Abstract
It is controversial whether a tumor located in the lower lobe is related with worse outcome of non-small cell lung cancer (NSCLC). This study aimed to clarify the prognostic role of primary tumor location in NSCLC. Patients newly diagnosed with NSCLC in a tertiary referral hospital from January 2011 to December 2014 were followed up for 5 years. Of the 2,289 NSCLC cases, 911 (39.8%) cases pertained to lower lobe cancers. Patients with lower lobe cancer showed a higher all-cause mortality rate than those with non-lower lobe cancer (48.6% vs. 40.3%, p < 0.001). Patients with lower lobe cancer had a lower proportion of adenocarcinoma histology and epidermal growth factor receptor (EGFR) mutations. Furthermore, compared to patients with non-lower lobe cancer, those with lower lobe cancer had a higher level of tumor markers (neuron-specific enolase and cytokeratin fragment 21-1). Mediation analysis revealed that the association between lower lobe cancer and higher all-cause mortality could be explained by an indirect pathway through EGFR mutations (percent mediated = 17.3%, p = 0.005). The sensitivity analysis for adenocarcinoma patients showed similar results (percent mediated = 18.8%, p = 0.021). Lower lobe cancer is associated with a higher all-cause mortality risk in patients with NSCLC, which is partly mediated by a lower proportion of EGFR mutations.
Similar content being viewed by others
Introduction
Lung cancer is the second most commonly detected cancer and the most leading cause of cancer death in the world, although incidence and mortality have been decreased in recent decades1. There have been advances in early detection and standard treatment for lung cancer, but the 5-year survival rate is still 4–55% according to localized or advanced stage2. Lung cancer is a heterogeneous disease with different clinicopathological features, and identifying subtypes by molecular abnormalities and biomarkers is mandatory for accurate prediction of treatment success and clinical prognosis. Exploring the differences in prognosis according to the lung cancer phenotypes is a fundamental step for elucidating the role of biologic markers.
The location of non-small cell lung cancer (NSCLC) is considered as an important factor in predicting treatment efficacy and clinical prognosis. Several studies have shown that the operation site or side influence the treatment outcome3. The location of NSCLC is related with the distribution of lymph node (LN) metastasis4,5. The unexpected upstaging by surgical LN evaluation is more frequently found in the lower lobe6. Differences of histologic type7,8 and epidermal growth factor receptor (EGFR) mutations9 were found according to tumor location. However, it has not been clearly explained how tumor location relates to clinical prognosis. In addition, it is unclear whether lower lobe cancer is significantly associated with worse prognosis. Several studies have revealed that the tumors located in non-upper lobes had poorer clinical outcomes in NSCLC with resectable stages10,11; contrasting results have been reported in some studies12,13.
We aimed to investigate whether a tumor located in the lower lobe is associated with higher mortality risk and to identify a plausible mediator between the tumor location and mortality in patients with NSCLC.
Methods
We confirmed that all methods were carried out in accordance with the guidelines and regulations for strengthening the reporting of observational studies in epidemiology (STROBE) statement14.
Study design and setting
This retrospective cohort study was conducted by reviewing the electronic medical records of patients newly diagnosed with NSCLC from January 2011 to December 2014 and followed up for 5 years at a tertiary teaching hospital in South Korea. After NSCLC diagnosis, the treatment plan was made by multidisciplinary discussion (MDD). Mortality data were obtained from the Ministry of Interior and Safety of Korea. Overall, the survival rate was assessed from the date of the diagnosis to the date of death or the last follow-up date.
Participants
During the study period, patients with pathologically proven NSCLC were recruited. Chest computed tomography (CT) at the initial stage of diagnosis was used to evaluate the location of the primary tumor (lower lobe or non-lower lobe). Cases in which the primary tumor location was difficult to identify because of multiple lesions or tumors involving two or more lobes were excluded. Non-lower lobes included the right upper lobe, the left upper lobe, and the right middle lobe. EGFR mutations and tumor markers such as neuron-specific enolase (NSE), cytokeratin fragment (CYFRA) 21-1 were conducted by the clinicians’ decision as a routine practice.
Variables and measures
The demographic information included age; sex, body mass index (BMI); smoking status; Eastern Cooperative Oncology Group (ECOG) performance status; presence of respiratory symptoms; pulmonary function test; pathology; tumor, node, metastases (TNM) stage; and initial treatments. Pulmonary function test included forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and the FEV1/FVC%. In our study population, a positron emission tomography-computed tomography (PET/CT) and a magnetic resonance imaging (MRI) of the brain was performed in most patients for clinical staging. An endobronchial ultrasound-guided transbronchial needle aspiration was conducted to evaluate mediastinal LN status if it was clinically indicated. The new 8th TNM staging system was applied to the clinical and pathologic stages. For purposes of accurate staging from I to IV, we combined clinical and pathologic staging. The pathologic stage was used in patients who underwent surgery, while the clinical stage was used on the remaining patients. Also, we verified if the TNM stage changed after surgery. Active treatment was defined as surgical resection, radiotherapy, and chemotherapy for curative purposes or for palliative care. The location of the primary tumor was classified into lower lobe versus non-lower lobe based on a chest CT. Information on the variables known to be prognostic factors was reviewed and included age, sex, BMI, smoking status, performance status, symptoms at the moment of diagnosis, lung function, histology, standardized uptake value (SUV) of the main mass, tumor markers (NSE, CYFRA 21-1, and carcinoembryonic antigen [CEA]), EGFR mutations, and anaplastic lymphoma kinase (ALK) translocation. The mediator candidates were determined among these variables considering differences between the lower lobe and non-lower lobe cancer. The outcomes were all-cause mortality and time to all-cause death.
Statistical methods
The chi-square test was used for categorical variables and the student t-test was used for continuous variables. The Kaplan–Meier method was used to compare the time to all-cause mortality between non-lower and the lower lobe cancer groups, and the difference was estimated by the log-rank test. A multivariable Cox proportional hazard assumption test was performed with model 1 and 2. Model 1 included covariates except for mediator candidates. Model 2 included the mediator candidates in addition to the covariates for model 1. We performed mediation analysis only for mediators (EGFR, NSE, CYFRA, or adenocarcinoma) that met the following requirements15: (1) the significant relationship between tumor location and the mediator; (2) the significant relationship between the mediator and mortality; (3) the significant relationship between tumor location and mortality in the absence of the mediator; and (4) the attenuated relationship between tumor location and mortality when the mediator was included in the model. Percent mediated was calculated as the ratio of the absolute value of the indirect effect to the absolute value of the total effect of metabolic components on the outcome16. P < 0.05 was considered significant difference. All the statistical analyses were performed using the Stata statistical software version 14.2 (StataCorp LP, College Station, TX, USA) and SAS 9.4 (SAS Institute, Cary, NC).
Ethics
This study was approved by the Institutional Review Board of Seoul National University Hospital (H-1611-047-807). Informed consent was waived.
Results
A total of 2,453 patients were diagnosed with NSCLC from January 2011 to December 2015. Of them, we excluded patients with small cell lung cancer and those who were transferred to other hospitals after the initial diagnosis or those who were lost to follow-up and whose primary location could not be assessed. Finally, 2,289 patients were included in our study (Fig. 1). Among them, 1,378 (60.2%) had a primary tumor located in non-lower lobes, while 911 (39.8%) had a primary tumor in the lower lobes. During mean 3.5(± 1.9) years of observation, we found 999 (43.6%) were died. The patients with NSCLC located in the lower lobes had a higher all-cause mortality rate than those with non-lower lobe cancers (48.6% and 40.3% respectively, P < 0.001).
Patients characteristics
The baseline characteristics were described according to the tumor location in Table 1. There were 911 patients (39.8%) with primary tumors in the lower lobes. There was no difference in age, sex, BMI, smoking status, ECOG performance status, accompanying symptoms, and pulmonary function test between the non-lower and the lower lobe group. We found no significant differences in lung cancer TNM stage and the SUV of the main mass. Active treatments were performed at a similar rate in both groups.
In pathology, adenocarcinomas are more frequently found in the non-lower lobe group, while squamous-cell carcinomas are more likely to be detected in the lower lobe group. Tumor markers such as NSE and CYFRA 21-1 were elevated in the lower lobe group. EGFR mutations were more frequently detected in the non-lower lobe group. Notably, we found that exon 21 mutations significantly contributed to the difference of EGFR mutation frequency between the non-lower lobe and the lower lobe groups (20.8% and 13.4% respectively; P < 0.001).
Comparison of survival rate between the non-lower lobe and the lower lobe groups
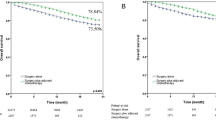
Covariates that had significant relationships with all-cause mortality were: pathology, EGFR mutations, serum NSE, and serum CYFRA 21-1 (Supplementary information 1). We determined these covariates as the mediator candidates. In the unadjusted Kaplan–Meier curve, a higher risk of all-cause mortality was observed in the lower lobe group than in the non-lower lobe group (P < 0.001, Fig. 2A). Similar results were found in the Kaplan–Meier curves adjusted by the covariates (Fig. 2B) and the mediator candidates (Fig. 2C). Multivariable Cox regression analysis in model 1 showed that the lower lobe cancer was associated with a higher risk of all-cause mortality (HR 1.34, 95% CI 1.14–1.59, P = 0.001) (Table 2). In addition, higher age (≥ 60), ever smoking, ECOG performance status ≥ 2, accompanying symptoms, higher SUV of the main mass ≥ 11.2, and higher stage were all related with a higher risk of all-cause mortality. In contrast, ALK translocation and active treatment were associated with a lower risk of all-cause mortality (Table 2). In model 2, multivariable analyses with the Cox proportional hazards regression model revealed that lower lobe cancer was associated with a higher risk of all-cause mortality (HR 1.31, 95% CI 1.01–1.70, P = 0.040). Also, NSE ≥ 16.3 ng/mL and CYFRA 21-1 ≥ 3.3 ng/mL increased the risk of all-cause mortality, while EGFR mutations decreased the risk of all-cause mortality. Sensitivity analysis with the patients who were diagnosed with adenocarcinomas showed similar results (Supplementary information 2).
Kaplan–Meier survival curve with univariate model and multivariate Cox proportional hazard models. (A) Kaplan–Meier survival curve with univariate model; (B) Cox proportional hazard model 1 with the covariates except for the mediator candidates; (C) Cox proportional hazard model 2 with the covariates including the mediator candidates. The covariates included age, sex, smoking status, performance status, presence of symptoms, body mass index, standardized uptake value of main mass, stage, anaplastic lymphoma kinase translocation, and active treatment. The mediator candidates included adenocarcinoma histology, serum neuron-specific enolase level, serum cytokeratin fragment level, and epidermal growth factor receptor mutations.
Stage change after complete surgical resection
Among the 2,289 patients, 1,072 (46.8%) underwent a complete surgical resection. TNM stage changed in 498 patients (46.5%) after surgery; pathologic upstage happened in 369 patients (34.4%), and downstaging happened in 129 patients (12.0%). The proportion of stage change from clinical to pathologic was not significantly different between the non-lower lobe and the lower lobe groups (Table 3).
Causal mediation analysis
In mediation analysis to identify causal associations between the tumor location and survival, EGFR mutations showed a statistically significant indirect effect (P = 0.005, Fig. 3). In the association between lower lobe location and higher mortality risk, 17.3% could be explained by lower expression of EGFR mutations. The sensitivity analysis of the patients diagnosed with adenocarcinomas showed that the percent of association mediated was 18.8% through EGFR mutations alone, and this indirect association was statistically significant (P = 0.021, Supplementary information 3).
Causal mediation analysis in non-small cell lung cancer patients. (A) Mediation analysis for indirect effect of EGFR mutations; (B) Mediation analysis for indirect effect of NSE; (C) Mediation analysis for indirect effect of CYFRA; (D) Mediation analysis for indirect effect of adenocarcinoma. CYFRA, cytokeratin fragment; EGFR, epidermal growth factor receptor mutations; NSE, neuron-specific enolase.
Discussion
In the present study, the patients with NSCLC in the lower lobes had a higher risk of all-cause mortality than those with non-lower lobe cancer. The patients with lower lobe cancer had a higher proportion of non-adenocarcinoma histology, a higher tumor marker level, and a lower proportion of EGFR mutations, which were also associated with an increased risk for 5-year all-cause mortality. In our knowledge, this is the first study that evaluated the relationship between lung cancer location and prognosis including patients with unresectable stage. Because of more permittable inclusion criterion in terms of lung cancer stage compared to previously published studies11,13,17,18, the 5-year survival rate was lower in our study subjects (44% vs. 62–74%). We found that lower lobe location and a lower expression of EGFR mutations were the independent factors linked to poor prognosis regardless of important clinical factors including lung cancer stage. In the mediation analysis, a significant indirect pathway through EGFR mutations in the relationship between the lower lobe location and all-cause mortality was observed. In the sensitivity analysis for adenocarcinoma patients, EGFR mutations were also identified as a significant mediator. These findings suggest that the lower frequency of EGFR mutations can partly mediate the higher all-cause mortality risk in the lower lobe NSCLC.
The prognostic role of the lobar location in NSCLC has not been well validated. In the early 2000s, two Japanese groups reported that the upper lobe location of a primary tumor allowed for better survival in patients with a completely resected stage IIIA19,20. In 2007, Ou et al. suggested that the non-upper lobe location was a risk factor for stage I patients10. There have been several efforts to determine why NSCLCs in lower lobes pose a worse prognosis when compared to those in the non-lower lobe. First, accurate clinical staging remains a challenge, especially in lower lobe cancers. A prospective study showed stage I or II NSCLCs located in the lower lobes were more likely to be upstaged in histologic diagnosis when compared to those in the upper lobes6. The main reason for stage misclassification in lower lobe cancers was a more advanced tumor (T) stage attributed by a radiologically uncertain pleural or chest wall invasion and an unsuspected spread to central airway or mediastinum. Second, the effectiveness of treatments may be different according to tumor location. Worse treatment outcomes for radiation therapy were reported in patients with lower lobe cancers17,21. The majority of the lower lobe cancers were not good candidates for radiation therapy than the non-lower lobe cancers, because there are more obstacles such as heart during the radiation treatment. Third, the predisposing location of underlying chronic lung disease may influence the prognosis according to the location of NSCLC. For example, idiopathic pulmonary fibrosis is frequently detected in lower lobes and is also associated with worse prognosis of NSCLC22. Fourth, EGFR mutations could be the link between tumor location and prognosis. EGFR mutations are less likely to be detected in the lower lobe cancers9. Considering that EGFR mutation is a favorable predictive marker23, lower lobe cancers are expected to have poor prognosis than non-lower lobe cancers. Therefore, our interest was to prove whether the relationship between lower lobe location and prognosis can be explained by expression of EGFR mutations in NSCLC.
EGFR mutation has been studied as a favorable prognostic marker in NSCLC. In the post-hoc analysis of phase III randomized controlled trial, EGFR mutations were related to a better survival rate, irrespective of treatment24. There is a higher rate of EGFR mutations in Asians25. In a large study, in which Asians were not included, scientists did not find a significant relationship between tumor location and clinical prognosis13. One plausible reason for inconsistent results about the prognostic role of cancer location is the different proportion of multiple EGFR mutations23. In our analyses on various EGFR mutations, exon 19 and 21 mutations were significantly related with survival, while exon 18 and 20 mutations were not. However, it is still unclear whether the differences in genetic abnormalities of the study population are the main reason for the difference in prognosis.
Our study has certain strengths. First, to our knowledge, this was the first mediation analysis study exploring why the survival difference was observed according to primary tumor location. Our results validate the reason why previous studies have shown similar outcomes. Second, we analyzed a large population with accurate lung cancer stage. In this study population, radiologic or interventional work-ups for lung cancer staging were fully available and determined by MDD. Similarly, covariates were evenly distributed according to the non-lower lobe and the lower lobe group, except for the mediator candidates. Sufficient patient data were available for the sensitivity analysis for the evaluated patients with lung adenocarcinomas. Third, our study included various prognostic factors as covariates to adjust for the association between tumor location and prognosis. In particular, our study was different in that a serum level of tumor markers was also assessed with clinicopathological features. Increased levels of NSE have also been reported in NSCLCs and reflects neuroendocrine components26. CYFRA 21-1 is highly expressed by all epithelial cells and represents a useful indicator of epithelial differentiation26. NSE and CYFRA 21-1 have been reported as predictive factors of clinical prognosis in NSCLC patients27,28.
In conclusion, our study showed that a lower lobe cancer is associated with a higher all-cause mortality risk in patients with NSCLC, which is partly mediated by a lower proportion of EGFR mutations in lower lobe cancers.
Abbreviations
- ALK:
-
Anaplastic lymphoma kinase
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- CYFRA:
-
Cytokeratin fragment
- EGFR:
-
Epidermal growth factor receptor
- FEV1:
-
Forced expiratory volume in 1 second
- FVC:
-
Forced vital capacity
- MDD:
-
Multidisciplinary discussion
- NSCLC:
-
Non-small cell lung cancer
- NSE:
-
Neuron-specific enolase
- PET:
-
Positron emission tomography
- SUV:
-
Standardized uptake value
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30. https://doi.org/10.3322/caac.21442 (2018).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2013 (National Cancer Institute, Bethesda, 2016).
Lipford, E. H. 3rd. et al. Prognostic factors in surgically resected limited-stage, nonsmall cell carcinoma of the lung. Am. J. Surg. Pathol. 8, 357–365 (1984).
Ketchedjian, A. et al. Location as an important predictor of lymph node involvement for pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 132, 544–548. https://doi.org/10.1016/j.jtcvs.2006.05.023 (2006).
Watanabe, S., Suzuki, K. & Asamura, H. Superior and basal segment lung cancers in the lower lobe have different lymph node metastatic pathways and prognosis. Ann. Thorac. Surg. 85, 1026–1031. https://doi.org/10.1016/j.athoracsur.2007.10.076 (2008).
Rocha, A. T., McCormack, M., Montana, G. & Schreiber, G. Association between lower lobe location and upstaging for early-stage non-small cell lung cancer. Chest 125, 1424–1430 (2004).
Lee, B. W., Wain, J. C., Kelsey, K. T., Wiencke, J. K. & Christiani, D. C. Association of cigarette smoking and asbestos exposure with location and histology of lung cancer. Am. J. Respir. Crit. Care Med. 157, 748–755. https://doi.org/10.1164/ajrccm.157.3.9707025 (1998).
Kinsey, C. M. et al. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer 84, 145–150. https://doi.org/10.1016/j.lungcan.2014.02.002 (2014).
Tseng, C. H. et al. EGFR mutation and lobar location of lung adenocarcinoma. Carcinogenesis 37, 157–162. https://doi.org/10.1093/carcin/bgv168 (2016).
Ou, S. H., Zell, J. A., Ziogas, A. & Anton-Culver, H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients : a population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 110, 1532–1541. https://doi.org/10.1002/cncr.22938 (2007).
Kudo, Y. et al. Do tumours located in the left lower lobe have worse outcomes in lymph node-positive non-small cell lung cancer than tumours in other lobes?. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 42, 414–419. https://doi.org/10.1093/ejcts/ezs065 (2012).
Puri, V. et al. Tumor location is not an independent prognostic factor in early stage non-small cell lung cancer. Ann. Thorac. Surg. 89, 1053–1059. https://doi.org/10.1016/j.athoracsur.2010.01.020 (2010).
Whitson, B. A. et al. T1/T2 non-small-cell lung cancer treated by lobectomy: does tumor anatomic location matter?. J. Surg. Res. 177, 185–190. https://doi.org/10.1016/j.jss.2012.05.022 (2012).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Baron, R. M. & Kenny, D. A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 51, 1173–1182. https://doi.org/10.1037//0022-3514.51.6.1173 (1986).
Park, S., Choi, N. K., Kim, S. & Lee, C. H. The relationship between metabolic syndrome and asthma in the elderly. Sci. Rep. 8, 9378. https://doi.org/10.1038/s41598-018-26621-z (2018).
Shaverdian, N. et al. Location matters: stage I non-small-cell carcinomas of the lower lobes treated with stereotactic body radiation therapy are associated with poor outcomes. Clin. Lung Cancer 18, e137–e142. https://doi.org/10.1016/j.cllc.2016.09.001 (2017).
Shien, K. et al. Lower lobe origin is a poor prognostic factor in locally advanced non-small-cell lung cancer patients treated with induction chemoradiotherapy. Mol. Clin. Oncol. 3, 706–712. https://doi.org/10.3892/mco.2015.509 (2015).
Ichinose, Y. et al. Completely resected stage IIIA non-small cell lung cancer: the significance of primary tumor location and N2 station. J. Thorac. Cardiovasc. Surg. 122, 803–808. https://doi.org/10.1067/mtc.2001.116473 (2001).
Inoue, M. et al. Results of surgical intervention for p-stage IIIA (N2) non-small cell lung cancer: acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. J. Thorac. Cardiovasc. Surg. 127, 1100–1106. https://doi.org/10.1016/j.jtcvs.2003.09.012 (2004).
Hayakawa, K. et al. Impact of tumor extent and location on treatment outcome in patients with stage III non-small cell lung cancer treated with radiation therapy. Jpn. J. Clin. Oncol. 26, 221–228. https://doi.org/10.1093/oxfordjournals.jjco.a023218 (1996).
Kanaji, N. et al. Impact of idiopathic pulmonary fibrosis on advanced non-small cell lung cancer survival. J. Cancer Res. Clin. Oncol. 142, 1855–1865. https://doi.org/10.1007/s00432-016-2199-z (2016).
Fang, S. & Wang, Z. EGFR mutations as a prognostic and predictive marker in non-small-cell lung cancer. Drug Des. Dev. Ther. 8, 1595–1611. https://doi.org/10.2147/DDDT.S69690 (2014).
Eberhard, D. A. et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 23, 5900–5909. https://doi.org/10.1200/jco.2005.02.857 (2005).
Shi, Y. et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 9, 154–162. https://doi.org/10.1097/jto.0000000000000033 (2014).
Zamay, T. N. et al. Current and prospective protein biomarkers of lung cancer. Cancers https://doi.org/10.3390/cancers9110155 (2017).
Yu, D., Du, K., Liu, T. & Chen, G. Prognostic value of tumor markers, NSE, CA125 and SCC, in operable NSCLC Patients. Int. J. Mol. Sci. 14, 11145–11156. https://doi.org/10.3390/ijms140611145 (2013).
Pujol, J. L., Boher, J. M., Grenier, J. & Quantin, X. Cyfra 21–1, neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 621 patients. Lung Cancer 31, 221–231 (2001).
Author information
Authors and Affiliations
Contributions
H.W.L. contributed substantially to analysis and interpretation of data AND drafted the article AND gave final approval of the version to be published AND agreed to act as guarantor of the work. Y.S.P. contributed substantially to conception and design and acquisition of data AND revised the article critically for important intellectual content AND gave final approval of the version to be published AND agreed to act as guarantor of the work. S.P. contributed substantially to analysis and interpretation of data AND revised the article critically for important intellectual content AND gave final approval of the version to be published AND agreed to act as guarantor of the work. C.-H.L. contributed substantially to conception and design and acquisition of data AND revised the article critically for important intellectual content AND gave final approval of the version to be published AND agreed to act as guarantor of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, H.W., Park, Y.S., Park, S. et al. Poor prognosis of NSCLC located in lower lobe is partly mediated by lower frequency of EGFR mutations. Sci Rep 10, 14933 (2020). https://doi.org/10.1038/s41598-020-71996-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71996-7
This article is cited by
-
Distribution and characteristics of malignant tumours by lung lobe
BMC Pulmonary Medicine (2024)
-
Predicting 2-year survival in stage I-III non-small cell lung cancer: the development and validation of a scoring system from an Australian cohort
Radiation Oncology (2022)
-
Quantification of the spatial distribution of primary tumors in the lung to develop new prognostic biomarkers for locally advanced NSCLC
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.