Abstract
In the run-up to biomechanical testing, fresh human tissue samples are often frozen in order to inhibit initial decomposition processes and to achieve a temporal independence of tissue acquisition from biomechanical testing. The aim of this study was to compare the mechanical properties of fresh tissue samples of the human iliotibial tract (IT) to fresh-frozen samples taken from the same IT and those modified with different concentrations of Dimethylsulfoxide (DMSO) prior to freezing. All samples were partial plastinated and destructive tensile tests were conducted with a uniaxial tensile test setup. A plastination technique already established in the laboratory was modified to improve the clamping behaviour of the samples. Material failure was caused by a gradual rupture of the load-bearing collagen fibre bundles. Contrary to our expectations, no significant difference was found between the tensile strength of fresh and fresh frozen specimens. The addition of 1 wt% DMSO did not increase the tensile strength compared to fresh-frozen samples; an addition of 10 wt% DMSO even resulted in a decrease. Based on our findings, the use of simple fresh-frozen specimens to determine the tensile strength is viable; however fresh specimens should be used to generate a complete property profile.
Similar content being viewed by others
Introduction
Tissue stored in fresh frozen condition is preferred for biomechanical testing of human soft tissue. This allows decoupling tissue acquisition, which usually has to be done shortly after donor death, and material testing. Especially in case of ligaments and aponeuroses, the influence of freezing and thawing processes on the mechanical properties of human tissue has not yet been fully investigated, despite current research in this field1,2,3,4,5. There is a large number of different research results in the field of biomechanical characterization of ligaments and tendons, particularly with regard to the influence of donor age and the influence of freezing effects on the mechanical properties6,7,8,9,10,11. The formation of ice crystals of varying size in intra- and extracellular water during the freezing process is known to cause tissue damage and is associated with penetration of cell membranes and extracellular structures.
In cell culture laboratories, various antifreeze agents such as dimethyl sulfoxide (DMSO) and glycerin are used to prevent tissue damage at the cellular level during deep-freeze storage. The aim of this study was to investigate the influence of freezing and thawing processes on the deformation behaviour and mechanical properties of the human iliotibial tract (IT) compared to the fresh state. The mechanical properties of fresh-frozen tissue pre-treated with DMSO were also investigated. The use of tissue in as fresh a condition as possible is a distinguishing feature of this study, which served as the basic tissue condition for the differently prepared sample variants. The IT was chosen for this study, as it has been extensively studied in the past12,13,14,15,16,17,18,19. Additionally, due to the size of this structure, sample acquisition is relatively simple and allows the extraction of several samples from individual IT, thus improving comparability.
Methods
Sample preparation
Twenty fresh tissue samples of human IT from ten body donors (six male and four female) were prepared.
All donors originated from the Institute of Anatomy of the Leipzig University and had given written consent to dedicate their bodies to medical education and research purposes. Being part of the body donor program regulated by the Saxonian Death and Funeral Act of 1994 (3rd section, paragraph 18, item 8), institutional approval for the use of the post-mortem tissues of human body donors was obtained. The authors declare that all experiments were performed according to the ethical principles of the Declaration of Helsinki.
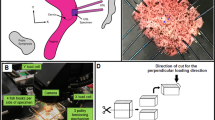
The mean donor age was 82.7 (SD 8.79) years, mean donor body mass was 73.2 (SD 17.78) kg. With a mean donor size of 1.68 (SD 0.09) m, the mean body mass index (BMI) was 25.73 (SD 5.18) kg/m2. The parallel main fibre orientation of the collagenous structure was determined visually. Each sample was cut lengthwise into four rectangular parts, according to the orientation of the load bearing collagen fibres. The upper and the lower part of the IT were removed first with a scalpel. The rectangular strips were manually cut by following the dominant fibre bundles with a scalpel until the upper and lower end of the extracted IT sample was reached (Fig. 1).
The widths of all specimen was documented with a mean width of 1.13 mm. A mean thickness of 0.9 mm was determined for all specimen. Each of the four parts was assigned to a different preparatory procedure prior to testing (Table 1). Samples prepared for unmodified deep freeze storage (Type II) were placed in physiological saline solution (PSS). For the treatment of sample types III and IV, DMSO in American Chemical Society purity grade (ACS) was used (Merck KGaA, Darmstadt, Deutschland) in combination with PSS.
In this study we used an already established freezing method and exposed tissue samples to different concentrations of DMSO. The freezing regime for sample categories II–IV after storage in the respective solution comprised four temperature levels. Starting from room temperature (20 °C), the samples were stored at 5 °C (30 min) in a first step, then at -20 °C (30 min) and finally at -80 °C. The further workflow of the preparation is shown in Fig. 2.
Fresh specimens were placed between two congruent aluminium plates, clamped and prepared for partial plastination. These plates had the purpose to shield the test areas of the specimens from the acetone during the subsequent dehydration step of the clamping zones. The length of the test zones was standardized to 50 mm.
To create a better contact zone between the samples and the applied resin, the plastination areas were immersed in pure acetone (J. T. Baker, Avantor, VWR International Global Exports, Radnor, PA, USA) for 20 min per side to degrease and dry the clamp areas. For plastination, two beech wood panels of congruent shape were sawn for each clamping area (Fig. 3).
The parts have been designed to provide maximum contact with the jaws of the Instron 5566A (Instron Corporation, Norwood, MA, USA) table-top testing machine. The resin used in this partial plastination technique was a two-component polyurethane rapid casting resin (RenCast FC 52/53 isocyanate/FC 52 polyol; Huntsman Advanced Materials, East Lansing, MI, USA) filled with aluminium hydroxide powder (Gössl + Pfaff GmbH, Karlskron, Deutschland). The mixing ratio was 1:1:3 (Isocyanate:Polyol:Al(OH)3). After mixing and thorough stirring, the resin-powder mixture was split into kneadable portions which were placed tightly around the dried clamping areas of the samples to ensure central placement. To increase the pull-out strength, the samples were additionally folded in the resin. The pre-cut pieces of beech wood were placed above and below before the complete stack was clamped for 30 min. In the last step, the aluminium plates which protected the fresh test areas of the sample were removed from acetone and the resin mixture. Immediately afterwards the testing was carried out.
Due to the fact that only complete sample series of states I–IV were considered for the final testing and evaluation, singular samples had to be discarded.
Testing
The tensile tests were performed on an Instron 5566A table-top testing machine using a uniaxial tensile test method and a 1 kN load cell. A low preload of 10 N was chosen prior to the tensile testing process in order to ensure alignment of the collagen fibres and thus to achieve easy preconditioning. As there is no ideal testing norm for the testing of human aponeurotic tissue, the test speed was based on the ISO 13934-1, which recommends a strain rate of 0.1 mm per mm sample length per minute for an expected failure strain of < 10%, which we expected here. Thus deformation was performed at a speed of 5 mm/min for our specimen length of 50 mm. The abort criterion of the procedure was a reduction in tensile stress by 30%, relative to the maximum value. The recording of the measured values was path-controlled. Force and displacement data were processed with the Bluehill 2 Software (Instron Corporation, Norwood, MA, USA). Engineering stress and engineering strain values were calculated based on this data using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The Young’s Modulus was calculated in the linear part of the stress–strain curves with E = (σ40% − s20%)/(ε40% − ε20%), with σ20% and σ40% being the stress values at 20% and 40% of the yield strength. Statistical analysis was performed with SPSS (IBM, Armonk, NY, USA) utilizing the Paired t Test.
Results
Sample preparation
The modified partial plastination process of the specimen clamping areas was successful for most samples. Two samples had to be discarded due to an inhomogenous resin mixture, which lead to insufficient processing properties of the resin-hydroxide-mix. Besides these two cases, the resin-hydroxide composite infiltrated the tissue and the beech wood equally and created a stable and firm connection between the specimen and the clamping material, as examined and determined in cross sections through the clamping zones (Fig. 4).
Some specimens had to be discarded due to dehydration and faulty sample cutting, which is why only complete specimen groups (states I, II, III & IV) were included in the evaluation.
Testing
In contrast to the classical tensile test, the material failure did not occur by an abrupt and complete tearing of the specimen, but by a gradual failure of the load-bearing collagen fibre bundles (Fig. 5). Two samples were affected by the problem of slippage, which is known to occur in this type of tissue 18.
Procedure for uniaxial tensile testing. Left: Under preload. Clearly visible at the edges left and right of the test area is the superficial tissue layer, which is not load bearing and masks the collagen fibre bundles, Mid: Immediately before failure, Right: After exceeding tensile strength and incipient failure of collagen fibre bundles at the bottom right area.
Specimen failure occurred with considerable donor-specific differences between 5.6 and 61.6 MPa; the mean values and moduli of elasticity determined are listed in Table 2.
Non-significant differences with slightly decreasing tensile strength values between the state F samples (37.66 (SD 18.87) MPa), FDF0 samples (34.64 (SD 12.08)) MPA) and FDF1 samples (30.96 (SD 13.12)) MPa) were determined, while the strengths of FDF10 samples were significantly lower (22.00 (SD 10.91) MPa). An exemplary stress–strain curve can be seen in Fig. 6. A slight increase in the Young’s Modulus of FDF0 samples (0.91 (SD 0.29) MPa) and FDF1 samples (0.83 (SD 0.28) MPa), compared to F samples (0.73 (SD 0.44) MPa) has been observed (Fig. 7). With a total of 10 samples (5 body donors), the entire process chain from cutting to size to plastination and final testing was successful, however three samples had to be excluded from the calculation of the mean ultimate tensile strength, as sample failure occurred close to the clamping area.
A significant decrease in tensile strength has been observed between the fresh state F (I) and the FDF10 state (IV) (p = 0.017), and between the FDF0 state (II) and the FDF10 samples (IV) (p = 0.006). No significant correlations were found for the comparison of the states F (I) with FDF0 (II) (p = 0.570), fresh (I) with FDF1 (III) (p = 0.432) and FDF0 (II) with FDF1 (III) (p = 0.065).
Discussion
The partial plastination technique used by Steinke et al.15 was modified to further minimize the slippage of the tissue samples. The use of wooden plates in the plastination process instead of polymer plates was advantageous for several reasons. The clamping behaviour improved because the metal clamps of the testing machine were able to penetrate the wood material and prevent the clamping areas from slipping. In addition, the resin was partially absorbed and integrated into the surficial fibre structure of the wood, resulting in a much stronger bond between the resin layer and the wood panels. The possibility of folding the tissue samples due to their long geometry probably also increased the pull-out strength and caused the reduced occurrence of slippage effects. However, this has to be verified in further experiments with the use of digital image correlation systems.
An essential difference in the IT investigated here compared to classical materials characterized by uniaxial tensile tests is the completely different failure pattern. An abrupt rupture (commonly observed in ceramic, brittle polymeric and metallic test specimens) or a characteristic failure pattern, which is typical for elastic polymers, was not observed. The material failure occurred by a cascading rupture of the load-bearing collagen fibre bundles, which manifested itself in gradual tension drops after exceeding the tensile strength. A similar failure behaviour can be found, for example, in the staggered rupture of a steel cable or braided rope, where the individual elements of the overall structure also fail in succession. In previously published studies on uniaxial tensile testing of ligaments and tendons, an hourglass shape is often chosen for the test areas, analogous to tensile test specimens made of metallic materials11,20. However, we deliberately chose a rectangular shape in order to reduce the number of interrupted collagen fibrils as much as possible, as these could in principle serve as initial defects for the material failure. The main objective was to obtain long strips of tissue with edges parallel to the internal, load bearing collagen structure, which has been used in similar studies20,21,22. This also assured that the direction of the tensile tests was colinear to this load bearing collagen structure. A final, comparative evaluation of the ideal sample shape should be made in further investigations, especially with regard to the staggered sample failure pattern.
We found in preliminary tests that the deformation behaviour of the load bearing structure was not completely reflected by the superficial, visible connective tissue layer, but was masked due to slipping of the tissue layers during testing. Thus, we did not use digital image correlation. Solving this problem could be content of a future study.
Contrary to our expectations, no significant difference was found between the tensile strength of fresh (I) and fresh frozen (II) specimens. Possibly the careful freezing was a partial cause for this, as the method used was optimized by a special cooling regime to minimize ice crystal growth in the tissue samples.
In this context, earlier studies by Lansdown et al. for ligament structures showed a negligible influence of freezing processes on the mechanical properties of human cruciate ligaments. Freezing–thawing cycles of up to eight times did not lead to any significant changes in strength23. This is consistent with our observations that, contrary to our initial hypothesis, the mean strength parameters of fresh, fresh-frozen and acetone-treated frozen iliotibial tract samples were in similar ranges.
Hochstraht and Müller et al. performed investigations on mouse tendons and compared on a microscopic level the condition of fresh, phosphate-buffered saline solution (PBS) treated and frozen as well as DMSO treated and frozen Achilles tendons. A change in the collagen fibre orientation due to freezing damage was observed at the molecular level, which is consistent with our hypothesis. In the biomechanical tests performed by Hochstraht and Müller et al., a significant correlation between the number of freezing cycles and the increase in elastic modulus was found. The described reduction of the static Young’s Modulus in samples frozen once is comparable to our results24.
A decrease in the tensile strength between group I and IV in our experiment could be attributed to a reduction in the collagen strength of the samples exposed to higher DMSO values. A splitting of the intermolecular cross-linking of collagen fibres is the most plausible cause, as Gries et al. showed25. An increase of the DMSO level could therefore reinforce this effect and lead to an earlier material failure of the load-bearing structure. However, despite their very similar molecular composition, the tissue samples in the mentioned publications were tissue from different species. The biomechanical test conditions also varied.
We deliberately refrained from preconditioning in order to prevent tissue damage in advance of the destructive material test. The reason for that was to be able to investigate the effects of the different sample preparation methods on the mechanical tissue properties on the tensile strength and the Young’s Modulus in as isolated a manner as possible.
Limitation
The limited number of samples due to the requirement to examine fresh donor tissue must be taken into account with regard to the results obtained. Donor-specific differences are therefore more impactful, compared to a larger sample size. In further investigations, the use of a digital image correlation technique is also useful to verify the quality of the slip reduction achieved by the modified plastination technique and to determine the detailed sample failure pattern. However, this could also lead to a deterioration in the visibility of the staggered sample failure, which would have to be assessed.
Conclusion
Non-significant differences with slightly decreasing tensile strength values of fresh (I), fresh-frozen samples without DMSO addition (II) and fresh-frozen samples with 1% DMSO addition (III) were determined. In contrast the tensile strengths of fresh-frozen samples with 10% DMSO addition were significantly lower. DMSO could to be responsible for the splitting of the crosslinks between the collagenous fibres, but this has to be proven in further studies. No positive effect on the mechanical properties of DMSO in cryopreservation was found. Likewise, no significant negative effect of freezing on tensile strength could be observed with single freeze–thaw cycles. On the other hand, a slight increase in the Young’s Modulus has been observed. The use of simple fresh-frozen specimens to determine the tensile strength can therefore be regarded as legitimate; fresh specimens should be used to establish a complete property profile including the Young's modulus, if possible.
Change history
25 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-81980-4
11 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-89952-4
References
Chen, L. et al. Effect of repeated freezing-thawing on the Achilles tendon of rabbits. Knee Surg. Sports Traumatol. Arthroscopy 19, 1028–1034. https://doi.org/10.1007/s00167-010-1278-y (2011).
Gottsauner-Wolf, F., Grabowski, J. J., Chao, E. Y. & An, K. N. Effects of freeze/thaw conditioning on the tensile properties and failure mode of bone-muscle-bone units: a biomechanical and histological study in dogs. J. Orthop. Res. 13, 90–95. https://doi.org/10.1002/jor.1100130114 (1995).
Lee, A. H. & Elliott, D. M. Freezing does not alter multiscale tendon mechanics and damage mechanisms in tension. Ann. N. Y. Acad. Sci. 1409, 85–94. https://doi.org/10.1111/nyas.13460 (2017).
Oswald, I. et al. The influence of cryopreservation and quick-freezing on the mechanical properties of tendons. J. Biomech. 64, 226–230. https://doi.org/10.1016/j.jbiomech.2017.08.018 (2017).
Quirk, N. P. et al. Effects of freeze-thaw on the biomechanical and structural properties of the rat Achilles tendon. J. Biomech. 81, 52–57. https://doi.org/10.1016/j.jbiomech.2018.09.012 (2018).
Blevins, F. T., Hecker, A. T., Bigler, G. T., Boland, A. L. & Hayes, W. C. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am. J. Sports Med. 22, 328–333. https://doi.org/10.1177/036354659402200306 (1994).
Greaves, L. L., Hecker, A. T. & Brown, C. H. The effect of donor age and low-dose gamma irradiation on the initial biomechanical properties of human tibialis tendon allografts. Am. J. Sports Med. 36, 1358–1366. https://doi.org/10.1177/0363546508314394 (2008).
Jones, D. B., Huddleston, P. M., Zobitz, M. E. & Stuart, M. J. Mechanical properties of patellar tendon allografts subjected to chemical sterilization. Arthroscopy 23, 400–404. https://doi.org/10.1016/j.arthro.2006.11.031 (2007).
Jung, H.-J. et al. The effects of multiple freeze-thaw cycles on the biomechanical properties of the human bone-patellar tendon-bone allograft. J. Orthop. Res. 29, 1193–1198. https://doi.org/10.1002/jor.21373 (2011).
Swank, K. R., Behn, A. W. & Dragoo, J. L. The effect of donor age on structural and mechanical properties of allograft tendons. Am. J. Sports Med. 43, 453–459. https://doi.org/10.1177/0363546514557246 (2015).
Zwirner, J. et al. Tensile properties of the human iliotibial tract depend on height and weight. Med. Eng. Phys. 69, 85–91. https://doi.org/10.1016/j.medengphy.2019.05.001 (2019).
Birnbaum, K. et al. Anatomical and biomechanical investigations of the iliotibial tract. Surg. Radiol. Anat. 26, 433–446. https://doi.org/10.1007/s00276-004-0265-8 (2004).
Vieira, E. L. C. et al. An anatomic study of the iliotibial tract. Arthroscopy 23, 269–274. https://doi.org/10.1016/j.arthro.2006.11.019 (2007).
Huang, B. K. et al. Injury of the gluteal aponeurotic fascia and proximal iliotibial band: anatomy, pathologic conditions, and MR imaging. Radiographics 33, 1437–1452. https://doi.org/10.1148/rg.335125171 (2013).
Hammer, N. et al. Ultimate stress and age-dependent deformation characteristics of the iliotibial tract. J. Mech. Behav. Biomed. Mater. 16, 81–86. https://doi.org/10.1016/j.jmbbm.2012.04.025 (2012).
Fairclough, J. et al. The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J. Anat. 208, 309–316. https://doi.org/10.1111/j.1469-7580.2006.00531.x (2006).
Flato, R. et al. The iliotibial tract: imaging, anatomy, injuries, and other pathology. Skeletal Radiol. 46, 605–622. https://doi.org/10.1007/s00256-017-2604-y (2017).
Sichting, F. et al. Quantification of material slippage in the iliotibial tract when applying the partial plastination clamping technique. J. Mech. Behav. Biomed. Mater. 49, 112–117. https://doi.org/10.1016/j.jmbbm.2015.04.028 (2015).
Yuan, B.-T. et al. Histology and molecular pathology of iliotibial tract contracture in patients with gluteal muscle contracture. Biosci. Rep. https://doi.org/10.1042/BSR20181351 (2019).
Cooney, G. M. et al. Uniaxial and biaxial tensile stress-stretch response of human linea alba. J. Mech. Behav. Biomed. Mater. 63, 134–140. https://doi.org/10.1016/j.jmbbm.2016.06.015 (2016).
Cooney, G. M., Moerman, K. M., Takaza, M., Des Winter, C. & Simms, C. K. Uniaxial and biaxial mechanical properties of porcine linea alba. J. Mech. Behav. Biomed. Mater. 41, 68–82. https://doi.org/10.1016/j.jmbbm.2014.09.026 (2015).
Schleifenbaum, S. et al. Tensile properties of the hip joint ligaments are largely variable and age-dependent - An in-vitro analysis in an age range of 14–93 years. J. Biomech. 49, 3437–3443. https://doi.org/10.1016/j.jbiomech.2016.09.001 (2016).
Lansdown, D. A., Riff, A. J., Meadows, M., Yanke, A. B. & Bach, B. R. What factors influence the biomechanical properties of allograft tissue for ACL reconstruction? A systematic review. Clin. Orthop. Relat. Res. 475, 2412–2426. https://doi.org/10.1007/s11999-017-5330-9 (2017).
Hochstrat, E. et al. Cryopreservation of tendon tissue using dimethyl sulfoxide combines conserved cell vitality with maintained biomechanical features. PLoS ONE 14, e0215595. https://doi.org/10.1371/journal.pone.0215595 (2019).
Gries, G., Bublitz, G. & Lindner, J. The effect of dimethyl sulfoxide on the components of connective tissue (Clinical and experimental investigations). Ann. N. Y. Acad. Sci. 141, 630–637 (1967).
Acknowledgement
We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Planned the experiments: B.F. and S.K. Carried out the experiments and provided technical support: B.F. and S.K. Evaluated and interpreted the data: B.F., S.K., A.H. and S.S. Wrote the paper: B.F., A.H. and S.K. Critically revised the manuscript: B.F., S.K., A.H. and S.S. B.F. and S.K. contributed equally to this publication and should be considered co-first authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, B., Kurz, S., Höch, A. et al. The influence of different sample preparation on mechanical properties of human iliotibial tract. Sci Rep 10, 14836 (2020). https://doi.org/10.1038/s41598-020-71790-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71790-5
This article is cited by
-
Evaluating suturing methods for surgical repair of muscle belly lacerations: a scoping review of biomechanical studies
European Journal of Plastic Surgery (2024)
-
The effect of gastrocnemius resection on knee flexion in a total knee arthroplasty model
Archives of Orthopaedic and Trauma Surgery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.