Abstract
It is known that ultrasonication has a certain effect on thermophysical properties and heat transfer of nanofluids. The present study is the continuation of the authors’ previous research on the effects of ultrasonication on the thermophysical properties of Multi-Walled Carbon Nanotubes (MWCNTs)-water nanofluid. Investigating the effects of ultrasonication time on samples’ stability, rheological properties, and pumping power of a water-based nanofluid containing MWCNTs nanoparticle is the main objective of the present study. The two-step method has been employed to prepared the samples. Moreover, a probe-type ultrasonic device has been used, and different ultrasonication times have been applied. The samples’ stability is investigated in different periods. The results revealed that prolonging the ultrasonication time to 60 min leads to improving the samples’ stability while prolonging ultrasonication time to higher than 60 min resulted in deteriorating the stability. As for dynamic viscosity, it is observed that increasing ultrasonication time to 60 min leads to decreasing the dynamic viscosity of the samples. As for pumping power, it is observed that the maximum increase in fanning friction factor ratio is less than 3%, which shows that adding MWCNTs to water does not impose a considerable penalty in the required energy for pumping power.
Similar content being viewed by others
Introduction
There is no denying the fact that introducing nanofluids (NFs), which are suspensions of nano-sized particles into the conventional working fluids, by Choi and Eastman1 in 1995 has opened new doors to improve the heat transfer performance in different applications. After this pioneering study by Choi and Eastman1, many researchers investigated the stability and thermophysical properties2,3,4, heat transfer5,6,7,8,9,10,11, applications of artificial intelligence in predicting the thermophysical properties of nanofluids12,13,14,15,16, and applications of various NFs17,18,19,20,21. It is known that thermophysical properties of each fluid play an important role in heat transfer performance of the fluids: viscosity directly affected the pumping power and the pressure loss, thermal conductivity indicates the heat transfer effectiveness of the fluid, and specific heat capacity shows the capability of the fluid in storing and moving heat away. Adding nano-sized particles to conventional coolants (i.e., water, oil, ethylene glycol, and so forth) leads to improving the thermal properties of the conventional coolants, which is highly desirable from the heat transfer point of view, although it causes some penalty in pumping power and pressure loss, which is not desirable from the energy management point of view.
It is accepted by all researchers that the most crucial step towards conducting experimental investigations using NFs is the sample preparation step. Suspending the nanoparticles (NPs) into the base fluid with the minimum sedimentation and agglomeration of the particles is a long-lasting challenge in employing NFs in practical applications. NPs naturally tend to sediment and agglomerate, which leads to having a non-uniform suspension. Uniform distribution of NPs into the base fluid leads to having higher thermal conductivity, and as a result, higher heat transfer performance while the non-uniform distribution of the NPs leads to deteriorating the thermophysical properties of the NFs or even leads to having lower thermophysical properties compared to the base fluids. Thus, the preparation step is a critical step in conducting an experimental study. Various methods and techniques have been applied so far by researchers to prepare long-time stable samples (at least two weeks) with minimum sedimentation and agglomeration. Generally, the samples are prepared using single-step or two-step methods. The simultaneous preparation and dispersion of nanomaterials into the base fluid is the procedure of the single-step method while in the two-step method, the nanomaterials are produced first, and then they disperse in the base fluid applying various mixing techniques22. Although single-step method leads to having more stable NFs, there are various disadvantages as well, such as limited application of the prepared samples which is limited to be used in low vapor pressure process and managing the size and structure of the NPs is challenging. As for the two-step method, which is a widely used method in literature, the main advantage is that it is the most economical method in preparing the samples22.
It is known that applying ultrasonication plays a crucial role in the two-step method. It is indicated in the literature that ultrasonication results in breaking down the large agglomeration of NPs into the smaller-sized agglomeration which results in having a more stable suspension with uniform distribution of NPs into the base fluid23,24,25,26. Literature showed that ultrasonication time, power, and type of ultrasonication device has certain effect on stability27,28 and thermophysical properties of NFs29,30,31. It is indicated that ultrasonic probe devices are more effective than that of the ultrasonic bath devices27,32, and prolonging the ultrasonication time leads to improving the thermal conductivity33 and decreasing the dynamic viscosity34 of the NFs. However, there are some references which reported an optimum ultrasonication time in which prolonging the ultrasonication time to higher than those values leads to deteriorating the stability and thermophysical properties of the NFs35,36,37. Thus, finding the optimum sonication time is of paramount importance in the preparation of NFs to achieve the best stability and thermophysical properties.
Based on what has been discussed thus far, it is evident that ultrasonication time has certain effects on the stability and thermophysical properties of NFs. Moreover, it is known that thermophysical properties affected the heat transfer performance and pressure loss in a system in which NFs employed as a heat transfer fluid. The present paper is the continuation of the authors' previous study on the effects of ultrasonication time on stability and thermal conductivity of the nanofluid38. In the present study, the effects of ultrasonication time on dynamic viscosity and pressure loss of MWCNT-water in three different solid concentrations of 0.1, 0.3, and 0.5 vol% and different temperatures ranging from 25 to 60 °C will be studied. The optimum ultrasonication time which leads to having the minimum dynamic viscosity will be found, and the dynamic viscosity of NF will be measured in different studied temperatures and solid concentrations. Finally, in order to show how adding the MWCNT to water affects the required pumping power of the system, the pressure loss has been studied.
Materials and methods
Sample preparation
As it is discussed in the introduction, there are two commonly used methods to prepare the NFs’ samples; single-step and two-step methods. The advantages of using the two-step method have been discussed in the introduction. Thus, in the present study, the two-step method has been employed to prepare the samples in three different solid concentrations of 0.1, 0.2, and 0.5 vol% containing MWCNT (US Research Nanomaterials Inc., USA) NPs. The detailed information of MWCNT NP has been presented in Tab. I, under the supporting information section. Moreover, the TEM image of the MWCNT, which has been provided by the manufacturer, has been presented in Fig. I (under the supporting information section).
A schematic view of the process of the two-step method to prepare the NFs has been depicted in Fig. II (under the supporting information section). First, the NPs were added to the base fluid. Employing a mechanical stirrer for 2 h, the NPs have been dispersed into the base fluid. In order to break down the large agglomeration of the NPs into the smaller size clusters or even single particle, a probe-type ultrasonic device (Hielscher UP400S, 400 W, 24 kHz, Germany) was employed. In order to study the effects of ultrasonication on the stability and rheological properties of the NF, different ultrasonication times of 10, 20, 40, 60, 70, 75, and 80 min were applied. The effectiveness of the ultrasonication process has been proven in the literature39,40,41,42. However, optimum ultrasonication time is still challenging. This way, the optimum ultrasonication time, which results in having long-time stable NF with the minimum sedimentation, has been investigated, and the results will be presented in the following sections.
Dynamic viscosity measurement
Searching the literature revealed that the most accurate and widely used device to measure the dynamic viscosity of different NFs is the Brookfield cone and plate viscometer manufactured by Brookfield Engineering Co., USA. In the present study, the CAP2000 + L viscometer has been employed to measure the dynamic viscosity of the MWCNT-water NF over different ranges of temperatures and solid concentrations. It should be noted that this series of viscometers are medium to high shear rate devices. It is interesting to know that the viscometer is equipped with an integrated temperature control system to control the temperature of the samples. The viscometer can operate in a wide range of rotational speeds ranging from 5 to 1,000 RPM, and the temperature control of the samples is possible between 5 to 75 °C with the accuracy of ± 0.5 °C. The calibration of the viscometer has been scrutinized before starting the experiments by measuring the dynamic viscosity of water and comparing the results whit those available in the ASHRAE handbook43. Figure 1 presents the results of the calibration test. As can be seen, there is a negligible difference (less than 8%) between the measured data and the data presented in the ASHRAE handbook43. Thus, it can be concluded that the viscometer is calibrated, and the measured data are reliable.
Stability analysis
It is known that the stability of NFs is a critical and challenging issue in implementing NFs in practical applications. Different characterization analysis can be employed to determine the stability of NFs, such as:
-
Electron Microscopy; Scanning electron microscopy (SEM), and transmission electron microscopy (TEM)
-
Sedimentation technique
-
Spectral analysis
-
Zeta potential
-
Dynamic light scattering (DLS).
In the present study, visual sedimentation and zeta potential analysis have been employed to investigate the effects of ultrasonication time on the stability of the samples over 30 days after the preparation of the samples. These two methods have been widely used in the literature by different researchers33,44,45. Figure III (under the supporting information section) shows the results of the visual observation of the samples in different periods after preparing the samples. It can be seen that the samples showed no sedimentation on the first day of preparation. The samples also showed good stability on the 5th day except for the sample, which was subjected to 80 min ultrasonication. After 10 days of preparation, the samples subjected to 75 and 80 min ultrasonication showed considerable sedimentation, and the sample subjected to 70 min ultrasonication showed negligible sedimentation. The rest of the samples are in good stability. As for the 30th day, massive sedimentation has been observed for the samples subjected to more than 70 min ultrasonication, but the rest of the samples are in good stability. From the visual observation, it can be concluded that prolonging the ultrasonication time to more than 60 min leads to deteriorating the stability of the samples. The results of the further accurate investigation by conducting zeta potential analysis on the stability of the samples subjected to different ultrasonication time will be presented in the following.
Zeta potential is a quantitative analysis that measures the interfacial potential between the dispersion medium (base fluid) and the thin layer attached to the surface of the NPs. The unit of the zeta potential (ζ) is mV, and higher values of zeta potential show better stability of the suspensions. It should be noted that the samples with the zeta potential lower than 30 mV consider as limited stability (unacceptable stability), between 30 to 60 mV considers as physical stability (good/moderately acceptable stability), and the samples with the zeta potential higher than 60 mV consider as excellent stability27,44. Figure 2 presents the results of zeta potential with respect to ultrasonication time at the solid concentration of 0.5 vol%, which is the highest studied solid concentration. As can be seen, increasing the ultrasonication time from 20 to 60 min resulted in increasing the value of zeta potential, which means higher stability. However, a further increase in ultrasonication time (higher than 60 min) leads to deteriorating the stability of the NF. The trend is approximately similar in different periods of time at which the zeta potential analysis has been performed. It can also be seen that the sample subjected to 60 min ultrasonication possesses the best stability even after 30 days of preparation. Thus, it can be concluded that 60 min ultrasonication is the optimum time in which the best stability can be achieved for the studied NF.
Results and discussion
In the following sections, first of all, the rheological behavior of the studied nanofluid will be investigated over a different range of temperatures, solid concentrations, and shear rates. Then, the effects of ultrasonication time on the variations of dynamic viscosity will be presented and discussed. Moreover, the results of the dynamic viscosity measurement in different temperatures and solid concentrations, at the optimum ultrasonication time will be presented and discussed. Apart from that, the effects of adding MWCNT to the water on the pressure loss will be studied.
Rheological behavior
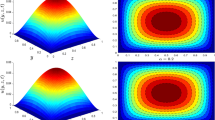
It is known that the rheological properties of nanofluids play a prominent role in dynamic viscosity and heat transfer. There are many debates on the rheological properties of the nanofluids containing carbon nanotubes (CNTs). While some researchers reported that CNT-based nanofluids show non-Newtonian behavior46, there are other researchers who observed that the CNT-based nanofluid with the solid concentrations less than 0.5 vol% show Newtonian behavior47,48,49, and increasing the solid concentration leads to changing the rheological behavior from Newtonian to non-Newtonian50. Thus, in the present study, the rheological properties of the studied nanofluid have been investigated over a different range of shear rates (100–800 1/s), temperatures, and solid concentration to realize that whether the studied nanofluid is a Newtonian or a non-Newtonian fluid. Figure 3 presents the results of dynamic viscosity variations versus shear rate in different temperatures and at the solid concentration of 0.5 vol%. As can be seen, the dynamic viscosity showed a linear trend in all the studied shear rates and temperature. Thus, it can be concluded that the studied nanofluid is a Newtonian fluid. The obtained results are in good agreement with the results presented by Halelfadel et al.48 and Afshari et al.50. It should also be noted that the dynamic viscosity versus shear rate showed the same trend in other studied solid concentrations.
Dynamic viscosity
Effects of ultrasonication time on dynamic viscosity
In order to investigate the effects of ultrasonication time on dynamic viscosity of MWCNT-water nanofluid, the dynamic viscosity of the samples has been measured over different ultrasonication times on the 30th day after samples preparation, and the results have been presented in Fig. 4. This figure reveals that increasing the ultrasonication time leads to a gentle decrease in the dynamic viscosity of the samples. It would be because of the fact that increasing the sonication time leads to breaking down the MWCNT particles into the smaller size particles, which leads to the de-clustering of the MWCNT bundles. However, prolonging the ultrasonication time to higher than 60 min results in increasing the dynamic viscosity. Other researchers have been previously reported the same results indicating that there is an optimum ultrasonication time in which the dynamic viscosity reached its minimum value, and prolonging the ultrasonication time leads to increasing the dynamic viscosity27,51. It is also seen in Sect. 2.3 that 60 min ultrasonication leads to having a more uniform distribution of nanoparticles, which would be the main reason for having the minimum value of dynamic viscosity by 60 min ultrasonication. The effects of ultrasonication on the distribution of NPs and viscosity measurement would be better understood by the aid of Fig. IV (under the supporting information section).
Effects of temperature and solid concentration on dynamic viscosity
After finding the optimum ultrasonication time in which the NF possesses the best stability and minimum dynamic viscosity, the dynamic viscosity of the MWCNT-water NF has been measured on different temperatures and solid concentrations, and at the ultrasonication time of 60 min. Figure 5 presents the variations of the dynamic viscosity with respect to temperature in the different studied solid concentrations. As can be seen, the dynamic viscosity showed a decreasing trend as the temperature started to rise. This trend is similar in all the solid concentrations. The main reason for this decrease would be that increasing the temperature leads to weakening the Van der Waals forces (inter-particles adhesion forces). It is interesting to note that the dynamic viscosity of the NF approached the dynamic viscosity of the base fluid in the temperatures higher than 45 °C. Moreover, it can be seen that increasing the solid concentration resulted in a slight increase in dynamic viscosity that is more noticeable in the temperatures of less than 40 °C. The maximum increase took place at the solid concentration of 0.5 vol% and the temperature of 25 °C by approximately 3%.
Effects of adding MWCNT on pressure loss and pumping power management
The effects of adding MWCNT to the base fluid and employing ultrasonication, in different solid concentrations and temperatures, on the pressure loss will be discussed and presented in this section. It is known that the pressure loss, which is due to viscous effects, can be calculated as follows52:
where ρV2avg/2 and f represent dynamic pressure and fanning friction factor, respectively. It must be noted that Eq. (1) is convenient to calculate the pressure loss for both the fully developed internal laminar and turbulent flow regimes52. The fanning friction factor can be calculated as follows52:
Moreover, ρnf can be calculated, as presented by Pak and Cho53, as follows:
where the indexes p and bf stand for particles and base fluid, respectively. It is known that adding NPs, which possess different density from the base fluid, to the base fluid affects the density of the resultant fluid (NF). Thus, in the present study, the density ratio is defined as the ratio of the NF’s density to that of the base fluid (water). Finally, investigating the effects of adding MWCNT to water on pressure loss, the fanning friction factor ratio (FFFR), which is the ratio of fanning friction factor of NF to that of the base fluid, is calculated for both the internal laminar and turbulent flow regimes54:
The variations of the density ratio with respect to temperature in different studied solid concentrations are presented in Fig. 6. It can be seen that the maximum increase in the density is just under 0.6%, which means that the increase in head loss and pumping power is negligible. Figure 7 presents the variations of FFFR with respect to temperature in different solid concentrations. As can be seen, adding MWCNT to water leads to increasing the FFFR in both the laminar and turbulent flow regimes. The maximum increase in FFFR for the laminar flow is just under 2.5%, while it is less than 0.7% for the turbulent flow regime. Moreover, increasing the temperature results in decreasing the FFFR for both the laminar and turbulent flow regimes. Thus, it can be concluded that using the MWCNT-water NF in the studied range of solid concentrations will not impose a considerable cost of pressure loss and pumping power.
Concluding remarks
In the present study, the effects of ultrasonication time on the stability, rheological properties, and pumping power management of MWCNT-water nanofluid have been studied over different ranges of temperature and solid concentrations. The stability of the prepared samples has been scrutinized, employing the visual observation method and zeta potential analysis throughout 30 days after preparation. It is found that the optimum ultrasonication time in which the samples showed good stability is 60 min, and prolonging the ultrasonication time leads to deteriorating the stability of the samples. After that, using a Brookfield cone and plate viscometer (CAP2000 +), the viscosity of the samples has been measured in different temperatures and solid concentrations. The rheological behavior of the nanofluid has been studied, and it is found that the prepared nanofluid is a Newtonian fluid. It is also revealed that adding nanoparticles to the base fluid results in increasing the dynamic viscosity of the base fluid. However, the increase in dynamic viscosity by increasing the solid concentration was negligible, and the maximum increase took place at the solid concentration of 0.5 vol% and temperature of 25 °C by just higher than 3%. It is also observed that the dynamic viscosity of the nanofluid approached the dynamic viscosity of the base fluid in the temperatures higher than 45 °C. The effects of adding MWCNT to the water on the pumping power management have also been investigated in both the laminar and turbulent flow regimes, and it is revealed that the nanofluid in the range of studied solid concentration will not impose a considerable cost for providing the required energy for pumping power. Thus, based on the dynamic viscosity of the studied nanofluid, which showed a negligible increase, it would be a good idea for future works to investigate the thermal and fluid dynamic performance of the nanofluid as well as the heat transfer performance in a practical application.
References
Choi J. A., S.U.S.; Eastman. Enhancing thermal conductivity of fluids with nanoparticles, ASME FED, 231: 99–103. In International Mechanical Engineering Congress and Exhibition, San Francisco, CA (United States) (1995).
Asadi, M., Asadi, A. & Aberoumand, S. An experimental and theoretical investigation on the effects of adding hybrid nanoparticles on heat transfer efficiency and pumping power of an oil-based nanofluid as a coolant fluid. Int. J. Refrig. 89, 83–92 (2018).
Alarifi, I. M., Alkouh, A. B., Ali, V., Nguyen, H. M. & Asadi, A. On the rheological properties of MWCNT-TiO2/oil hybrid nanofluid: an experimental investigation on the effects of shear rate, temperature, and solid concentration of nanoparticles. Powder Technol. 355, 157–162 (2019).
Hemmat Esfe, M., Saedodin, S., Asadi, A. & Karimipour, A. Thermal conductivity and viscosity of Mg(OH)2-ethylene glycol nanofluids. J. Thermal Anal. Calorimetry 120(2), 1145–1149 (2015).
Pourfattah, F., Abbasian Arani, A. A., Babaie, M. R., Nguyen, H. M. & Asadi, A. On the thermal characteristics of a manifold microchannel heat sink subjected to nanofluid using two-phase flow simulation. Int. J. Heat Mass Transf. 143, 118518 (2019).
Asadi, A. A guideline towards easing the decision-making process in selecting an effective nanofluid as a heat transfer fluid. Energy Convers. Manag. 175, 1–10 (2018).
Asadi, A., Asadi, M., Rezaniakolaei, A., Rosendahl, L. A. & Wongwises, S. An experimental and theoretical investigation on heat transfer capability of Mg (OH)2/MWCNT-engine oil hybrid nano-lubricant adopted as a coolant and lubricant fluid. Appl. Therm. Eng. 129, 577–586 (2018).
Hasan, H. A., Alquziweeni, Z. & Sopian, K. Heat transfer enhancement using nanofluids for cooling a central processing unit (CPU) system. J. Adv. Res. Fluid Mech. Thermal Sci. 51, 145–157 (2018).
Alshayji, A., Asadi, A. & Alarifi, I. M. On the heat transfer effectiveness and pumping power assessment of a diamond-water nanofluid based on thermophysical properties: an experimental study. Powder Technol. 373, 397–410 (2020).
Lyu, Z., Pourfattah, F., Arani, A. A. A., Asadi, A. & Foong, L. K. On the thermal performance of a fractal microchannel subjected to water and kerosene carbon nanotube nanofluid. Sci. Rep. 10(1), 1–16 (2020).
Lyu, Z., Asadi, A., Alarifi, I. M., Ali, V. & Foong, L. K. Thermal and fluid dynamics performance of MWCNT-water nanofluid based on thermophysical properties: an experimental and theoretical study. Sci. Rep. 10(1), 5185 (2020).
Alarifi, I. M., Nguyen, H. M., Naderi Bakhtiyari, A. & Asadi, A. Feasibility of ANFIS-PSO and ANFIS-GA models in predicting thermophysical properties of Al2O3-MWCNT/oil hybrid nanofluid. Materials 12(21), 3628 (2019).
Hemmat Esfe, M. et al. Applications of feedforward multilayer perceptron artificial neural networks and empirical correlation for prediction of thermal conductivity of Mg(OH)2-EG using experimental data. Int. Commun. Heat Mass Transf. 67, 46–50 (2015).
Moradikazerouni, A. et al. Assessment of thermal conductivity enhancement of nano-antifreeze containing single-walled carbon nanotubes: optimal artificial neural network and curve-fitting. Phys. A 521, 138–145 (2019).
Asadi, A., Alarifi, I. M., Nguyen, H. M. & Moayedi, H. Feasibility of least-square support vector machine in predicting the effects of shear rate on the rheological properties and pumping power of MWCNT–MgO/oil hybrid nanofluid based on experimental data. J. Therm. Anal. Calorim. (2020).
Asadi, A., Bakhtiyari, A. N. & Alarifi, I. M. Predictability evaluation of support vector regression methods for thermophysical properties, heat transfer performance, and pumping power estimation of MWCNT/ZnO–engine oil hybrid nanofluid. Eng. Comput. 1–11 (2020).
Bazdar, H., Toghraie, D., Pourfattah, F., Akbari, O. A., Nguyen, H. M. & Asadi, A. Numerical investigation of turbulent flow and heat transfer of nanofluid inside a wavy microchannel with different wavelengths. J. Therm. Anal. Calorim. 1–16 (2019).
Hadavand, M., Yousefzadeh, S., Ali Akbari, O., Pourfattah, F., Minh Nguyen, H. & Asadi, A. A numerical investigation on the effects of mixed convection of ag-water nanofluid inside a sim-circular lid-driven cavity on the temperature of an electronic silicon chip. Appl. Therm. Eng. 114298 (2019).
Liew, P. J., Shaaroni, A., Razak, J. A., Hadzley, M. & Bakar, A. Comparison between carbon nanofiber (cnf) nanofluid with deionized water on tool life and surface roughness in turning of D2 steel. J. Adv. Res. Fluid Mech. Therm. Sci. 46, 169–174 (2018).
Hilo, A. et al. Heat transfer and thermal conductivity enhancement using graphene nanofluid: a review. J. Adv. Res. Fluid Mech. Therm. Sci. 55, 74–87 (2019).
Pourfattah, F., Sabzpooshani, M., Bayer, Ö., Toghraie, D. & Asadi, A. On the optimization of a vertical twisted tape arrangement in a channel subjected to MWCNT–water nanofluid by coupling numerical simulation and genetic algorithm. J. Therm. Anal. Calorim. 1–13 (2020).
Bergmann, C. P., Sharma, V., Nor, K. & Hamid, H. B. Topics in mining, metallurgy and materials engineering series editor: engineering applications of nanotechnology from energy to drug delivery.
Asadi, A. & Pourfattah, F. Heat transfer performance of two oil-based nanofluids containing ZnO and MgO nanoparticles; a comparative experimental investigation. Powder Technol. 343, 296–308 (2019).
Asadi, M., Asadi, A. & Aberoumand, S. An experimental and theoretical investigation on the effects of adding hybrid nanoparticles on heat transfer efficiency and pumping power of an oil-based nanofluid as a coolant fluid. Int. J. Refrig. 89 (2018).
Kamalgharibi, M., Hormozi, F., Zamzamian, S. A. H. & Sarafraz, M. M. Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: influence of stirring, sonication and surface active agents. Heat Mass Transf. 52(1), 55–62 (2016).
Sarafraz, M. M., Hormozi, F. & Kamalgharibi, M. Sedimentation and convective boiling heat transfer of CuO-water/ethylene glycol nanofluids. Heat Mass Transf. 50(9), 1237–1249 (2014).
Mahbubul, I. M., Elcioglu, E. B., Saidur, R. & Amalina, M. A. Optimization of ultrasonication period for better dispersion and stability of TiO2–water nanofluid. Ultrason. Sonochem. 37, 360–367 (2017).
Kumar, P. G., Kumaresan, V. & Velraj, R. Stability, viscosity, thermal conductivity, and electrical conductivity enhancement of multi-walled carbon nanotube nanofluid using gum arabic. Fullerenes Nanotubes Carbon Nanostruct. 25(4), 230–240 (2017).
Asadi, A., Asadi, M., Siahmargoi, M., Asadi, T. & Gholami Andarati, M. The effect of surfactant and sonication time on the stability and thermal conductivity of water-based nanofluid containing Mg(OH)2 nanoparticles: An experimental investigation. Int. J. Heat Mass Transf. 108, 191–198 (2017).
Mahbubul, I. M., Saidur, R., Hepbasli, A. & Amalina, M. A. Experimental investigation of the relation between yield stress and ultrasonication period of nanofluid. Int. J. Heat Mass Transf. 93, 1169–1174 (2016).
Shah, J., Ranjan, M., Gupta, S. K. & Sonvane, Y. Ultrasonication effect on thermophysical properties of Al2O3 nanofluids. AIP Conf. Proc. 1951(1), 020008 (2018).
Sharifalhoseini, Z., Entezari, M. H. & Jalal, R. Direct and indirect sonication affect differently the microstructure and the morphology of ZnO nanoparticles: optical behavior and its antibacterial activity. Ultrason. Sonochem. 27, 466–473 (2015).
Ghadimi, A. & Metselaar, I. H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Thermal Fluid Sci. 51, 1–9 (2013).
Ruan, B. & Jacobi, A. M. Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res. Lett. 7(1), 127 (2012).
Mahbubul, I. M. et al. Effect of ultrasonication duration on colloidal structure and viscosity of alumina-water nanofluid. Ind. Eng. Chem. Res. 53(16), 6677–6684 (2014).
Mahbubul, I. M. et al. Experimental investigation on effect of ultrasonication duration on colloidal dispersion and thermophysical properties of alumina–water nanofluid. Int. J. Heat Mass Transf. 88, 73–81 (2015).
Shahsavar, A., Salimpour, M. R., Saghafian, M. & Shafii, M. B. An experimental study on the effect of ultrasonication on thermal conductivity of ferrofluid loaded with carbon nanotubes. Thermochim. Acta 617, 102–110 (2015).
Asadi, A., Alarifi, I. M., Ali, V. & Nguyen, H. M. An experimental investigation on the effects of ultrasonication time on stability and thermal conductivity of MWCNT-water nanofluid: finding the optimum ultrasonication time. Ultrason. Sonochem. 104639 (2019).
Yu, L., Bian, Y., Liu, Y. & Xu, X. Experimental investigation on rheological properties of water based nanofluids with low MWCNT concentrations. Int. J. Heat Mass Transf. 135, 175–185 (2019).
Hamid, K. A., Azmi, W. H., Nabil, M. F., Mamat, R. & Sharma, K. V. Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf. 116, 1143–1152 (2018).
Siddiqui, F. R., Tso, C. Y., Chan, K. C., Fu, S. C. & Chao, C. Y. H. On trade-off for dispersion stability and thermal transport of Cu-Al2O3 hybrid nanofluid for various mixing ratios. Int. J. Heat Mass Transf. 132, 1200–1216 (2019).
Asadi, A. et al. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: a comprehensive review. Ultrasonics Sonochem. 58 (2019).
ASHRAE Handbook-Fundamentals. Atlanta, GA (2009).
Wei, B., Zou, C. & Li, X. Experimental investigation on stability and thermal conductivity of diathermic oil based TiO2 nanofluids. Int. J. Heat Mass Transf. 104, 537–543 (2017).
Choudhary, R., Khurana, D., Kumar, A. & Subudhi, S. Stability analysis of Al2O3/water nanofluids. J. Exp. Nanosci. 12(1), 140–151 (2017).
Abbasi, S., Zebarjad, S. M., Baghban, S. H. N., Youssefi, A. & Ekrami-Kakhki, M. S. Experimental investigation of the rheological behavior and viscosity of decorated multi-walled carbon nanotubes with TiO2 nanoparticles/water nanofluids. J. Therm. Anal. Calorim. 123(1), 81–89 (2016).
Akhavan-Behabadi, M. A., Shahidi, M., Aligoodarz, M. R. & Fakoor-Pakdaman, M. An experimental investigation on rheological properties and heat transfer performance of MWCNT-water nanofluid flow inside vertical tubes. Appl. Therm. Eng. 106, 916–924 (2016).
Halelfadl, S., Maré, T., Estellé, P. & Mohd-Ghazali, N. Experimental investigation of Rheological behavior and pressure drop of aqueous suspensions of carbon nanotubes in a horizontal tube. Procedia Eng. 56, 344–349 (2013).
Chen, L., Xie, H., Yu, W. & Li, Y. Rheological behaviors of nanofluids containing multi-walled carbon nanotube. J. Dispersion Sci. Technol. 32(4), 550–554 (2011).
Afshari, A., Akbari, M., Toghraie, D. & Yazdi, M. E. Experimental investigation of rheological behavior of the hybrid nanofluid of MWCNT–alumina/water (80%)–ethylene-glycol (20%): new correlation and margin of deviation. J. Therm. Anal. Calorim. 132(2), 1001–1015 (2018).
Li, F., Li, L., Zhong, G., Zhai, Y. & Li, Z. Effects of ultrasonic time, size of aggregates and temperature on the stability and viscosity of Cu-ethylene glycol (EG) nanofluids. Int. J. Heat Mass Transf. 129, 278–286 (2019).
Çengel, Y. A. Fluid Mechanics (2000).
Pak, B. C. & Cho, Y. I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 11(2), 151–170 (1998).
Dardan, E., Afrand, M. & Meghdadi Isfahani, A. H. Effect of suspending hybrid nano-additives on rheological behavior of engine oil and pumping power. Appl. Therm. Eng. 109, 524–534 (2016).
Asadi, A. et al. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. (2019).
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Majmaah University for funding this work under project number No (RGP-2019-15).
Funding
The funding was provided by Majmaah University (MU) (Grant No. RGP-2019-15).
Author information
Authors and Affiliations
Contributions
All authors contributed to discussions and preparation of the manuscript, A.A. was responsible for performing the experiments and analyzing the data, and I.M.A. proposed the methodology of the research and led the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asadi, A., Alarifi, I.M. Effects of ultrasonication time on stability, dynamic viscosity, and pumping power management of MWCNT-water nanofluid: an experimental study. Sci Rep 10, 15182 (2020). https://doi.org/10.1038/s41598-020-71978-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71978-9
This article is cited by
-
An overview of fused filament fabrication technology and the advancement in PLA-biocomposites
The International Journal of Advanced Manufacturing Technology (2024)
-
A guide to the preparation techniques of six classes of metal-, metal oxide-, and carbon-based nanofluids and the implications for their stability
Journal of Thermal Analysis and Calorimetry (2023)
-
Exploring the Variation of Sonication Amplitude and Time Parameters on the Ultrasonic Disperse Dyeing of Polyacrylonitrile Fibers
Fibers and Polymers (2023)
-
Characterization of nanofluids using multifractal analysis of a liquid droplet trace
Scientific Reports (2022)
-
Examining rheological behavior of CeO2-GO-SA/10W40 ternary hybrid nanofluid based on experiments and COMBI/ANN/RSM modeling
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.