both azithromycin and spiramycin (macrolide antibiotics) could control T. gondii infection in BeWo cells by inducing anti-inflammatory response with increased of MIF, IL-4, and IL-10 levels29,78. Acute T. gondii infection is marked by a high production of pro-inflammatory abortogenic cytokines, such as IFN-γ and TNF-α, which are deleterious for conception79. Thus, oleoresin treatment induces an anti-inflammatory response, which may be essential for the maintenance of gestation as well as to control the parasite infection.
In conclusion, the present study demonstrated that the oleoresins from C. reticulata, C. duckei, C. paupera, and C. pubiflora could control T. gondii infection as shown through both in vitro and ex vivo experimental models. We concluded that the anti-T. gondii effects triggered by these oleoresins are probably related to the immunomodulation of the host cells as well as the direct effect on parasites. Thus, our findings provide evidence of the potential for oleoresins to be an alternative source for the treatment for congenital toxoplasmosis by reducing the proliferation rate of T. gondii with low cytotoxicity effects to the host cells. Additional mechanisms by which the Copaifera oleoresins affect T. gondii need to be thoroughly evaluated in further studies.
Methods
Cell culture and parasite maintenance
Human trophoblast cells (BeWo lineage) were commercially purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained as previously described32. RPMI 1,640 medium (Cultilab, Campinas, SP, Brazil) supplemented with 100 U/mL penicillin (Sigma Chemical Co., St. Louis, MO, USA), 100 μg/mL streptomycin (Sigma) and 10% fetal bovine serum (FBS) (Cultilab) was used for cell culture maintenance. Cultures were kept at 37 °C under a humidified atmosphere containing CO2 (5%). Toxoplasma gondii tachyzoites (virulent RH strain, 2F1 clone) constitutively expressing the β-galactosidase gene were cultured as described elsewhere73,80. Briefly, tachyzoites were maintained by serial passages in BeWo cells cultured in RPMI 1,640 medium containing 2% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2.
Copaifera spp. oleoresins collection
The oleoresins used in the present study were collected between 2011 and 2014 in Northern region of Brazil, as following: Copaifera reticulata Ducke (Brasil Novo, Pará State, 03° 22.028′ S, 52° 29.947′ W), Copaifera duckei Dwyer (Belém, Pará State, 01° 06.933′ S, 48° 19.781′ W), Copaifera paupera Herzog (Xapuri, Acre State, 10° 27.861′ S, 68° 35.815′ W), and Copaifera publifora Benth (Mucajaí, Roraima State, 02° 36.205′ N, 60° 56.767′ W). The botanical identification was carried out by Silvane Tavares Rodrigues at the IAN Herbarium (EMBRAPA Amazônia Oriental), from who a taxonomic certification is available upon request, and the voucher specimens were deposited under the registry numbers 175266, 175206, 175983, and 180231, respectively for C. reticulata, C. duckei, C. paupera, and C. pubiflora. Brazilian Council for Authorization and Information on Biodiversity (SIBIO/ICMBio/MMA/BRASIL) and Genetic Heritage Management (CGEN/MMA/BRASIL) provided the legal permits for research activities with these plant materials in this paper. Permit numbers 35143-1 and 010225/2014-5, respectively.
Host cell viability
We evaluated the host cell viability in the presence of the oleoresins to establish the non-toxic concentration of each oleoresin. The viability of BeWo cells treated with different concentrations of oleoresins was assessed by MTT colorimetric assay81. In summary, we tested four oleoresins from different species from Copaifera genus as follows: the oleoresins of Copaifera reticulata, Copaifera duckei, Copaifera paupera, and Copaifera pubiflora were solubilized in dimethyl sulfoxide (DMSO) and diluted in supplemented RPMI 1,640 medium to form a stock solution of 640 µg/mL51,82. BeWo cells were seeded at 3.0 × 104 cells/well in 96-well microplates. After adhesion, the culture monolayer was carefully rinsed, and solutions of oleoresins ranging from 256 to 4 µg/mL (twofold serial dilutions) were added to the microplate in the total volume of 200 μL/well. To assess whether DMSO toxicity in BeWo cells, we treated the cells with DMSO 1.2% in RPMI 1,640 medium in the total volume of 200 μL/well, which is the percentage of DMSO used in the highest oleoresin concentration (256 µg/mL). Culture medium alone was used as a positive control of viability, and cells considered to be 100% viable. We referred to published data to establish the work concentration of sulfadiazine (Sigma) and pyrimethamine (Sigma) (200 + 8 μg/mL, respectively), the chosen concentrations have been reported as non-toxic for BeWo cells33. Microplates were incubated for 24 h at 37 °C under a humidified atmosphere and 5% CO2. Next, we remove the supernatants and incubate the cells with MTT reagent (5 mg/mL, 10 µL) plus 90 µL of supplemented RPMI 1,640 medium for four hours at 37 °C and 5% CO2, followed by addition of 10% sodium dodecyl sulfate (SDS, Sigma) and 50% N,N-dimethyl formamide (Sigma) and further incubation for 30 min. MTT reduction was measured at 570 nm absorbance with a multi-well scanning spectrophotometer (Titertek Multiskan Plus, Flow Laboratories, McLean, VA, USA). We reported cell viability in percentages (cell viability %), with the absorbance of cells incubated only with culture medium considered to be 100% viable.
T. gondii intracellular proliferation assay: β-galactosidase activity-based screen
We screened different oleoresin concentrations of C. reticulata, C. duckei, C. paupera, and C. pubiflora for their effect on growth modulation of a highly virulent strain (RH strain, 2F1 clone) of T. gondii using BeWo cells as host cell and a β-galactosidase colorimetric assay as previously described72,73,77. Briefly, BeWo cells were plated at 3.0 × 104 cells/well in 96-well microplates. After adhesion, cells were infected with T. gondii tachyzoites at a multiplicity of infection (MOI) of 3:1 (ratio of parasites per cell) in supplemented RPMI 1,640 medium. After three hours, the medium was discarded, and the cells rinsed with culture medium in order to remove the excess of extracellular parasites. Next, we incubated the cells for 24 h at 37 °C and 5% CO2 with non-toxic oleoresin concentrations in twofold serial dilutions as follows: C. reticulata and C. paupera (128 to 4 µg/mL), C. duckei and C. pubiflora (64 to 4 µg/mL). According to the literature, sulfadiazine, and pyrimethamine (SDZ + PYR) are considered classic drugs in the treatment of congenital toxoplasmosis. In this sense, the present work used this classic drug combination as gold-standard to evaluate the effect of the oleoresins33. Infected BeWo cells were incubated with RPMI 1,640 medium in the absence of any drug and used as negative treatment control. After 24 h of treatment, culture supernatants were collected and stored at − 80 °C for further measurement of cytokines. Next, T. gondii intracellular proliferation was analyzed using a colorimetric β-galactosidase assay. We quantified T. gondii intracellular proliferation in percentage (% of T. gondii proliferation) and calculated the number of tachyzoites in comparison to a standard curve produced with free tachyzoites (ranging from 1 × 106 to 15.625 × 103 parasites). Infected BeWo cells treated with only culture medium (negative control) represented non-inhibited parasite growth. We measured the efficiency of each treatment condition by comparison with the negative control (100% parasite proliferation).
Adhesion assay of oleoresins-pretreated T. gondii
To investigate whether the effects of oleoresins occurs directly on the parasite, we performed an adhesion assay83,84 with minor modifications. BeWo cells were seeded at a density of 1.0 × 105 cells in 24-well microplates containing 13 mm coverslips on each well. After adhesion, cells were fixed with paraformaldehyde (4%) for 30 min at room temperature and washed three times with 1 × phosphate-buffered saline (PBS). Next, T. gondii tachyzoites at an MOI of 3:1 were pre-incubated with oleoresins (64 and 32 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora; SDZ + PYR (200 + 8 μg/mL) or culture medium only (untreated group) for one hour at 37 °C and 5% CO2. We used the oleoresin concentrations stipulated on our preliminary results from the β-galactosidase assay. The pretreated parasites were rinsed and resuspended in the medium before incubation with cells for three hours at 37 °C and 5% CO2. After removing the excess of T. gondii that did not adhere to cells with repeated rinsing with 1 × PBS, parasites were fixed in the same conditions as mentioned above. The coverslips were then incubated overnight with a mouse monoclonal primary anti-Toxoplasma gondii antibody [SAG1/p30] (Abcam TP3 #ab8313) (diluted 1: 500 in PGN-0.01% saponin solution). Finally, coverslips were rinsed three times with 1 × PBS, and incubated with Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen, USA #A11001) (diluted 1:500 in PGN-0.01% saponin solution), tetramethylrhodamine isothiocyanate (TRITC)-conjugated phalloidin (Sigma, P1951) (diluted 1:50 in PGN + saponin) and 4,6′-diamidino-2-phenylindole dilactate (DAPI, Invitrogen, USA) (diluted 1:500 in PGN + saponin) for one h in the dark at room temperature to label tachyzoites of T. gondii, F-actin and nuclei, respectively. We mounted the Coverslips onto glass slides, and samples were analyzed using confocal fluorescence microscopy (Zeiss, LSM 510 Meta, Germany) with an inverted microscope (Zeiss Axiovert 200 M). We analyzed the following parameters: the number of BeWo cells with adhered parasites and the total number of attached parasites per cell in a total of 20 fields chosen randomly.
Invasion and intracellular proliferation of oleoresins-pretreated T. gondii
After verifying the adhesion of pretreated parasites in BeWo cells, we also investigated the invasion and intracellular proliferation of oleoresins-pretreated parasites. The experimental procedures to assess the possible direct effect of oleoresins on T. gondii tachyzoites were performed according to Barbosa et al. (2012)32 and Adeyemi et al. (2017)36, with minor modifications. In the first set of experiments, BeWo cells were seeded at 3.0 × 104 cells/well in 96-well microplates for 24 h in supplemented RPMI 1,640 medium. Next, cell culture-derived tachyzoites of T. gondii at a ratio of 3 parasites per cell (3:1) were pre-incubated for one hour at 37 °C and 5% CO2 with two oleoresin concentrations (64 and 32 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora. At the same conditions, parasites were also pre-incubated with SDZ + PYR (200 + 8 μg/mL) or with only culture medium (untreated group). Afterward, purified parasite suspension in a treatment-free medium was placed in 96-well tissue culture plates containing previously adhered BeWo cells, and invasion was allowed to occur for three hours. Following this basic design setup, we tested two experimental conditions; (1) for the invasion analysis, after 3 h of incubation, the monolayers were thoroughly rinsed in order to remove the extracellular parasites; (2) for intracellular proliferation analysis, after 3 h of incubation, the monolayers were rinsed, and a fresh supplemented culture medium was added for an additional 24 h at 37 °C and 5% CO2. In both experiments, intracellular T. gondii was quantified using the β-galactosidase assay.
Reversibility assay
In order to evaluate the maintenance of the antiparasitic effects of oleoresins on T. gondii proliferation, we performed the reversibility assay as previously described36,85, with minor modifications. Briefly, BeWo cells were seeded at 3.0 × 104 cells/well in 96-well microplates. After adhesion, cells were infected with T. gondii tachyzoites at an MOI of 3:1 (parasites: cell) in supplemented RPMI 1,640 medium. After three hours of invasion, we rinsed the cells to remove the unattached parasites and used this basic experimental setup to test two conditions, as follows; (1) the invading parasites (after three h of invasion) were allowed to grow in the presence of oleoresins (64 and 32 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora, SDZ + PYR (200 + 8 μg/mL) or culture medium only (untreated group) for 24 h at 37 °C and 5% CO2. In the second condition tested, (2) the invading parasites were grown at the same conditions of treatments (as mentioned above on item 1), however after 24 h of treatment, the cells were rinsed, the media replaced by complete RPMI 1,640, and the parasites allowed to growth for an additional 24 h. In both situations, we quantified T. gondii intracellular proliferation using the β-galactosidase assay, as mentioned above. Finally, we measure the reversibility rate in percentage (reversibility of treatment %) in 24 h after treatment removal by comparison with both the untreated group (considered as 100% of reversibility) and the corresponding treatment condition in 24 h of treatment (baseline for comparison).
To corroborate the reversibility data, we investigated whether the oleoresin treatment of infected BeWo cells with T. gondii tachyzoites would interfere in the ability of these parasites to invade and replicate inside new fresh cells. In summary, BeWo cells were seeded at 1.0 × 106 cells/well in 6-well microplates. After adhesion, cells were infected with T. gondii tachyzoites at an MOI of 3:1 (parasites: cell) in supplemented RPMI 1,640 medium for three h at 37 °C and 5% CO2. Next, we treated the infected cells with oleoresins (64 and 32 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora; SDZ + PYR (200 + 8 μg/mL) or culture medium only (untreated group) for 24 h at 37 °C and 5% CO2. After, we isolated the intracellular parasites from infected cells by multiple passages through a 21 and 26-gauge needle. Finally, T. gondii tachyzoites from each experimental condition were then allowed to reinfect monolayers of BeWo cells previously seeded in 96-well microplates (3.0 × 104 cells/well). We used the β-galactosidase assay to quantify the tachyzoites after 24 h incubation.
Parasite cell cycle analysis
The parasite cell cycle was analyzed by flow cytometry as previously described69,70,86, with some modifications. Briefly, BeWo cells were seeded at 1.0 × 106 cells/well in 6-well microplates. After adhesion, cells were infected with T. gondii tachyzoites at an MOI of 3:1 (parasites: cell) in supplemented RPMI 1,640 medium for three h at 37 °C and 5% CO2. Next, we treated the cells with oleoresins (64 and 32 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora; SDZ + PYR (200 + 8 μg/mL) or culture medium only (untreated group) for 24 h at 37 °C and 5% CO2. After, we isolated the intracellular parasites from infected cells by multiple passages through a 21 and 26-gauge needle. T. gondii tachyzoites from each experimental condition were rinsed with PBS and then fixed overnight with ethanol (70%) at 4 °C. Next, fixed parasites were rinsed and incubated overnight with a mouse monoclonal primary anti-Toxoplasma gondii antibody [SAG1/p30] (Abcam TP3 #ab8313) (diluted in 1 × PBS). Finally, parasites were rinsed with 1 × PBS, incubated with Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen, USA #A11001), 100 mg/mL of RNase A and 10 µg/mL of PI, all reagents diluted in 1 × PBS. We labeled the parasites in the dark for 45 min at room temperature. Quantification of the DNA content for parasite cycle analysis was performed using the Cytoflex flow cytometer (Beckman Coulter, United States), and the data collected by using FlowJo software (version 7.6.3).
Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM) studies were carried out as previously described33,87, with the following minor changes. Briefly, BeWo cells were seeded at 1.0 × 106 cells/well in 6-well microplates. After adhesion, cells were infected with T. gondii tachyzoites at an MOI of 3:1 (parasites: cell) in supplemented RPMI 1,640 medium. After three h of invasion, cells were rinsed and incubated for 24 h at 37 °C and 5% CO2 with oleoresins (64 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora; SDZ + PYR (200 + 8 μg/mL) or culture medium only (untreated group). Next, we harvested the cells and fixed them in Karnovsky solution containing 2% paraformaldehyde and glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7.4) for 24 h. Samples were incubated for one h in 1% osmium tetroxide (OsO4) in cacodylate solution and treated with potassium ferrocyanide for an additional 30 min at room temperature., followed by dehydration in increasing concentrations of ethanol and propylene oxide, and inclusion in Epon resin. Ultrathin sections were stained with uranyl acetate and lead citrate and then analyzed using a transmission electron microscope (Hitachi, TM 3000).
Measurement of intracellular reactive oxygen species (ROS) by BeWo cells
The influence of oleoresins from different species of Copaifera in ROS production by BeWo cells was based on the intracellular peroxide-dependent oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Invitrogen|catalog number: D399) to form the fluorescent compound 2′,7′-dichlorofluorescein (DCF), as previously described53,88, with some modifications. Briefly, BeWo cells were seeded at 0.7 × 105 cells/well in 24-well microplates. Then, cells were infected or not with T. gondii tachyzoites in a ratio of 3:1 (parasites: cell) for 3 h in a supplemented RPMI 1,640 medium at 37 °C and 5% CO2. Subsequently, cells were rinsed thoroughly with culture medium and treated with oleoresins (64 and 32 µg/mL) from C. reticulata, C. duckei, C. paupera, and C. pubiflora; SDZ + PYR (200 + 8 μg/mL) or culture medium only (untreated group) for 24 h at 37 °C and 5% CO2. Hydrogen peroxide (H2O2) was used as a positive control of ROS production. After treatment, cells were harvested, rinsed with 1 × PBS, and incubated with 150 μL of H2DCF-DA (10 μM; diluted in 1 × PBS containing 10% FBS) for 45 min at 37 °C and 5% CO2 under darkness. Finally, DCF fluorescence intensity was detected immediately using a Cytoflex flow cytometer (Beckman Coulter, United States) and FlowJo software (version 7.6.3). Data are presented as median fluorescence intensity (MFI).
Human chorionic villous explant culture
In order to extrapolate our in vitro findings obtained in human trophoblast cells, we assessed the effects of oleoresins from different species of Copaifera during T. gondii infection using an ex vivo model. We used human villous explants from the placentas in the third trimester, as this model is a good representation of the maternal–fetal interface30,76. Firstly, third-trimester human placentas (36 to 40 weeks of pregnancy) were collected after elective cesarean section deliveries at the Clinics Hospital of the Federal University of Uberlândia (HC-UFU), MG, Brazil. Placental tissues were collected following exclusion criteria, as follows: pre-eclampsia, chronic hypertension, infectious disease including toxoplasmosis, chorioamnionitis, chronic renal disease, cardiac disease, connective tissue disease, pre-existing diabetes mellitus, gestational diabetes mellitus, and other pathological manifestations. In brief, placental tissues were washed in ice-cold sterile PBS (pH = 7.2) in order to remove excess blood, and then aseptically dissected to remove endometrial tissue and fetal membranes up to 1 h after collection. Terminal chorionic villi containing five to seven free tips per explant were collected as described previously89 and added to 96-well microplates (one villus per well) in 200 µL/well of a fresh RPMI 1,640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Sigma) and 10% FBS for 24 h at 37 °C under a humidified atmosphere containing CO2 (5%) until the experimental procedure.
Viability of human chorionic villous explant treated with oleoresins
Oleoresin toxicity in human villous explants was performed using viability assays with both a lactate dehydrogenase (LDH) and MTT assays, according to published protocols30,81,90. In both assays, villous explants were collected and cultured in a supplemented RMPI 1,640 medium for 24 h at 37 °C and 5% CO2, as mentioned above. After 24 h, villi were treated oleoresin of C. reticulata, C. duckei, C. paupera, and C. pubiflora at 256, 128, and 64 µg/mL or SDZ + PYR (150 and 200 µg/mL, respectively). These drug concentrations were chosen based on the previous study by our research group, showing that the drugs at the mentioned concentration have no significant toxicity in human villous explants33. As vehicle control, we treated the villi with DMSO (1.2%) in RPMI 1,640 medium, which is the percentage of DMSO used in the highest oleoresin concentration (256 µg/mL). As control of viability, villous explants were treated with culture medium alone. Following 24 h of incubation, the culture supernatants were collected, and LDH concentration measured according to the manufacturer’s instructions30 with minor modifications (LDH Liquiform, Labtest Diagnostica S.A., Lagoa Santa, MG, Brazil|Ref 86-2/30, Lot 7010). This assay is based on the consumption and decrease of absorption of NADH at 340 nm, as measured by a microplate reader (Versa Max ELISA Microplate Reader, Molecular Devices, Sunnyvale, CA, USA). LDH released into the culture media was expressed in U/L of LDH enzymatic activity and was used as a marker for tissue integrity.
In parallel, at the same experimental condition, as described above, tissue viability was also assessed by the MTT assay. After 24 h of treatment, culture supernatants were discarded and replaced by 200 µL MTT solution [180 µL culture medium plus 20 µL MTT (5 mg/mL)] for 4 h at 37 °C and 5% CO2. Subsequently, formazan crystal resulting from MTT reduction was solubilized by the addition of 100 µL PBS containing sodium dodecyl sulfate (SDS, 10%) and HCl (0.01 M, 18 h, 37 °C, 5% CO2). Finally, villi were removed from each well, and the absorbance (570 nm) was measured with a multi-well scanning spectrophotometer (Titertek Multiskan Plus, Flow Laboratories, McLean, VA, USA). Tissue viability was expressed in percentages (viability % by MTT incorporation), with the absorbance of villi incubated with culture medium alone (untreated group) considered to be 100% viable. Furthermore, we performed morphological analysis of treated villi to corroborate the viability assays. Villi tissue sections were stained with hematoxylin/eosin and examined using a light microscope (BX40 Olympus, Tokyo, Japan)30.
T. gondii infection of human chorionic villous explants and oleoresin treatments
We quantified T. gondii intracellular proliferation in human villous explants treated with oleoresins using a colorimetric β-galactosidase assay30,76, with minor modifications. In brief, villi were collected and cultured in 96-well microplates (one villus per well/200 µL) in a supplemented culture medium for 24 h at 37 °C and 5% CO2. Next, the villi were infected with T. gondii tachyzoites of RH strain-2F1 (1 × 106 parasites per well/200 µL) and incubated for 24 h at 37 °C and 5% CO2. Afterward, villous explants were rinsed thoroughly with culture medium in order to remove non-adhered parasites. Following our data from tissue viability (LDH and MTT assays), the villi were treated for an additional 24 h with oleoresins (128 µg/mL) from C. reticulata, C. duckei, C. paupera and C. pubiflora, and SDZ + PYR (150 + 200 μg/mL). At the same experimental conditions, uninfected and untreated villous explants or infected and untreated villous explants were cultured with culture medium alone. Finally, culture supernatants were collected and stored at − 80 °C for cytokines measurement. In addition, villous explants were also collected and stored at − 80 °C for the following analyzes: protein content determination using Bradford reagent and T. gondii intracellular proliferation by β-galactosidase assay.
Initially, frozen villous explants were homogenized by addition of 150 µL of radioimmunoprecipitation assay buffer (RIPA) [50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% (w/v) sodium deoxycholate, and 0.1% (w/v) SDS, pH 7.5] containing protease inhibitor cocktail (Complete, Roche Diagnostic, Mannheim, Germany). The homogenate was centrifuged at 21,000× g for 15 min at 4 °C, and the supernatant was collected to measure the total amount of protein using the Bradford method91. Determination of T. gondii intracellular proliferation in villous explants was carried out with 20 µL of supernatants from each sample. The supernatants were incubated with 160 μL of assay buffer (100 mM 1 × PBS, pH 7.3, 102 mM β-mercaptoethanol and 9 mM MgCl2) and 40 μL of 6.25 mM CPRG (chlorophenol red-β-D-galactopyranoside; Roche, Indianapolis, IN) for approximately 30 min. We measured the absorbance at 570 nm using a kinetic plate reader (Titertek Multiskan Plus, Flow Laboratories, McLean, VA, USA). The number of T. gondii tachyzoites were normalized according to the total protein concentration (µg/mL) of each villous obtained by Bradford assay, and expressed by the number of parasites per µg of tissue. Toxoplasma gondii intracellular proliferation in villous explants was expressed in percentage (% of T. gondii proliferation), and the number of tachyzoites calculated in comparison to a standard curve of free tachyzoites (ranging from 1 × 106 to 15.625 × 103 parasites). As controls we quantified the number of tachyzoites from untreated and infected villous incubated with culture medium alone (negative control), this condition was considered as 100% of parasite proliferation, and the number of parasites from each treatment condition was expressed in percentage of T. gondii proliferation in comparison with the negative control.
Cytokine determination
We measured the release of cytokines in supernatants of BeWo cells and villous explants cultures using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA). Assays for IL-6, TNF-α, IL-10, TGF-β1 (OpTEIA, BD Bioscience, San Diego, CA, USA) and MIF (Duoset R&D Systems, Minneapolis, MN, USA) were performed according to the manufacturer’s instructions. Cytokine concentrations for experiments with BeWo cells were expressed in pg/mL. In contrast, we normalized the concentration fo cytokines in the villous experiments using a ratio between cytokine production (pg/mL) and its corresponding total protein content (mg/mL) of each villous, as obtained by the Bradford method for protein quantification and results are shown as pg/mg of tissue. The limits of detection of each cytokine were determined from standard curves: IL-6 (4.7 pg/mL), TNF-α (7.8 pg/mL), MIF (7.8 pg/mL), IL-10 (7.8 pg/mL) and TGF-β1 (125 pg/mL).
Statistical analysis
All data were expressed as means ± standard deviations (SD). Data were checked first for normal distribution. Significance differences were compared to controls by using One-way ANOVA, Tukey’s, or Sidak’s multiple comparisons post-test for the parametric data. Nonparametric data were analyzed by the Kruskal–Wallis test and Dunn’s multiple comparison post-test. Data were considered statistically significant at P < 0.05, according to the experimental design (GraphPad Prism Software version 6.01).
Ethical approval
The present research protocol using human tissue samples was performed in accordance with relevant guidelines and regulations, and the experimental protocols were approved by the Ethics Committee of the Federal University of Uberlandia, MG, Brazil, with Approval Number 1.585.342. A consent term was obtained from all subjects, and when the subjects were under 18, a parent and/or legal guardian was acquired. In addition, informed consent was obtained from all participants and/or their legal guardians.
References
Beck, H. P. et al. Molecular approaches to diversity of populations of apicomplexan parasites. Int. J. Parasitol. 39, 175–189. https://doi.org/10.1016/j.ijpara.2008.10.001 (2009).
Pappas, G., Roussos, N. & Falagas, M. E. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 39, 1385–1394. https://doi.org/10.1016/j.ijpara.2009.04.003 (2009).
Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet (London, England) 363, 1965–1976. https://doi.org/10.1016/s0140-6736(04)16412-x (2004).
Dubey, J. P. & Jones, J. L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 38, 1257–1278. https://doi.org/10.1016/j.ijpara.2008.03.007 (2008).
Dubey, J. P., Lago, E. G., Gennari, S. M., Su, C. & Jones, J. L. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology 139, 1375–1424. https://doi.org/10.1017/s0031182012000765 (2012).
Minbaeva, G. et al. Toxoplasma gondii infection in Kyrgyzstan: seroprevalence, risk factor analysis, and estimate of congenital and AIDS-related toxoplasmosis. PLoS Negl. Trop. Dis. 7, e2043. https://doi.org/10.1371/journal.pntd.0002043 (2013).
Bigna, J. J. & Tochie, J. N. Global, regional and national estimates of Toxoplasma gondii seroprevalence in pregnant women: a protocol for a systematic review and modelling analysis. BMJ Open 9, e030472. https://doi.org/10.1136/bmjopen-2019-030472 (2019).
Peyron, F. et al. Congenital Toxoplasmosis in France and the United States: one parasite, two diverging approaches. PLoS Negl. Trop. Dis. 11, e0005222. https://doi.org/10.1371/journal.pntd.0005222 (2017).
Aguirre, A. A. et al. The one health approach to Toxoplasmosis: epidemiology, control, and prevention strategies. EcoHealth 16, 378–390. https://doi.org/10.1007/s10393-019-01405-7 (2019).
Dunn, D. et al. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet (London, England) 353, 1829–1833. https://doi.org/10.1016/s0140-6736(98)08220-8 (1999).
Robert-Gangneux, F. & Darde, M. L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296. https://doi.org/10.1128/cmr.05013-11 (2012).
Fallahi, S., Rostami, A., Nourollahpour Shiadeh, M., Behniafar, H. & Paktinat, S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J. Gynecol. Obst. Hum. Reprod. 47, 133–140. https://doi.org/10.1016/j.jogoh.2017.12.003 (2018).
Carlier, Y., Truyens, C., Deloron, P. & Peyron, F. Congenital parasitic infections: a review. Acta Trop. 121, 55–70. https://doi.org/10.1016/j.actatropica.2011.10.018 (2012).
Wyrosdick, H. M. & Schaefer, J. J. Toxoplasma gondii: history and diagnostic test development. Anim. Health Res. reviews 16, 150–162. https://doi.org/10.1017/s1466252315000183 (2015).
Robert-Gangneux, F. et al. The placenta: a main role in congenital toxoplasmosis?. Trends Parasitol. 27, 530–536. https://doi.org/10.1016/j.pt.2011.09.005 (2011).
Cortina-Borja, M. et al. Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. PLoS Med. https://doi.org/10.1371/journal.pmed.1000351 (2010).
Campello Porto, L. & Duarte, E. C. Association between the risk of congenital toxoplasmosis and the classification of toxoplasmosis in pregnant women and prenatal treatment in Brazil, 1994–2009. Int. J. Infect. Dis. IJID Off Publ. Int. Soc. Infect. Dis. 16, e480–e486. https://doi.org/10.1016/j.ijid.2012.01.016 (2012).
Wei, H. X., Wei, S. S., Lindsay, D. S. & Peng, H. J. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS ONE 10, e0138204. https://doi.org/10.1371/journal.pone.0138204 (2015).
Elsheikha, H. M. Congenital toxoplasmosis: priorities for further health promotion action. Public health 122, 335–353. https://doi.org/10.1016/j.puhe.2007.08.009 (2008).
Tamaru, S. et al. Fetal therapy of severe symptomatic toxoplasmosis using azithromycin. J. Obst. Gynaecol. Res. 37, 953–957. https://doi.org/10.1111/j.1447-0756.2010.01459.x (2011).
Montoya, J. G. & Remington, J. S. Management of Toxoplasma gondii infection during pregnancy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 47, 554–566. https://doi.org/10.1086/590149 (2008).
Anderson, A. C. Targeting DHFR in parasitic protozoa. Drug Discov. Today 10, 121–128. https://doi.org/10.1016/s1359-6446(04)03308-2 (2005).
Villena, I. et al. Pyrimethamine-sulfadoxine treatment of congenital toxoplasmosis: follow-up of 78 cases between 1980 and 1997. Reims Toxoplasmosis Group. Scand. J. Infect. Dis. 30, 295–300. https://doi.org/10.1080/00365549850160963 (1998).
Doliwa, C. et al. Sulfadiazine resistance in Toxoplasma gondii: no involvement of overexpression or polymorphisms in genes of therapeutic targets and ABC transporters. Parasite (Paris, France) 20, 19. https://doi.org/10.1051/parasite/2013020 (2013).
Vijayalaxmi, K. K. & Vishalakshi, M. Evaluation of the genotoxic effects of pyrimethamine, an antimalarial drug, in the in vivo mouse. Teratog. Carcinog. Mutagen. 20, 65–71. https://doi.org/10.1002/(sici)1520-6866(2000)20:2%3c65::aid-tcm2%3e3.0.co;2-k (2000).
Hernandez-Diaz, S., Werler, M. M., Walker, A. M. & Mitchell, A. A. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am. J. Epidemiol. 153, 961–968. https://doi.org/10.1093/aje/153.10.961 (2001).
Oz, H. S. Toxoplasmosis complications and novel therapeutic synergism combination of diclazuril plus atovaquone. Front. Microbiol. 5, 484. https://doi.org/10.3389/fmicb.2014.00484 (2014).
Costa, I. N. et al. Azithromycin inhibits vertical transmission of Toxoplasma gondii in Calomys callosus (Rodentia: Cricetidae). Placenta 30, 884–890. https://doi.org/10.1016/j.placenta.2009.08.002 (2009).
Franco, P. S. et al. Azithromycin and spiramycin induce anti-inflammatory response in human trophoblastic (BeWo) cells infected by Toxoplasma gondii but are able to control infection. Placenta 32, 838–844. https://doi.org/10.1016/j.placenta.2011.08.012 (2011).
Castro-Filice, L. S. et al. Azithromycin is able to control Toxoplasma gondii infection in human villous explants. J. Transl. Med. 12, 132. https://doi.org/10.1186/1479-5876-12-132 (2014).
Franco, P. S. et al. Brazilian strains of Toxoplasma gondii are controlled by azithromycin and modulate cytokine production in human placental explants. J. Biomed. Sci. 26, 10. https://doi.org/10.1186/s12929-019-0503-3 (2019).
Barbosa, B. F. et al. Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Vet. Parasitol. 187, 44–52. https://doi.org/10.1016/j.vetpar.2011.12.039 (2012).
da Silva, R. J. et al. Enrofloxacin and toltrazuril are able to reduce Toxoplasma gondii growth in human BeWo trophoblastic cells and villous explants from human third trimester pregnancy. Front. Cell. Infect. Microbiol. 7, 340. https://doi.org/10.3389/fcimb.2017.00340 (2017).
Black, M. W. & Boothroyd, J. C. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. MMBR 64, 607–623. https://doi.org/10.1128/mmbr.64.3.607-623.2000 (2000).
Boothroyd, J. C. & Dubremetz, J. F. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat. Rev. Microbiol. 6, 79–88. https://doi.org/10.1038/nrmicro1800 (2008).
Adeyemi, O. S., Murata, Y., Sugi, T. & Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 12, 1647–1661. https://doi.org/10.2147/ijn.s122178 (2017).
Benoit-Vical, F., Santillana-Hayat, M., Kone-Bamba, D., Mallie, M. & Derouin, F. Anti-toxoplasma activity of vegetal extracts used in West African traditional medicine. Parasite (Paris, France) 7, 3–7. https://doi.org/10.1051/parasite/2000071003 (2000).
Youn, H. J., Lakritz, J., Kim, D. Y., Rottinghaus, G. E. & Marsh, A. E. Anti-protozoal efficacy of medicinal herb extracts against Toxoplasma gondii and Neospora caninum. Vet. Parasitol. 116, 7–14. https://doi.org/10.1016/s0304-4017(03)00154-7 (2003).
Choi, K. M., Gang, J. & Yun, J. Anti-Toxoplasma gondii RH strain activity of herbal extracts used in traditional medicine. Int. J. Antimicrob. Agents 32, 360–362. https://doi.org/10.1016/j.ijantimicag.2008.04.012 (2008).
Kavitha, N., Noordin, R., Chan, K. L. & Sasidharan, S. In vitro anti-Toxoplasma gondii activity of root extract/fractions of Eurycoma longifolia Jack. BMC Complement. Altern. Med. 12, 91. https://doi.org/10.1186/1472-6882-12-91 (2012).
Al Nasr, I., Ahmed, F., Pullishery, F., El-Ashram, S. & Ramaiah, V. V. Toxoplasmosis and anti-Toxoplasma effects of medicinal plant extracts—a mini-review. Asian Pac. J. Trop. Med. 9, 730–734. https://doi.org/10.1016/j.apjtm.2016.06.012 (2016).
Mui, E. J. et al. Triazine inhibits Toxoplasma gondii tachyzoites in vitro and in vivo. Antimicrob. Agents Chemother. 49, 3463–3467. https://doi.org/10.1128/aac.49.8.3463-3467.2005 (2005).
Al-Zanbagi, N. A. & Zelai, N. T. Two methods for attenuating Toxoplasma gondii tachyzoites RH strain by using ethanol extract of Curcuma longa. J. Egypt. Soc. Parasitol. 38, 965–976 (2008).
Al-Zanbagi, N. A. In vivo effect of some home spices extracts on the Toxoplasma gondii tachyzoites. J. Fam. Commun. Med. 16, 59–65 (2009).
Efferth, T., Herrmann, F., Tahrani, A. & Wink, M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomed. Int. J. Phytother. Phytopharmacol. 18, 959–969. https://doi.org/10.1016/j.phymed.2011.06.008 (2011).
Leesombun, A., Boonmasawai, S., Shimoda, N. & Nishikawa, Y. Effects of extracts from thai piperaceae plants against infection with Toxoplasma gondii. PLoS ONE 11, e0156116. https://doi.org/10.1371/journal.pone.0156116 (2016).
Sharif, M. et al. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: a systematic review. Can. J. Physiol. Pharmacol. 94, 1237–1248. https://doi.org/10.1139/cjpp-2016-0039 (2016).
Degbe, M. et al. Extracts of tectona grandis and vernonia amygdalina have anti-toxoplasma and pro-inflammatory properties in vitro. Parasite (Paris, France) 25, 11. https://doi.org/10.1051/parasite/2018014 (2018).
Izumi, E., Ueda-Nakamura, T., Veiga-Junior, V. F. & Nakamura, C. V. Toxicity of oleoresins from the genus Copaifera in Trypanosoma cruzi: a comparative study. Planta Med. 79, 952–958. https://doi.org/10.1055/s-0032-1328712 (2013).
de Souza, G. A. et al. In vitro and in vivo antimalarial potential of oleoresin obtained from Copaifera reticulata Ducke (Fabaceae) in the Brazilian Amazon rainforest. Phytomed. Int. J. Phytother. Phytopharmacol. 24, 111–118. https://doi.org/10.1016/j.phymed.2016.11.021 (2017).
Vieira, R. G. L. et al. In vitro studies of the antibacterial activity of Copaifera spp. oleoresins, sodium hypochlorite, and peracetic acid against clinical and environmental isolates recovered from a hemodialysis unit. Antimicrob. Resist. Infect. Control 7, 14. https://doi.org/10.1186/s13756-018-0307-3 (2018).
Rodrigues, I. A. & Ramos, A. S. Development of nanoemulsions to enhance the antileishmanial activity of copaifera paupera oleoresins. BioMed Res. Int. 2018, 9781724. https://doi.org/10.1155/2018/9781724 (2018).
Kian, D. et al. Trypanocidal activity of copaiba oil and kaurenoic acid does not depend on macrophage killing machinery. Biomed. Pharmacother. Biomed. Pharmacother. 103, 1294–1301. https://doi.org/10.1016/j.biopha.2018.04.164 (2018).
Ravindran, S., Lodoen, M. B., Verhelst, S. H., Bogyo, M. & Boothroyd, J. C. 4-Bromophenacyl bromide specifically inhibits rhoptry secretion during Toxoplasma invasion. PLoS ONE 4, e8143. https://doi.org/10.1371/journal.pone.0008143 (2009).
Hall, C. I. et al. Chemical genetic screen identifies Toxoplasma DJ-1 as a regulator of parasite secretion, attachment, and invasion. Proc. Natl. Acad. Sci. USA 108, 10568–10573. https://doi.org/10.1073/pnas.1105622108 (2011).
Montazeri, M. et al. Drug resistance in Toxoplasma gondii. Front. Microbiol. 9, 2587. https://doi.org/10.3389/fmicb.2018.02587 (2018).
Meneceur, P. et al. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob. Agents Chemother. 52, 1269–1277. https://doi.org/10.1128/aac.01203-07 (2008).
Silva, L. A., Reis-Cunha, J. L., Bartholomeu, D. C. & Vitor, R. W. Genetic polymorphisms and phenotypic profiles of sulfadiazine-resistant and sensitive Toxoplasma gondii Isolates obtained from newborns with congenital toxoplasmosis in minas gerais Brazil. PLoS ONE 12, e0170689. https://doi.org/10.1371/journal.pone.0170689 (2017).
Sepulveda-Arias, J. C., Veloza, L. A. & Mantilla-Muriel, L. E. Anti-Toxoplasma activity of natural products: a review. Recent Pat. Anti-Infect. Drug Discov. 9, 186–194. https://doi.org/10.2174/1574891x10666150410120321 (2014).
Santos, A. O. et al. Effect of Brazilian copaiba oils on Leishmania amazonensis. J. Ethnopharmacol. 120, 204–208. https://doi.org/10.1016/j.jep.2008.08.007 (2008).
dos Santos, A. O. et al. Leishmania amazonensis: effects of oral treatment with copaiba oil in mice. Exp. Parasitol. 129, 145–151. https://doi.org/10.1016/j.exppara.2011.06.016 (2011).
Dos Santos, A. O., Ueda-Nakamura, T., Dias Filho, B. P., da Veiga Junior, V. F. & Nakamura, C. V. Copaiba oil: an alternative to development of new drugs against Leishmaniasis. Evid. Based Complement. Altern. Med. eCAM 2012, 898419. https://doi.org/10.1155/2012/898419 (2012).
Santos, A. O. et al. Antileishmanial activity of diterpene acids in copaiba oil. Mem. Inst. Oswaldo Cruz 108, 59–64. https://doi.org/10.1590/s0074-02762013000100010 (2013).
Izumi, E., Ueda-Nakamura, T., Veiga, V. F. Jr., Pinto, A. C. & Nakamura, C. V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 55, 2994–3001. https://doi.org/10.1021/jm201451h (2012).
Hernandez, S. M., Sanchez, M. S. & de Tarlovsky, M. N. Polyamines as a defense mechanism against lipoperoxidation in Trypanosoma cruzi. Acta Trop. 98, 94–102. https://doi.org/10.1016/j.actatropica.2006.02.005 (2006).
Scherz-Shouval, R. & Elazar, Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 17, 422–427. https://doi.org/10.1016/j.tcb.2007.07.009 (2007).
da Trindade, R. & da Silva, J. K. Copaifera of the neotropics: a review of the phytochemistry and pharmacology. Int. J. Mol. Sci. 19, 1511. https://doi.org/10.3390/ijms19051511 (2018).
Lavine, M. D. & Arrizabalaga, G. The antibiotic monensin causes cell cycle disruption of Toxoplasma gondii mediated through the DNA repair enzyme TgMSH-1. Antimicrob. Agents Chemother. 55, 745–755. https://doi.org/10.1128/aac.01092-10 (2011).
Kamau, E. et al. A novel benzodioxole-containing inhibitor of Toxoplasma gondii growth alters the parasite cell cycle. Antimicrob. Agents Chemother. 55, 5438–5451. https://doi.org/10.1128/aac.00455-11 (2011).
Charvat, R. A. & Arrizabalaga, G. Oxidative stress generated during monensin treatment contributes to altered Toxoplasma gondii mitochondrial function. Sci. Rep. 6, 22997. https://doi.org/10.1038/srep22997 (2016).
Lavine, M. D. & Arrizabalaga, G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS ONE 7, e42107. https://doi.org/10.1371/journal.pone.0042107 (2012).
Castro, A. S. et al. Trophoblast cells are able to regulate monocyte activity to control Toxoplasma gondii infection. Placenta 34, 240–247. https://doi.org/10.1016/j.placenta.2012.12.006 (2013).
Barbosa, B. F. et al. IL10, TGF beta1, and IFN gamma modulate intracellular signaling pathways and cytokine production to control Toxoplasma gondii infection in BeWo trophoblast cells. Biol. Reprod. 92, 82. https://doi.org/10.1095/biolreprod.114.124115 (2015).
Ferro, E. A. et al. Macrophage migration inhibitory factor is up-regulated in human first-trimester placenta stimulated by soluble antigen of Toxoplasma gondii, resulting in increased monocyte adhesion on villous explants. Am. J. Pathol. 172, 50–58. https://doi.org/10.2353/ajpath.2008.070432 (2008).
Flores, M. et al. Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 22, 3661–3671. https://doi.org/10.1096/fj.08-111666 (2008).
de Oliveira, G. A. et al. Effect of macrophage migration inhibitory factor (MIF) in human placental explants infected with Toxoplasma gondii depends on gestational age. Am. J. Pathol. 178, 2792–2801. https://doi.org/10.1016/j.ajpath.2011.02.005 (2011).
Barbosa, B. F. et al. Susceptibility to Toxoplasma gondii proliferation in BeWo human trophoblast cells is dose-dependent of macrophage migration inhibitory factor (MIF), via ERK1/2 phosphorylation and prostaglandin E2 production. Placenta 35, 152–162. https://doi.org/10.1016/j.placenta.2013.12.013 (2014).
Rubin, B. K. Immunomodulatory properties of macrolides: overview and historical perspective. Am. J. Med. 117(Suppl 9A), 2s–4s. https://doi.org/10.1016/j.amjmed.2004.07.021 (2004).
Shiono, Y. et al. Maternal-fetal transmission of Toxoplasma gondii in interferon-gamma deficient pregnant mice. Parasitol. Int. 56, 141–148. https://doi.org/10.1016/j.parint.2007.01.008 (2007).
Angeloni, M. B. et al. Differential apoptosis in BeWo cells after infection with highly (RH) or moderately (ME49) virulent strains of Toxoplasma gondii is related to the cytokine profile secreted, the death receptor Fas expression and phosphorylated ERK1/2 expression. Placenta 34, 973–982. https://doi.org/10.1016/j.placenta.2013.09.005 (2013).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 (1983).
Furtado, F. B., Borges, B. C., Teixeira, T. L. & Garces, H. G. Chemical composition and bioactivity of essential oil from Blepharocalyx salicifolius. Int. J. Mol. Sci. 19, 33. https://doi.org/10.3390/ijms19010033 (2018).
Oliveira, J. G. et al. BeWo trophoblasts are unable to control replication of Toxoplasma gondii, even in the presence of exogenous IFN-gamma. Placenta 27, 691–698. https://doi.org/10.1016/j.placenta.2005.06.006 (2006).
Borges, I. P. et al. Anti-parasitic effect on Toxoplasma gondii induced by BnSP-7, a Lys49-phospholipase A2 homologue from Bothrops pauloensis venom. Toxicon Off. J. Int. Soc. Toxinol. 119, 84–91. https://doi.org/10.1016/j.toxicon.2016.05.010 (2016).
Kamau, E. T. et al. A focused small-molecule screen identifies 14 compounds with distinct effects on Toxoplasma gondii. Antimicrob. Agents Chemother. 56, 5581–5590. https://doi.org/10.1128/aac.00868-12 (2012).
Cota Teixeira, S. et al. Pentachloropseudilin impairs angiogenesis by disrupting the actin cytoskeleton, integrin trafficking and the cell cycle. ChemBioChem 20, 2390–2401. https://doi.org/10.1002/cbic.201900203 (2019).
Dos Santos, M. A. et al. Human B cells infected by Trypanosoma cruzi undergo F-actin disruption and cell death via caspase-7 activation and cleavage of phospholipase Cgamma1. Immunobiology https://doi.org/10.1016/j.imbio.2020.151904 (2020).
Costa, M. S. et al. Increased ROS generation causes apoptosis-like death: mechanistic insights into the anti-Leishmania activity of a potent ruthenium(II) complex. J. Inorg. Biochem. 195, 1–12. https://doi.org/10.1016/j.jinorgbio.2019.03.005 (2019).
Caniggia, I., Lye, S. J. & Cross, J. C. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 138, 3976–3986. https://doi.org/10.1210/endo.138.9.5403 (1997).
Vaidya, S. S., Walsh, S. W. & Gerk, P. M. Application of human placental villous tissue explants to study ABC transporter mediated efflux of 2,4-dinitrophenyl-S-glutathione. Curr. Pharm. Biotechnol. 12, 814–823. https://doi.org/10.2174/138920111795470976 (2011).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Acknowledgements
The authors gratefully acknowledge the financial support by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), São Paulo Research Foundation (FAPESP) [Grant Number 2011/13630-7] and “Grupo Luta pela Vida”. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. This manuscript has been edited by native English-speaking experts. We would like to thank Professor Silvio Favoreto for his insightful comments and for the English editing.
Author information
Authors and Affiliations
Contributions
S.C.T. wrote the first drafts, was responsible for project development, designed the experimental approaches, performed experiments, and interpreted the data. G.S., B.C.B., T.E.A., A.M.R. contributed to the experimental manipulations. F.A.A., S.R.A., R.C.S.V., and J.K.B were responsible for the identification and collection of Copaifera spp. oleoresins. M.J.B.S., C.H.G.M., and B.F.B designed the biological experiments, analyzed the data, and participated in the data interpretation. E.A.V.F coordinated and designed the biological experiments, analyzed and interpreted the data, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teixeira, S.C., de Souza, G., Borges, B.C. et al. Copaifera spp. oleoresins impair Toxoplasma gondii infection in both human trophoblastic cells and human placental explants. Sci Rep 10, 15158 (2020). https://doi.org/10.1038/s41598-020-72230-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72230-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.