Abstract
The oceanic uptake of atmospheric carbon dioxide (CO2) emitted by human activities alters the seawater carbonate system. Here, the chemical status of the Northeast Atlantic is examined by means of a high-quality database of carbon variables based on the GO-SHIP A25 section (1997–2018). The increase of atmospheric CO2 leads to an increase in ocean anthropogenic carbon (Cant) and a decrease in carbonate that is unequivocal in the upper and mid-layers (0–2,500 m depth). In the mid-layer, the carbonate content in the Northeast Atlantic is maintained by the interplay between the northward spreading of recently conveyed Mediterranean Water with excess of carbonate and the arrival of subpolar-origin waters close to carbonate undersaturation. In this study we show a progression to undersaturation with respect to aragonite that could compromise the conservation of the habitats and ecosystem services developed by benthic marine calcifiers inhabiting that depth-range, such as the cold-water corals (CWC) communities. For each additional ppm in atmospheric pCO2 the waters surrounding CWC communities lose carbonate at a rate of − 0.17 ± 0.02 μmol kg−1 ppm−1. The accomplishment of global climate policies to limit global warming below 1.5–2 ℃ will avoid the exhaustion of excess carbonate in the Northeast Atlantic.
Similar content being viewed by others
Introduction
The uptake of anthropogenic CO2 by the ocean (Cant) creates a series of chemical changes known as ocean acidification1. The North Atlantic stores the largest amount of Cant in the ocean2,3, with the Atlantic Meridional Overturning Circulation (AMOC) conveying and exporting acidified Cant-loaded waters to the deep ocean4. At basin-scale, North Atlantic Ocean acidification is a well-known process5, usually reported with pH decrease rates of ~ 1–2 × 10–3 pH units yr−16,7,8,9. The reduction in the concentration of carbonate ions ([CO32−]) is also a consequence of ocean acidification10, usually expressed as the change in the calcium carbonate (CaCO3) saturation state. The availability of carbonate connects the chemistry of the seawater with the biological activity, since carbonate is used by calcifying organisms to create the different forms of biogenic CaCO3: aragonite (corals, pteropods) or calcite (coccolithophores, foraminifera). The difference between the in situ [CO32−] and the concentration at saturation is the excess of carbonate (xc[CO32−])10,11. Positive values of xc[CO32−] indicate supersaturated waters, while negative values indicate undersaturation and the tendency for the biogenic mineral to dissolve12,13. Ocean acidification decreases xc[CO32−], compromising the fitness of marine calcifiers and even their survival when waters reach negative values of xc[CO32−].
Cold-water corals (CWC) with biogenic CaCO3 skeletons made of aragonite14 are important ecosystem engineers of deep-sea habitats15,16. Ocean acidification is recognized as one of the most challenging threats that CWC will face with global change17. At global scale, large CWC reefs are more abundant in the North Atlantic, since the depth at which aragonite becomes susceptible of dissolution is deeper than elsewhere in the world ocean4,18,19. In the Northeast Atlantic, the relationship between reefs and hydrography is such that living CWC reefs are located in the potential density range 27.35 > σ0 > 27.65 kg m−320. Recently, several Marine Protected Areas (MPA) have been proposed in European waters based on the presence of Lophelia pertusa sp. communities21,22. An assessment of the long-term viability of these hotspots of biodiversity and ecosystem services14 in the context of a changing ocean is therefore necessary.
This study relies on repeated marine chemical surveys across the A25 section of the Global Ocean Ship-Based Hydrographic Investigations Program (GO-SHIP, https://www.go-ship.org) to explore the implications of ocean acidification for marine calcifiers from a biogeochemical perspective (Fig. 1). The separation between natural and anthropogenic carbon allows the identification of ocean acidification trends driven by atmospheric CO2 increase. Although xc[CO32−] decrease rates are scarce in the current literature of ocean acidification23, the use of this variable is particularly relevant to assess quantitatively the chemical conditions to which marine calcifiers are exposed. The current and future xc[CO32−] for the main water masses of the Northeast Atlantic and for the waters where current living communities of CWC exist are reported.
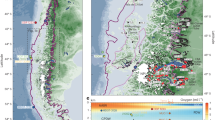
Map of the Northeast Atlantic zone of study close to the Iberian Peninsula. The location of the OVIDE (FOUREX) stations used in this study are represented with orange (red) diamonds (circles). The FOUREX section was carried out in 1997 and the OVIDE section has nine repeats, biennially from 2002 to 2018. The Azores-Biscay Ridge is the northern limit of the measurements used in this study. The locations with reported CWC64 communities where Lophelia pertusa is present are represented with stars. The stars are coloured according to the depth of the CWC location (see legend in Figure). A schematic diagram of the large-scale circulation of the main water masses is also shown: North Atlantic Central Water (NACW), Mediterranean Water (MW) and Labrador Sea Water (LSW), adapted from32,65.
Results
The vertical distribution of xc[CO32−] along the OVIDE cruise section for the year 2018 is shown in Fig. 2. The xc[CO32−] values decrease with depth, from supersaturated (xc[CO32−] > 0) surface waters with more than 100 μmol kg−1 of xc[CO32−], towards abyssal values of 50 μmol kg−1 below saturation (xc[CO32−] < 0). At mid-depths there are larger xc[CO32−] values close to the Iberian Peninsula, creating a gradual eastward upward tilt of the isolines. From 3,000 m depth to the bottom, the isolines are horizontal. The isoline of 0 μmol kg−1 of xc[CO32−], known as the Aragonite Saturation Horizon (ASH, red dashed line in Fig. 2), is around 2,500 m depth. With respect to the water masses distribution (Table 1), NACW occupies the top layer down to 700 m at the westward end of the section (that is also the northernmost station, Fig. 1) and to around 500 m close to the Iberian Peninsula. Below, MW extends down to about 1,500 m, encompassing entirely the layer where CWC inhabit between 600 and 1,000 m depth range. Beneath MW, LSW reaches 2,500 m. The lower limit of LSW (σ2 = 37.00 kg m−3) and the ASH are pretty similar. The limit between the upper and lower NADW is around 3,800 m depth, where waters are already undersaturated (xc[CO32−] < 0).
Vertical distribution of excess carbonate with respect to aragonite saturation for the year 2018 at the OVIDE section south of Azores-Biscay Ridge. The plot is the vertical distribution (m) between surface and bottom (maximum depth around 5,500 m at longitude 14° W). The orange dash line is the 50 μmol kg−1 isoline and the red dash line is the Aragonite Saturation Horizon (ASH, 0 μmol kg−1). The water masses are separated by black lines of potential density according to the layer separation (acronyms and density values are detailed in Table 1). The layer of living cold-water corals (CWC) is represented within the green isopycnals. Note that the depth-scale is not linear.
The total dissolved inorganic carbon (DIC) mean concentration by water mass (Fig. 3a) shows that the upper layer (NACW) has the lowest values and the bottom layer (lNADW) the largest. In the ocean, the amount of DIC usually increases with depth, but in the Northeast Atlantic the MW influence modifies such vertical distribution. The mid-layer MW has a mean DIC concentration higher than the underlying LSW and very similar to uNADW. The concentration of atmospheric CO2 for the period 1997–2018 increased from 364 to 409 ppm (Fig. 3), with an annual mean increase larger than 2 ppm yr−1. For DIC, the increase trend with increasing atmospheric CO2 concentration is only found in the uppermost water mass, NACW (results of statistical hypothesis test in Supplementary information Table S1).
Mean layer concentration trends of dissolved inorganic carbon components in Northeast Atlantic water masses versus atmospheric CO2 concentration. Mean layer (a) total DIC concentration (μmol kg−1) and its separation into (b) natural (DICnat, μmol kg−1) and (c) anthropogenic (Cant, μmol kg−1) components versus atmospheric CO2 concentration (ppm) for the main water masses in the Northeast Atlantic: NACW (cyan), MW (orange), LSW (purple) and upper and lower NADW (pink and light green). Only linear trends (μmol kg−1 ppm−1) with a statistical p-value < 0.001 (Supplementary Information Table S1) have been depicted, and the grey shading accounts for the trend errors. Uncertainties in the mean properties are two times the standard error of the mean (i.e., 95% confidence interval). The year of the cruise is represented in the upper x-axis.
DIC includes the natural (DICnat) and the anthropogenic (Cant) components. DICnat (Fig. 3b) shows a more monotonous increase from surface to bottom than DIC, although DICnat concentrations for MW and LSW are very similar. In addition, contrary to the total DIC, DICnat does not show a trend with increasing atmospheric CO2 in any layer. When Cant is analyzed (Fig. 3c), its vertical distribution shows that the surface-most NACW has the highest concentration, which decreases until values slightly above zero in the deeper layers. The increase in Cant with increasing atmospheric CO2 is unequivocal in the upper and mid-layers (Supplementary information Table S1), but not for NADW layers. As expected from direct air-sea CO2 exchange, the largest increase in Cant occurs in the surface-most NACW layer (0.38 ± 0.02 μmol kg−1 ppm−1), with concentrations exceeding 60 μmol kg−1 by the end of the observational period. MW and LSW mid-layers show rather similar increase in Cant concentration rates (0.15 ± 0.02 μmol kg−1 ppm−1 and 0.09 ± 0.01 μmol kg−1 ppm−1, respectively). Note that when time (in years) is used as dependent variable (Fig. S1), the results remain consistent. The vertical distributions of DIC, Cant and DICnat along the OVIDE cruise section for the year 2018 are shown in Fig. S2.
The vertical distributions of total alkalinity (TA) and TA normalized by salinity (S = 35) along the OVIDE cruise section for the year 2018 are shown in Figure S3. The TA mean concentration by water mass does not show any trend with increasing atmospheric CO2 concentration (Fig. S3a). The variation of TA is highly correlated with salinity. The distribution of TA normalized (Fig. S3b) shows a steady increase towards the bottom that is due to CaCO3 dissolution but has a very low effect on [CO32−]24.
The trends of the mean xc[CO32−] by water mass and associated uncertainties with respect to the atmospheric CO2 concentration for 1997–2018 are represented in Fig. 4. The pattern of xc[CO32−] values between water masses follows a vertical distribution, with positive xc[CO32−] values at the surface and negative values in deep waters. The xc[CO32−] decreases with the excess of atmospheric CO2 in the upper (NACW, − 0.27 ± 0.05 μmol kg−1 ppm−1) and mid-layers (MW − 0.18 ± 0.02 μmol kg−1 ppm−1, and LSW − 0.10 ± 0.01 μmol kg−1 ppm−1) of the Northeast Atlantic. Such decrease in xc[CO32−] does not exist in the two layers of NADW (p-values of 0.13 and 0.29 for upper and lower NADW, respectively). The rate of decrease in xc[CO32−] is almost three times larger at surface than in LSW, with its physicochemical properties and its position in the water column making LSW to be close to the limit of no excess. If these trends persist in time, LSW will become undersatured in the Northeast Atlantic when a concentration of 514 ± 31 ppm of atmospheric CO2 is reached.
Mean water mass xc[CO32−] (μmol kg−1) versus atmospheric CO2 concentration (ppm) in the Northeast Atlantic: NACW (cyan), MW (orange), LSW (purple) and upper and lower NADW (pink and light green). Only linear trends (μmol kg−1 ppm−1) with a statistical p-value < 0.001 have been depicted (Supplementary Information Table S1), and the grey shading accounts for the trend errors. Uncertainties in the mean properties are two times the standard error of the mean (i.e., 95% confidence interval). The year of the cruise is represented in the upper x-axis. The light red zone below the limit of 0 xc[CO32−] represents undersaturated waters with respect to aragonite.
In the depth range of living CWC, the change in xc[CO32−] with increasing atmospheric CO2 shows a decrease from 56 to less than 50 μmol kg−1 during the observing time frame (inset in Fig. 5). The rate of decrease is − 0.17 ± 0.02 μmol kg−1 ppm−1 (p-value = 4.3 × 10–5). Conserving this linear trend, the current layer of living CWC in the Northeast Atlantic would then be in undersaturated waters at a concentration of 702 ± 53 ppm of atmospheric CO2 (Fig. 5). The nonlinearity between ocean carbon variables requires a thermodynamical approach to infer distant projections10. However, within a thermodynamic equilibrium, the layer of living CWC approximately follows the linear trend (Fig. 5). The large loss of linearity occurs below the undersaturation level (xc[CO32−] < 0), so the linear trend is a valid approach for the study purposes.
Mean xc[CO32−] (μmol kg−1) versus atmospheric CO2 concentration (ppm) for the living cold-water coral (CWC) layer (σ0 = 27.35–27.65 kg m−3) observed (inset) and predicted. The inset shows the observed measurements and the grey shading accounts for the trend errors. The linear trend in the inset (green line, μmol kg−1 ppm−1) has a statistical p-value = 4.3 × 10–5. In the inset, the uncertainties in the mean properties are two times the standard error of the mean (i.e., 95% confidence interval), and the year of the cruise is represented in the upper x-axis. The predictions (dashed lines) are based on the linear rate of decrease for the observational period (green) and on the thermodynamic equilibrium trend (blue, Orr et al.10).
Discussion
In the huge pool of inorganic carbon existing in the ocean, the anthropogenic component represents a relatively small fraction of it (< 4%)2. Current anthropogenic perturbation in NACW layer (< 600 m depth, approximately) is large enough to be detected in the DIC pool within a 21-year timespan (1997–2018) (Fig. 3a). The detection of trends in DIC, however, may require longer time series if the measurements are not normalized by salinity6 or alkalinity. In the Northeast Atlantic, the increase in DIC is not mediated by a change in total alkalinity (Fig. S3). And even without salinity normalization, the large anthropogenic perturbation, combined with the fact that all measurements were made in the same season (summer), favors the detection of trends5. This 21-year time-scale agrees well with the 14-year period required to detect the emergence of the anthropogenic signal from natural variability in another carbon variable, the surface pH25. In contrast, the natural DIC shows no change at any layer (Fig. 3b, Supplementary Information Table S1) suggesting that, within this approach, we cannot discard the steady-state of the natural carbon cycle in the Northeast Atlantic. Nevertheless, the capability of current anthropogenic carbon methods to discern changes in the natural DIC is still a debated subject26 that is out of the scope of the present study. For the Cant, increasing trends are unequivocally observed not only in the upper NACW layer but also in the mid-layer water masses, i.e., until 2,500 m depth. There is a consistent increase in Cant in the upper and mid-layers of the Northeast Atlantic that responds to the atmospheric CO2 increase over time. These results agree, and update by more than a decade, the previously reported Cant trends in the Northeast Atlantic27. The Cant increase in the NACW layer is among the largest trends reported in the literature9,27, in agreement with the view that the subtropical areas are the places where the upper water masses increase its Cant burden3,28,29,30. South of the study zone, in the Gulf of Cadiz region (Fig. 2), the downwelling of central waters with high Cant concentrations and the subsequent formation of MW exports Cant to the Northeast Atlantic31. This convection of the anthropic signal at mid-depths is then advected, spreading acidified waters to the North Atlantic. Although the anthropogenic influence has already reached the old water masses (NADW) of the Iberian Abyssal Plain, a longer monitoring period is required to identify it. In summary, the combination of the natural component of DIC in steady-state with the unequivocal trends for Cant in NACW, MW and LSW, makes it reasonable to assume that the existence of perturbations in other marine carbonate system variables are also produced by human influence, as seen for pH, CO3 (Fig. S4, Table S1) and xc[CO32−] (Fig. 4). The latter is the variable that we are going to consider hereafter.
xc[CO32−] decreases with depth due to solubility (high pressure and low temperature increases aragonite solubility). The anthropogenic decline of xc[CO32−] exists in all the supersaturated water masses, that is, NACW, MW and LSW. The decline is happening three times faster in NACW than in LSW, as expected from the atmospheric source of the driver for ocean acidification (atmospheric CO2) and the age of these water masses9. The relatively young and recently formed NACW has the largest trend, whereas LSW, ventilated in western subpolar latitudes, reaches the Northeast Atlantic basin with a greater mean age and hence has the lowest trend among the acidified water masses. MW, formed at the southeast of the Iberian Peninsula (Gulf of Cadiz, Fig. 2) by mixing of NACW, Mediterranean Outflow Water (MOW) and a diluted form of Antarctic Intermediate Water32,33, shows intermediate trends. At the latitudes of the measurements, the layer with living CWC is comprised within the MW layer, between 600 and 1,000 m depth. Therefore, the living CWC are in waters with excess of available carbonate, in agreement with the idea that suitable habitats for CWC development are supersaturated for aragonite18. MOW contributes for around a third (~ 34%) in the composition of MW32. Due its intrinsic properties of high salinity and alkalinity34,35, MW is therefore crucial to keep the Northeast Atlantic CWC in chemically optimal waters. In the Gulf of Cadiz, the ocean acidification rates in MW are relatively high36. Currently, living CWC are located between the fast-acidifying surface waters from above and the rising ASH from below. A potential reduction in MOW by global change37 would involve a greater influence of subpolar origin waters that could accelerate the exhaustion of excess carbonate in the Northeast Atlantic.
Reaching undersaturation for aragonite in the upper and mid-layers of the Northeast Atlantic would take less than a century in worst scenarios (SSP5.85, SSP4.60 and SSP3.70, Fig. 6). Fossil-fueled development pathway SSP5.85 is similar to that projected by the previous IPCC business-as-usual scenario (RCP8.5)38. The scenarios that best represent the limitation of global warming to 1.5 or 2 °C of the Paris Agreement are the SSP1-19 and the SSP1-26 respectively39. For both scenarios, the decline of xc[CO32−] will not reach undersaturation values not even in LSW layer (Fig. 6). Therefore, if the CO2 emission targets for limiting global warming to 1.5 or 2 ℃ are accomplished, the Northeast Atlantic will remain in chemically optimal conditions for CWC communities the following century. However, if the atmospheric CO2 concentration reached 700 ppm, then the living CWC communities in the Northeast Atlantic would be exposed to waters that are chemically hostile to their carbonate structures. Our projection based in measurements is in agreement with previous model forecasts10,18.
Projections of atmospheric CO2 concentration (ppm) versus time (years) as modelled by the eight Shared Socioeconomic pathways (SSPs) considered. Atmospheric CO2 data from39. The horizontal dashed lines are the predicted atmospheric concentration at the moment of undersaturation for each layer. The predicted uncertainty for the LSW and CWC layers is the 95% confidence interval at the moment of undersaturation for the projected mean properties linear trends; and it is represented with the light-colored horizontal band. For specific pathways, the expected year of reaching undersaturation along with their uncertainty is included in the upper axis.
Many species of benthic calcifiers, even CWC other than Lophelia pertusa, inhabit undersaturated seawaters40,41. Although living CWC can have net calcification in undersaturated conditions42,43, they do it at expenses of stored energy reserves44. Three-dimensional CWC reefs structures are composed by an important fraction (> 70%) of dead corals framework45. In contrast to the living CWC, the dissolution of the dead skeletons is a predicted chemical reaction46 because the skeletons have no capacity to cope with dissolution. The net dissolution will be directly proportional to the time CWC remain in undersaturated waters and that could happen within a century if the Paris Agreement is not accomplished. To the best of our knowledge, there are no long-term in situ experiments that confirm net dissolution of dead CWC framework available at this time. However, the combination of bioerosion proceeding faster in substrates weakened by ocean acidification47 with the close relationship between carbonate availability and the location of healthy CWC communities4,18,19 suggests that CWC development might be compromised. Furthermore, the decrease in available carbonate saturation level may combine with, and potentially exacerbated by, other climate change pressures such as warming and deoxygenation48,49.
Conclusions
The uptake of anthropogenic carbon from the atmosphere is unequivocally decreasing the amount of carbonate available for marine calcifiers in the upper (NACW) and intermediate water masses (MW and LSW) of the Northeast Atlantic Ocean. The northward spreading of MW plays a key role in limiting the arrival of subpolar-origin waters with low excess of carbonate available. The chemical conditions that made the Northeast Atlantic a region favorable for CWC development in preindustrial time are changing fast due to ocean acidification. Currently, provided the water masses contribution remain the same, living CWC would inhabit undersaturated waters when the atmospheric CO2 concentration reaches 702 ± 57 ppm. If greenhouse gas emissions maintain the path of fossil-fuel development, the running out of excess carbonate in the Northeast Atlantic will take place during this century. The efficiency of high seas Marine Protected Areas (MPA) created for the long-term conservation of CWC and their associated ecosystems is ultimately associated with the accomplishment of the Paris Agreement and the limitation of global warming below 2 ℃.
Materials and methods
Two-decades of ocean acidification trends across the whole water column of the Northeast Atlantic are evaluated by means of high-quality CO2 measurements. The observations came from ten hydrographic cruises across the Iberian Abyssal Plain between 1997 and 2018 (Fig. 2): the 1997 FOUREX cruise (CLIVAR Carbon Hydrographic Data Office site, https://cchdo.ucsd.edu/cruise/74DI230_1) and 9 repeats of the OVIDE section (OVIDE group, 2020). They are almost evenly spaced in time since all cruises except the first one belong to the OVIDE sampling program, a high-resolution hydrographic survey that has been carried out every other year during spring–summer since 2002 (https://www.umr-lops.fr/en/Projets/Projets-actifs/OVIDE). The assembled high-quality CO2 system database between the Azores Biscay Ridge (45° N, 18° W) and the Iberian Peninsula therefore spans 21 years (1997–2018; Fig. 2) and the whole-water column (Fig. 1). Note the cruise track is identical for the nine repetitions of the OVIDE section, but it is slightly southern for the 1997 cruise. Both coast-to-coast hydrographic sections belong to GO-SHIP (Global Ocean Ship-Based Hydrographic Investigations Program), which is part of the global ocean/climate observing systems (GOOS/GCOS)50. Note here we are analyzing their easternmost part (< 18° W) exclusively.
Measured carbon variables: pH and total alkalinity
In this study, the carbon analysis for all the hydrographic data involved followed the same analytical methodology and were in situ calibrated against Certified Reference Materials (CRMs).
pH was determined with a spectrophotometric method51. The protocols of measurements developed and followed during the cruises, including periodical CRM checks, allowed to achieve an internal consistency and reproducibility of ± 0.0014 pH units9,27. The large amount of measurements from the deeper waters sampled at the Iberian Abyssal Plain (n = 1,633) show a very low standard deviation (7.9146 ± 0.0006 pH at in situ conditions of temperature and pressure in the total scale), which is a useful indicator of consistency and reproducibility since these old waters are expected to be in near steady state9. Total alkalinity (TA) was analyzed by single point titration52 and calibrated with CRM with a measurement precision of ± 2 μmol kg−1. Over 3,200 samples of TA were analyzed for this study, with around three hundred per cruise for the stations shown in Fig. 2. The amount of total alkalinity measurements with WOCE flags values different from “Acceptable” is less than 1%. The datasets were subject to primary and secondary quality control procedures53 consistent with the GLODAP data products54,55 and neither carbon related variables (pH and total alkalinity) nor oxygen have been modified at the secondary quality control procedure55. Then, results are supported by high-quality and low-uncertainty carbon measurements spanning 21 years.
Computed carbon variables
Concentrations of dissolved inorganic carbon (DIC) and in situ carbonate ions ([CO32−]is) were calculated with the CO2SYS toolbox56 using the acid dissociation constants of Mehrbach et al.57 refitted by Dickson and Millero58, and the aragonite solubility of Mucci59. The uncertainty in the concentrations is ± 4.6 μmol kg−1 and ± 3.7 μmol kg−1 for DIC and [CO32−]is, respectively60.
The in situ degree of aragonite saturation (ΩArg) is the product of the ion concentrations of calcium ([Ca2+]) and carbonate ([CO32−]) divided by the aragonite solubility product (KArg) at in situ conditions (subscript “is”) of temperature, salinity, and pressure12. Following the ΩArg definition:
we can infer [CO32−] at saturation (i.e., when ΩArg equals one):
where the concentration of the conservative ion [Ca2+] is determined only by salinity61. Both KArg and [Ca2+] were calculated with the CO2SYS toolbox56. The difference between [CO32−]is and [CO32−]sat(ΩArg = 1) (Eq. 2) is the excess of carbonate ion concentration over aragonite saturation: xc[CO32−]4,11. Note that an alternative estimate is the xc[CO32−] over calcite saturation, computed with the degree of calcite saturation (ΩCalcite). Since calcite is more resistant to dissolution than aragonite11, calcifying organisms that form their structures with calcite will be less affected by ocean acidification at shorter time-scales62. Therefore, in this work, we refer exclusively to excess carbonate and the saturation state with respect to aragonite.
Finally, anthropogenic carbon (Cant) was estimated with the biogeochemical back-calculation ϕCT° method63, which has an overall uncertainty of ± 5.2 μmol kg−1. The natural fraction in the total DIC (DICnat) is the difference between DIC and Cant.
Layer separation
The whole-water column was separated following a vertical water mass distribution into five layers delimited by potential density (σrefpressure) isopycnals (Table 1, Fig. 1), following previous studies in the area8,27. The reference pressure level for the isopycnals (refpressure) varies among 0, 1, 2 and 4 (× 103 dbar).
In addition, we also delimited the range of water column where living CWC exist in the Northeast Atlantic20, i.e. 27.35 ≤ σ0 ≤ 27.65 kg m−3 (Fig. 1).
For each layer, the mean property concentrations (xc[CO32−], DIC, DICnat, Cant, TA pHisT and [CO32−]is) were based on interpolated bottle data at dbar resolution. Interpolation was done on an area-weighted basis, considering the thickness of the layer and the distance between measurements9. The mean properties are represented along with the 95% confidence interval (error bars are two times the standard error of the mean, 2x(std/√n); where n is the number of bottle measurements in that layer and cruise, Figs. 3, 4, 5).
Atmospheric CO2: past concentrations and shared socioeconomic pathways projections
In this study we contrasted the anthropogenic changes in the ocean carbon cycle with respect to its main driver, the atmospheric CO2 concentration, in contrast to previous studies in the region using time as dependent variable8,9. Note that this methodology develops rates with respect to atmospheric CO2 rather than time, so the units of the rates are μmol kg−1 ppm−1. Atmospheric CO2 concentration is a more meaningful variable than time, since it allows to make projections directly based on greenhouse gas concentrations resulting from different policy decisions and socioeconomic pathways. Past atmospheric CO2 concentrations were taken from the Mauna Loa database (https://www.esrl.noaa.gov/gmd/ccgg/trends/) [last time accessed: 28/12/2019]. In this study we used the mean annual concentration of CO2 (in parts per million of volume, p.p.m.) for the year of the cruise. Shared Socioeconomic Pathways (SSP) are climate scenario frameworks that take into account a wide range of socio-economic futures and policy decisions38. Atmospheric CO2 concentrations for eight representative SSP scenarios belonging to the Coupled Model Intercomparison Project phase 6 (CMIP6) were downloaded from https://greenhousegases.science.unimelb.edu.au [last time accessed: 28/12/2019]39. This data is used in the atmospheric CO2 concentration projections.
Data availability
Data were collected and made publicly available by the International Global Ship-based Hydrographic Investigations Program (GO-SHIP; https://www.go-ship.org/) and the national programs that contribute to it. Global Distribution of Cold-water Corals version 5.0 (June 2018) is distributed under UNEP-WCMC's General Data License (excluding WDPA). URL: https://data.unep-wcmc.org/datasets/3.
References
Caldeira, K. & Wickett, M. E. Anthropogenic carbon and ocean pH. Nature 425, 365 (2003).
Khatiwala, S. et al. Global ocean storage of anthropogenic carbon. Biogeosciences 10, 2169–2191 (2013).
Gruber, N. et al. The oceanic sink for anthropogenic CO2 from 1994 to 2007. Science 363, 1193–1199 (2019).
Perez, F. F. et al. Meridional overturning circulation conveys fast acidification to the deep Atlantic Ocean. Nature 554, 515–518 (2018).
Bates, N. R. et al. A time-series view of changing surface ocean chemistry due to ocean uptake of anthropogenic CO2 and ocean acidification. Oceanography https://doi.org/10.5670/oceanog.2014.16 (2014).
Santana-Casiano, J. M., González-Dávila, M., Rueda, M. J., Llinás, O. & González-Dávila, E. F. The interannual variability of oceanic CO2 parameters in the northeast Atlantic subtropical gyre at the ESTOC site. Glob. Biogeochem. Cycles 21, 1–16 (2007).
Olafsson, J. et al. Rate of Iceland Sea acidification from time series measurements. Biogeosciences 6, 2661–2668 (2009).
Vázquez-Rodríguez, M., Pérez, F. F., Velo, A., Ríos, A. F. & Mercier, H. Observed acidification trends in North Atlantic water masses. Biogeosciences 9, 5217–5230 (2012).
García-Ibáñez, M. I. et al. Ocean acidification in the subpolar North Atlantic: Rates and mechanisms controlling pH changes. Biogeosciences 13, 3701–3715 (2016).
Orr, J. C. et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (2005).
Broecker, W. S. & Peng, T. H. Tracers in the Sea. (1983). https://doi.org/10.1016/0016-7037(83)90075-3
Feely, R. A., Byrne, R. H., Betzer, P. R., Gendron, J. F. & Acker, J. G. Factors influencing the degree of saturation of the surface and intermediate waters of the North Pacific ocean with respect to aragonite. J. Geophys. Res. 89, 631–640 (1984).
Subhas, A. V. et al. A novel determination of calcite dissolution kinetics in seawater. Geochim. Cosmochim. Acta https://doi.org/10.1016/j.gca.2015.08.011 (2015).
Roberts, J. M., Wheeler, A. J., Freiwald, A. & Cairns, S. D. Cold-water corals: The biology and geology of deep-sea coral habitats. Cold-Water Corals Biol. Geol. Deep-Sea Coral Habitats https://doi.org/10.1017/CBO9780511581588 (2009).
Jones, C. G., Lawron, J. H. & Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78, 1946–1957 (1997).
Hennige, S. J. et al. Self-recognition in corals facilitates deep-sea habitat engineering. Sci. Rep. 4, 1–7 (2014).
Roberts, J. M. et al. Cold-water corals in an era of rapid global change: Are these the deep ocean’s most vulnerable ecosystems? in The Cnidaria, Past, Present and Future (2016). https://doi.org/10.1007/978-3-319-31305-4_36
Guinotte, J. M. et al. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals?. Front. Ecol. Environ. 4, 141–146 (2006).
Davies, A. J., Wisshak, M., Orr, J. C. & Murray Roberts, J. Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia). Deep. Res. Part I Oceanogr. Res. Pap. 55, 1048–1062 (2008).
Dullo, W. C., Flögel, S. & Rüggeberg, A. Cold-water coral growth in relation to the hydrography of the Celtic and Nordic European continental margin. Mar. Ecol. Prog. Ser. 371, 165–176 (2008).
Sánchez, F. et al. Habitat characterization of deep-water coral reefs in La Gaviera Canyon (Avilés Canyon System, Cantabrian Sea). Deep. Res. Part II Top. Stud. Oceanogr. 106, 118–140 (2014).
van den Beld, I. M. J. et al. Cold-water coral habitats in submarine canyons of the Bay of Biscay. Front. Mar. Sci. 4, 118 (2017).
Mostofa, K. M. G. et al. Reviews and Syntheses: Ocean acidification and its potential impacts on marine ecosystems. Biogeosciences https://doi.org/10.5194/bg-13-1767-2016 (2016).
Milliman, J. D. Production and accumulation of calcium carbonate in the ocean: Budget of a nonsteady state. Glob. Biogeochem. Cycles https://doi.org/10.1029/93GB02524 (1993).
Henson, S. A. et al. Rapid emergence of climate change in environmental drivers of marine ecosystems. Nat. Commun. 8, 1–9 (2017).
Clement, D. & Gruber, N. The eMLR(C*) method to determine decadal changes in the global ocean storage of anthropogenic CO2. Glob. Biogeochem. Cycles https://doi.org/10.1002/2017GB005819 (2018).
Pérez, F. F. et al. Trends of anthropogenic CO2 storage in North Atlantic water masses. Biogeosciences 7, 1789–1807 (2010).
Pérez, F. F. et al. Atlantic Ocean CO2 uptake reduced by weakening of the meridional overturning circulation. Nat. Geosci. 6, 146–152 (2013).
Zunino, P. et al. Dissolved inorganic carbon budgets in the eastern subpolar North Atlantic in the 2000s from in situ data. Geophys. Res. Lett. 42, 9853–9861 (2015).
Guallart, E. F. et al. Trends in anthropogenic CO2 in water masses of the Subtropical North Atlantic Ocean. Prog. Oceanogr. 131, 21–32 (2015).
Carracedo, L. I. et al. Role of the circulation on the anthropogenic CO2 inventory in the North-East Atlantic: A climatological analysis. Prog. Oceanogr. 161, 78–86 (2018).
Carracedo, L. I., Pardo, P. C., Flecha, S. & Pérez, F. F. On the mediterranean water composition. J. Phys. Oceanogr. 46, 1339–1358 (2016).
Ríos, A. F., Pérez, F. F. & Fraga, F. Long-term (1977–1997) measurements of carbon dioxide in the Eastern North Atlantic: Evaluation of anthropogenic input. Deep. Res. Part II Top. Stud. Oceanogr. https://doi.org/10.1016/S0967-0645(00)00182-X (2001).
García-Lafuente, J., Sánchez-Román, A., Naranjo, C. & Snchez-Garrido, J. C. The very first transformation of the Mediterranean outflow in the Strait of Gibraltar. J. Geophys. Res. Ocean. https://doi.org/10.1029/2011JC006967 (2011).
Pérez, F. F., Álvarez, M. & Ríos, A. F. Improvements on the back calculation technique for estimating anthropogenic CO2. Deep. Res. Part I Oceanogr. Res. Pap. https://doi.org/10.1016/S0967-0637(02)00002-X (2002).
Huertas, I. E. et al. Anthropogenic and natural CO2 exchange through the strait of gibraltar. Biogeosciences 6, 647–662 (2009).
Thorpe, R. B. & Bigg, G. R. Modelling the sensitivity of Mediterranean Outflow to anthropogenically forced climate change. Clim. Dyn. https://doi.org/10.1007/s003820050333 (2000).
Kriegler, E. et al. Fossil-fueled development (SSP5): An energy and resource intensive scenario for the 21st century. Glob. Environ. Chang. 42, 297–315 (2017).
Meinshausen, M. et al. The SSP greenhouse gas concentrations and their extensions to 2500. (2019).
Lebrato, M. et al. Benthic marine calcifiers coexist with CaCO3-undersaturated seawater worldwide. Glob. Biogeochem. Cycles https://doi.org/10.1002/2015GB005260 (2016).
Baco, A. R. et al. Defying dissolution: Discovery of deep-sea scleractinian coral reefs in the North Pacific. Sci. Rep. https://doi.org/10.1038/s41598-017-05492-w (2017).
Form, A. U. & Riebesell, U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Chang. Biol. 18, 843–853 (2012).
Maier, C., Hegeman, J., Weinbauer, M. G. & Gattuso, J. P. Calcification of the cold-water coral Lophelia pertusa under ambient and reduced pH. Biogeosciences 6, 1671–1680 (2009).
Hennige, S. J. et al. Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to ocean acidification. Deep. Res. Part II Top. Stud. Oceanogr. 99, 27–35 (2014).
Vad, J., Orejas, C., Moreno-Navas, J., Findlay, H. S. & Roberts, J. M. Assessing the living and dead proportions of cold-water coral colonies: Implications for deep-water Marine Protected Area monitoring in a changing ocean. PeerJ 2017, 1–20 (2017).
Hennige, S. J. et al. Hidden impacts of ocean acidification to live and dead coral framework. Proc. R. Soc. B Biol. Sci. 282, 20150990 (2015).
Schönberg, C. H. L., Fang, J. K. H., Carreiro-Silva, M., Tribollet, A. & Wisshak, M. Bioerosion: The other ocean acidification problem. ICES J. Mar. Sci. https://doi.org/10.1093/icesjms/fsw254 (2017).
Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. Science https://doi.org/10.1126/science.aam7240 (2018).
Durack, P. J. et al. Ocean warming from the surface to the deep in observations and models. Oceanography 31, 41–51 (2018).
Sloyan, B. M. et al. The global ocean ship-base hydrographic investigations program (GO-SHIP): A platform for integrated multidisciplinary ocean science. Front. Mar. Sci. 6, 445 (2019).
Clayton, T. D. & Byrne, R. H. Spectrophotometric seawater pH measurements: Total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep. Res. Part I 40, 2115–2129 (1993).
Perez, F. F. & Fraga, F. A precise and rapid analytical procedure for alkalinity determination. Mar. Chem. https://doi.org/10.1016/0304-4203(87)90037-5 (1987).
Velo, A. et al. CARINA data synthesis project: PH data scale unification and cruise adjustments. Earth Syst. Sci. Data https://doi.org/10.5194/essd-2-133-2010 (2010).
Olsen, A. et al. The global ocean data analysis project version 2 (GLODAPv2)—An internally consistent data product for the world ocean. Earth Syst. Sci. Data https://doi.org/10.5194/essd-8-297-2016 (2016).
Olsen, A. et al. GLODAPv2.2019: An update of GLODAPv2. Earth Syst. Sci. Data Discuss. https://doi.org/10.5194/essd-2019-66 (2019).
Van Heuven, S., Pierrot, D., Rae, J. W. B., Lewis, E. & Wallace, D. W. R. CO2SYS v 1.1, MATLAB program developed for CO2 system calculations. ORNL/CDIAC-105b. Carbon Dioxide Inf. Anal. Center, Oak Ridge Natl. Lab. U.S. DoE, Oak Ridge, TN. (2011). https://doi.org/10.1017/CBO9781107415324.004
Mehrbach, C., Culberson, C. H., Hawley, J. E. & Pytkowicx, R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. https://doi.org/10.4319/lo.1973.18.6.0897 (1973).
Dickson, A. G. & Millero, F. J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. https://doi.org/10.1016/0198-0149(87)90021-5 (1987).
Mucci, A. The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. https://doi.org/10.2475/ajs.283.7.780 (1983).
Orr, J. C., Epitalon, J. M., Dickson, A. G. & Gattuso, J. P. Routine uncertainty propagation for the marine carbon dioxide system. Mar. Chem. https://doi.org/10.1016/j.marchem.2018.10.006 (2018).
Millero, F. J., Feistel, R., Wright, D. G. & McDougall, T. J. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep. Res. Part I Oceanogr. Res. Pap. https://doi.org/10.1016/j.dsr.2007.10.001 (2008).
Raven, J. et al. Ocean acidification due to increasing atmospheric carbon dioxide. R. Soc. 5, 60 (2005).
Vázquez-Rodríguez, M. et al. Anthropogenic carbon distributions in the Atlantic Ocean: Data-based estimates from the Arctic to the Antarctic. Biogeosciences 6, 439–451 (2009).
Freiwald, A. et al. Global distribution of cold-water corals (version 5.0). Fifth update to the dataset in Freiwald et al. (2004) by UNEP-WCMC. (UN Environment World Conservation Monitoring Centre, Cambridge, 2017).
Ríos, A. F., Pérez, F. F. & Fraga, F. Water masses in the upper and middle North Atlantic Ocean east of the Azores. Deep Sea Res. Part A Oceanogr. Res. Pap. https://doi.org/10.1016/0198-0149(92)90093-9 (1992).
Lherminier, P. et al. Transports across the 2002 Greenland-Portugal Ovide section and comparison with 1997. J. Geophys. Res. Ocean. 112, 1–20 (2007).
Acknowledgements
For this work M. Fontela was funded by the Spanish Ministry of Economy and Competitiveness (BES-2014-070449) supported by the Spanish Government and co-funded by the Fondo Europeo de Desarrollo Regional 2007–2012 (FEDER) and by Portuguese national funds from FCT—Foundation for Science and Technology through project UID/Multi/04326/2019 and CEECINST/00114/2018. F.F. Pérez was supported by the BOCATS Project (CTM2013-41048-P) and ARIOS project (CTM2016-76146-C3-1-R) both co-funded by the Spanish Government and the Fondo Europeo de Desarrollo Regional (FEDER). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 820989 (project COMFORT, Our common future ocean in the Earth system—quantifying coupled cycles of carbon, oxygen, and nutrients for determining and achieving safe operating spaces with respect to tipping points). We thank Evan Edinger and Mario Lebrato for their constructive and useful review that greatly helped to improve the paper.
Author information
Authors and Affiliations
Contributions
M.F., F.F.P. and L.I.C wrote the manuscript. M.F. prepared all the figures. M.F. and F.F.P. analyzed the data. M.F., F.F.P., L.I.C., X.A.P., A.V., M.I.G-I. and P.L. have contributed to the acquisition of data, have participated in the results discussion and have reviewed the manuscript and supporting information.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fontela, M., Pérez, F.F., Carracedo, L.I. et al. The Northeast Atlantic is running out of excess carbonate in the horizon of cold-water corals communities. Sci Rep 10, 14714 (2020). https://doi.org/10.1038/s41598-020-71793-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71793-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.