Abstract
The long saphenous vein (LSV) is commonly used as a conduit in coronary artery bypass grafting. However, long term patency remains limited by the development of vascular inflammation, intimal hyperplasia and accelerated atherosclerosis. The impact of acute exposure of venous endothelial cells (ECs) to acute arterial wall shear stress (WSS) in the arterial circulation, and the subsequent activation of inflammatory pathways, remain poorly defined. Here, we tested the hypothesis that acute exposure of venous ECs to high shear stress is associated with inflammatory responses that are regulated by NF-κB both in-vitro and ex-vivo. Analysis of the LSV endothelium revealed that activation of NF-κB occurred within 30 min after exposure to arterial rates of shear stress. Activation of NF-κB was associated with increased levels of CCL2 production and enhanced binding of monocytes in LSVECs exposed to 6 h acute arterial WSS. Consistent with this, ex vivo exposure of LSVs to acute arterial WSS promoted monocyte interactions with the vessel lumen. Inhibition of the NF-κB pathway prevented acute arterial WSS-induced CCL2 production and reduced monocyte adhesion, both in vitro and in human LSV ex vivo, demonstrating that this pathway is necessary for the induction of the acute arterial WSS-induced pro-inflammatory response. We have identified NF-κB as a critical regulator of acute endothelial inflammation in saphenous vein in response to acute arterial WSS. Localised endothelial-specific inhibition of the NF-κB pathway may be beneficial to prevent vein graft inflammation and consequent failure.
Similar content being viewed by others
Introduction
Ischaemic heart disease (IHD) is one of the leading causes of mortality in the UK and worldwide1. Coronary artery bypass grafting (CABG) remains the gold standard intervention in the presence of complex coronary disease, diabetes and poor ventricular function2,3,4,5,6. The long saphenous vein (LSV) is the most used conduit in many patients7,8, however, its use is complicated by considerable rates of late stenosis or occlusion due to the development of intimal hyperplasia (IH) and superimposed atherosclerosis9. IH is a chronic inflammatory process that starts with endothelial cell (EC) activation, followed by the migration and proliferation of smooth muscle cells (SMC) to the intima, which is then accompanied by alterations in extracellular matrix10 (ECM). Vascular inflammation is regulated by signalling intermediaries, including Nuclear Factor-κB, NF-κB (p65), that trigger EC expression of chemokines such as monocyte chemotactic protein-1 (CCL2) and other pro-inflammatory molecules.
The susceptibility of vessels to inflammatory processes may, in part, be related to the vascular bed from which they originate and the types of haemodynamic environment to which they are chronically exposed11. Venous ECs in situ, are adapted to chronic levels of low shear stress and, following grafting, they become exposed to shear stresses (defined as force/wall area and measured in dyn/cm2) up to tenfold higher in the arterial system12. Acute onset of increased rates of shear stress is known to alter the phenotype of venous EC; however, the mechanisms that underpin this response have not been fully elucidated. To gain better insight into the intrinsic molecular mechanisms underlying pro-inflammatory responses of veins to grafting, we investigated the impact of acute shear changes on NF-κB activation and associated vECs response in vitro, ex vivo and in vivo.
Results
Acute arterial WSS induces pro-inflammatory responses in LSV ECs ex vivo
Freshly harvested segments of human LSV were acutely exposed to arterial shear stress, ex vivo, at 12 dyn/cm2, a rate which has previously been associated with EC activation and vascular remodelling in ex vivo venous bypass graft models12,13. We noted a significant increase in CCL2 mRNA levels in LSV ECs following exposure to acute high shear stress (acute arterial WSS) (Fig. 1A). En face immunofluorescent staining revealed that exposure of veins to acute arterial WSS increased CCL2 expression in ECs at the protein level (Fig. 1B,C and Supplementary videos 2 and 3).
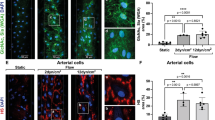
Activation of the LSV endothelium by acute arterial wall shear stress. The LSV endothelium was activated following exposure to acute arterial rates of shear stress, shown by NF-κB translocation and increased CCL2 protein levels. LSV sections were exposed to acute arterial WSS at 12 dyn/cm2 for 30 and 90 min or maintained in static conditions and immunostained en face for the localisation of NF-κB in LSV ECs. (A) LSV portions were exposed to acute arterial WSS for 90 and 120 min or maintained in static conditions, following shear exposure EC RNA was extracted and mRNA levels of pro-inflammatory genes (CCL2, IL-6, IL-8 and ICAM-1) were evaluated by RT-qPCR. Data shown represents independent experiments with 4 different segments of LSV. (B) Mean arbitrary fluorescence intensity of CCL2 immunostaining calculated using FIJI. (C) Representative images of independent experiments with 3 different segments of LSV, white arrows indicate perinuclear localisation of CCL2 and scale bar represents 10 µm. (D) Mean arbitrary fluorescence intensity of NF-κB en face immunostaining in endothelial nuclei 3D volumes shown for all 3 conditions, as calculated by Imaris. (E) Representative images of independent experiments with 5 different segments of LSV immunostained en face for the quantification of nuclear NF-κB, scale bar represents 10 µm. Two-tailed two-sample t-test (B) and One-way ANOVA followed by post-hoc pairwise comparisons with Bonferroni correction (D) were used to calculate significance, where, **** indicates p < 0.0001, ** indicates p < 0.01 and * indicates p < 0.05.
NF-κB inhibition prevented a pro-inflammatory response to acute arterial WSS in LSV endothelium
To investigate the possible role of NF-κB, we analysed the expression of p65 NF-kB sub-units in LSVs exposed to arterial shear stress. acute arterial WSS enhanced p65 nuclear translocation in ECs (Fig. 1D,E and Supplementary video 1), indicating that acute arterial shear stress activates NF-kB in LSV endothelium. To investigate the function of NF-κB on pro-inflammatory responses to acute arterial WSS, a selective small-molecule NF-kB inhibitor, BAY11-7085 (BAY) was used (Supplementary Figure 9). The pre-treatment of LSV with 50 µmol/L of BAY modulated EC responses to acute arterial WSS by reducing the levels of CCL2 (Fig. 2A,B). Furthermore, NF-κB inhibition reduced acute arterial WSS induced monocyte adhesion to the LSV endothelium (Fig. 2C,D). Thus, we conclude that NF-κB inhibition can suppress the initiation of monocyte adhesion to ECs in veins under acute arterial WSS.
Inhibition of NF-κB and endothelial pro-inflammatory response to acute high shear stress—ex vivo. Inhibition of NF-κB prevented the pro-inflammatory response associated with acute arterial WSS in LSV ECs ex vivo. (A) Segments of LSV were incubated for 3 h with 50 µmol/L NF-κB inhibitor, BAY11-7085, or 0.5% (v/v) DMSO control and were then exposed to acute arterial WSS for 6 h, or maintained in static conditions. Segments were then immunostained en face for the quantification of CCL2 in LSV ECs. (A) Mean arbitrary fluorescence intensity of CCL2 immunostaining calculated using FIJI. (B) Representative images of independent experiments with 4 different segments of LSV, white arrows indicate perinuclear localisation of CCL2 and scale bar represents 10 µm. Sections of LSV were exposed pre-treated for 3 h with 50 µM NF-κB inhibitor, BAY11-7085, or 0.5% (v/v) DMSO control and were then exposed to acute arterial WSS for 6 h. Following acute arterial WSS exposure, LSV sections were immediately prepared en face and incubated with 1 × 106 Calcein-labelled monocytes per mL for 15 min then imaged immediately. (C) Total number of monocytes was calculated using CellProfiler software. (D) Images represent independent experiments with 4 different segments of LSV and scale bar represents 50 µm. Two-way ANOVA followed by post-hoc pairwise comparisons with Bonferroni correction (A,C) was used to calculate significance, where, * indicates p < 0.05.
Acute arterial WSS activates pro-inflammatory responses and the NF-κB classical pathway in vitro
To validate our ex vivo findings and further investigate the molecular basis involved in the venous EC response to arterial rates of shear stress, we studied the effects of acute arterial WSS on HUVECs in vitro. Acute high shear stress significantly increased nuclear translocation of p65 (Fig. 3A,D) which was associated with reciprocally decreased IκBα expression (Fig. 3B,D) indicating the activation of the NF-κB classical pathway. Furthermore, NF-κB was phosphorylated at Serine residue 276 (Ser-276) following 30 min of acute arterial WSS (Fig. 3C,D), whilst also showing increased DNA binding to the NF-κB consensus oligonucleotide (Fig. 3E), both of which are indicative of transcriptional activation of NF-κB. This transcriptional activation was transient, with levels peaking at 30 min, which is likely indicative of the feed-forward mechanism of negative regulation of NF-κB by IκBα following 60 min acute arterial WSS exposure13. We observed that acute arterial WSS, but not low shear, was associated with up regulation of CCL2 (Fig. 4A–D). The suppression of the NF-κB classical pathway using 20 μmol/L BAY pre-treatment resulted in reduced pro-inflammatory activation, similar to what was observed ex vivo (Fig. 5A).
Acute high shear stress and activation of the NF-κB classical pathway—in vitro. The NF-κB classical pathway was activated under acute high shear stress as indicated firstly by the nuclear translocation of NF-κB and reciprocal reduction in cytosolic levels of IκB⍺, as well as phosphorylation and increased DNA binding affinity. HUVECs were exposed to acute arterial WSS for 30 and 90 min or maintained in static conditions, then cellular fractions were collected to be analysed by Western blotting. Total amounts of protein were quantified relative to stain-free loading controls and expressed as a fold change of static control. (A) Nuclear NF-κB, (B) cytosolic levels of IκB⍺ and (C) Phospho-NF-κB (Serine residue 276 (Ser276)) from whole cell lysates were analysed by WB, and normalised to stain-free loading controls from nuclear, cytosolic and whole cell lysates, respectively. Western blots shown are representative of 5 or 6 independent experiments showing, in addition to NF-κB and IκB⍺, markers of nuclear and cytosolic fractions, Lamin A/C and GAPDH respectively (D). The transcriptional activation of NF-κB under acute arterial WSS, for both 30 and 90 min, was assessed by phosphorylation at Serine residue 276 (C) and DNA binding activity (E) in whole cell lysates. ELISA based DNA binding activity assay was performed using total cell lysates and was expressed relative to static controls and were representative of 3 independent experiments. One-way ANOVA followed by post-hoc pairwise comparisons with Bonferroni correction was used to calculate significance, where, ** indicates p < 0.01 and * indicates p < 0.05.
NF-κB target gene response to acute high shear stress—in vitro. Pro-inflammatory, NF-κB target genes were increased in ECs exposed to acute arterial WSS, in vitro. (C,D) HUVECs were exposed to AASS at 12 dyn/cm2 (A) and acute low shear stress (ALSS) at 0.5 dyn/cm2 (B) for 90 and 120 min in order to compare pro-inflammatory gene transcript levels by RT-qPCR. Data represent 6 independent experiments. (C,D) HUVECs were exposed to AASS at 12 dyn/cm2 for 4 h or maintained in static conditions, then immunostained for quantification of total CCL2 using CellProfiler. (C) Mean arbitrary fluorescence intensity of CCL2 staining was evaluated in whole cells. (D) Images are representative of 3 independent experiments and scale bar represents 15 µm. One-way ANOVA followed by post-hoc pairwise comparisons with Bonferroni correction (A,B) and two-tailed two-sample t-test (C) were used to calculate significance, where, * indicates p < 0.05.
Inhibition of NF-κB and endothelial pro-inflammatory response to acute arterial WSS—in vitro. Inhibition of NF-κB prevented the pro-inflammatory response associated with acute arterial WSS in ECs in vitro, as shown by pro-inflammatory mRNA expression, CCL2 protein levels, EC-monocyte interactions and VE-Cadherin cell–cell contact. HUVECs were pre-treated for 1 h with 20 µmol/L NF-κB inhibitor, BAY11-7085 (represented as squares and dashed lines), or 0.2% (v/v) DMSO control (represented as circles and solid lines) and were then exposed to acute arterial WSS for 90 and 120 min or maintained in static conditions. (A) mRNA levels of pro-inflammatory genes (CCL2, IL-6, IL-8 and ICAM-1) were then analysed by RT-qPCR. HUVECs were infected with Adenoviruses expressing WT-IκB⍺ transgene (represented as squares and dashed lines) or empty vector viral control (rAd66; represented as circles and solid lines) at 1 × 108 pfu/mL for 18 h, then cultured for a further 48 h and finally exposed to acute arterial WSS for 90 and 120 min (B) or 4 h (C) and compared to static controls. Adenoviral-mediated overexpression of WT-IκB⍺ was firstly evaluated at the transcript level by RT-qPCR (B) and, secondly, at the protein level, by immunocytochemical analysis of CCL2 mean arbitrary fluorescence intensity (C,D). All data and images represent 3 independent experiments and scale bar represents 15 µm. (E,F) HUVECs were infected with Adenoviruses expressing WT-IκB⍺ transgene or empty vector viral control (rAd66) at 1 × 108 pfu/mL for 18 h, then cultured for a further 48 h and finally exposed to acute arterial WSS for 4 h or maintained in static conditions then analysed with immunocytochemistry for VE-Cadherin. (E) VE-Cadherin-based EC cell–cell contact was measured as a percentage of objects in contact with one another, representing a surrogate marker of barrier disruption. (F) Images are representative of 3 independent experiments and scale bar represents 15 µm. (G,H) HUVECs were pre-treated for 3 h with 20 µmol/L NF-κB inhibitor, BAY11-7085, or 0.2% (v/v) DMSO control and were then exposed to pulsatile acute arterial WSS at 12 dyn/cm2 and 1 Hz for 6 h using the integrated, microfluidic capillary-based Bioflux 200 system. Following acute arterial WSS exposure, 3 × 106 Calcein-labelled monocytes per mL, were flowed over the endothelial monolayer and imaged in real time (temporal schematic represented in (F), scale bar represents 100 µm). (G) Number of monocytes adhered to ECs were enumerated after 10 min and represented as a percentage of the total number of ECs in the channel, data represent 3 independent experiments. Two-way ANOVA followed by post-hoc pairwise comparisons with Bonferroni correction (A,B) and two-tailed two-sample t-test (C,E,G) were used to calculate significance, where, * indicates p < 0.05, ** indicates p < 0.01 vs. untreated control samples (equivalent time-points), $ indicates p < 0.05 vs. untreated static control.
We further validated these findings using adenoviral-mediated over-expression of WT-IκBα (Supplementary Figure 9), which demonstrated that the overexpression of WT-IκBα prevented induction of CCL2 following exposure to acute arterial WSS (Fig. 5B). Moreover, immunoanalysis showed a significant reduction in the expression of CCL2 following 4 h of acute arterial WSS, as compared with static controls (Fig. 5C,D). Whilst, overexpression of WT-IκBα prevented the loss of VE-Cadherin cell–cell contacts following acute arterial WSS exposure (Fig. 5E,F), indicative of an intact endothelial barrier.
We next looked at the impact of NF-κB inhibition on monocyte-EC interactions in vitro, dynamically and in real-time, following exposure to acute arterial WSS. We noted that the pre-treatment of HUVECs with 20 μmol/L BAY significantly reduced acute arterial WSS-induced dynamic monocyte adhesion to the vEC monolayer (Fig. 5G,H).
NF-κB pathway-related gene expression was promoted by acute arterial WSS in vivo
Having observed a significant increase in CCL2 mRNA and protein in the LSV endothelium, we then analysed a publicly available gene expression microarray dataset, generated from a rabbit bilateral interposition vein graft model14. Grafts were maintained under low or high shear stress, for 2 and 24 h each. Log expression values (Log Fold Change (LogFC)) of differentially expressed genes (1602 genes) significantly different (p < 0.01) from baseline, in any of the 4 graft conditions were hierarchically clustered and plotted (Supplementary Figure 8A). Interestingly, gene set enrichment analysis between the two acute groups (high and low shear) revealed highly significant enrichment of biological process GO-terms involved in the inflammatory response, cytokine-mediated signalling and neutrophil activity, in grafts exposed to high shear, but not low (Supplementary Figure 8B,C). In addition, the high shear-exposed grafts showed the least overlap with any other graft condition, in terms of both gene overlap and GO-terms, suggesting that grafts exposed to high shear had the largest functionally unique change of any other condition (Supplementary Figure 8D,E). Within grafts exposed to high shear stress for 2 h, significantly up-regulated genes with a LogFC > 1.5 (a total of 114 genes) were analysed further for functional pathway and motif enrichment. Transcriptional regulatory inference, performed using LISA15, ranked NF-κB as the most probable transcriptional regulator of this gene-set, which was further supported by the observation that the NF-κB motif was highly enriched within the promoter regions of these genes (Supplementary Figure 8F,G). Finally, KEGG and REACTOME pathway analysis revealed that this gene list was strongly associated with the TNFα- and NF-kappaB signalling pathways, further supporting the role for NF-κB activation in vein grafts following acute shear stress exposure (Supplementary Figure 8H).
Discussion
Vein graft disease following CABG remains a problem of serious clinical significance despite decades of surgical advances16,17. Haemodynamics influence the inflammatory process by controlling leukocyte margination and adhesive interactions and by generating shear stress, which in turn alters EC physiology18,19. Increased shear stress rates in the LSV graft have long been considered to directly affect graft patency and survival; however, at present, there is no clinical consensus as to the extent of this effect, perhaps due to the diverse range of rates of graft WSS exposure, postoperatively20,21. Furthermore, the molecular cascades that occur after acute increases in WSS rates in LSV graft ECs remain poorly understood. We, and others, have previously shown that arterial and venous EC respond differently to acute high shear stress, in both in vivo and ex vivo graft models13,22,23,24. Here, we show that the NF-κB classical pathway is activated in the endothelium of LSV in response to acute exposure to arterial rates of shear stress and is a critical regulator of vascular inflammation in veins. In vitro experiments further demonstrated that targeting NF-κB prior to acute flow induction was sufficient to prevent endothelial CCL2 expression and monocyte recruitment, as well as EC cell–cell contact disruption, another hallmark of the dysfunctional endothelium.
These findings in the LSV were further substantiated by evidence from analysis of a previously published14 in vivo rabbit vein graft model and DNA microarray. Differential expression analysis revealed significantly up-regulated NF-κB and inflammatory pathway-related genes between acute high and low shear-exposed vein grafts. Together, these data provide a possible mechanism for the regulation of acute vein graft inflammation, a factor which is inextricably linked to downstream pathologies. Furthermore, owing to the unique window of opportunity to locally pre-treat the autologous vein graft prior to implantation, inhibition of NF-κB may represent an exciting possibility in the resolution of vascular inflammation within the graft.
Acute onset of increased rates of shear stress is known to promote inflammatory transcriptional responses in ECs in vitro, a process mediated through NF-κB, p38-MAPK and activating protein 1 (AP-1)17,24,25,26,27. However, no studies so far have directly addressed the role of acute shear stress on NF-κB pathway activation in the vein graft endothelium.
Our findings with regards to activation of CCL2 are in concordance with previously published small animal in vivo data28,29. Several previous studies have shown that in vivo, local NF-κB inhibition, using either a decoy cis-element (κB-oligodeoxynucleotide), siRNA-mediated knockdown or an alternate gene therapy approach to overexpress IκBα, limits IH development in multiple vein graft models30,31,32. However, all such previous models propose the same conclusions for their observed results; that it is due to the actions of NF-κB inhibition on reduction of medial VSMC proliferation and migration, and not the role of NF-κB in the acute adaptive response of the vein graft to its new haemodynamic environment. Following this acute haemodynamic adaptation, ECs typically promote endogenous mediators that promote quiescence and dampen inflammation, through the activation of KLF2 and Nrf2 and repression of NF-κB, within 12 h of high WSS exposure33. Our novel findings demonstrate a potential role of acute NF-κB inhibition in maintaining this quiescent, anti-inflammatory endothelium prior to and under conditions of arteriovenous transposition that may further help to ameliorate the pathogenic progression to late-stage graft failure.
In conclusion, we identified that acute arterial WSS is responsible for early pro-inflammatory activation of the LVG EC, a process regulated by NF-κB (p65) activation, resulting in upregulation of pro-inflammatory mediators and increased monocyte recruitment, thus, providing the first evidence for the mechanistic involvement of NF-κB in shear-induced inflammation in the vein graft endothelium.
Methods
Detailed descriptions of all materials and methods can be found in the Supplementary Methods.
Ex vivo perfusion of veins
The study was approved by the NRES Committee East of England—Norfolk ethics number (REC14/EE/1097) and all use of human tissue conformed to the principles outlined in the Declaration of Helsinki. Surplus segments of surgically prepared human long saphenous vein (LSV), resected during coronary artery bypass graft surgery, from anonymised consenting patients, were exposed to acute arterial WSS, at 12 ± 0.2 dyn/cm2, for between 30 min to 6 h using an in-house designed bioreactor system.
En face immunostaining
Briefly, segments of LSV were assessed for the cellular localisation of NF-κB and quantity of CCL2 after exposure to acute arterial WSS by immunostaining of en face prepared vessels (for detailed description, see Supplementary Methods). The LSV endothelium was imaged en face, using confocal microscopy (Leica, Germany) and image analysis was all performed in 3D, either using Imaris (Supplementary Figure 3) or ImageJ (Supplementary Figure 4).
Ex vivo monocyte adhesion
Following a 3-h incubation with 50 µmol/L NF-κB inhibitor, BAY11-7085, and exposure to acute arterial WSS for 6 h, LSV segments were dissected longitudinally, pinned with the luminal surface facing upwards and co-cultured, in static conditions, with 1 × 106 Calcein AM-labelled (10 µmol/L) THP-1 cells for 15 min. Immediately after co-culture and washing, in situ adhered monocytes and LSV segments were imaged using fluorescence microscopy (Zeiss, Germany).
Intimal RNA extraction
For extraction of RNA from LSV ECs, a technique was used which involves the flushing of the lumen of the vessel with a severe lysis buffer, in this case, Qiazol (Qiagen), to disrupt cells in the intimal layer, which were predominantly ECs34. Briefly, segments were cut transversely into 2–3 cm lengths and washed in ice-cold DPBS. Using a 1 mL syringe and 18-gauge unbevelled needle with the tip inserted a into the vessel lumen, the vessel was flushed with ice-cold DPBS. Finally, the lumen was quickly flushed (3–6 s) with 350 μL of ice-cold Qiazol and the eluate was collected in a 1.5 mL Eppendorf tube. This eluate was subjected to the RNA isolation protocol described later.
EC culture and shear stress
HUVECs were exposed to laminar, unidirectional shear stress (at 0.5 or 12 dyn/cm2 to simulate venous and arterial rates of shear stress, respectively) for varying times, using parallel plate flow chambers designed in-house (Supplementary Figure 2), as described previously35, or maintained in static conditions. Following HUVEC dynamic culture for variable time-points, cells were subjected to analysis for immunocytochemistry, qPCR or Western Blotting.
In vitro real-time monocyte adhesion
HUVECs were exposed to acute arterial WSS using the microfluidic capillary Bioflux 200 system, which allowed for dynamic co-culture with THP-1 cells and visualisation in real-time (Supplementary video 4). Briefly, following a 3 h incubation with 20 µmol/L NF-κB inhibitor, BAY11-7085, CmDiI-labelled HUVECs were exposed to acute arterial WSS at 12 dyn/cm2 for 4 h, after which point, Calcein-labelled THP-1 cells were introduced into the system and co-cultured, under shear stress (at 1 dyn/cm2), for a further 10 min, and imaged in real time (for detailed description, see Supplementary methods). Total number of adhered monocytes were then enumerated.
Immunocytochemistry
Briefly, HUVECs cultured on glass slides and subsequently exposed to shear stress, or maintained in static conditions, were fixed in 3% Paraformaldehyde (PFA). Following fixation, HUVECs were stained with primary antibodies against either NF-κB, CCL2 or VE-Cadherin, followed by appropriate secondary antibodies. Slides were then imaged using the Zeiss AxioObserver Z1 fluorescent microscope and automated quantification was performed with CellProfiler analysis software (for detailed description, see supplementary methods).
NF-κB activity assay
Total HUVEC lysates were used to detect NF-κB binding activity using a TransAM p65 DNA-binding ELISA kit (Active Motif, USA). The manufacturer’s instructions were followed and the colourimetric reaction endpoint was read at 450 nm.
Statistical analyses
For experiments where only two groups were analysed, data were subjected to a paired, two- tailed t-test. For experiments where more than two groups were analysed, a one- or two-way ANOVA was used depending on the number of independent variables, followed by post-hoc pairwise comparisons with Bonferroni correction for multiple comparisons. If datasets were large enough (for example for immocytochemical analyses, where 20 images per sample were analysed), normal distribution was assessed with the D’Agostino-Pearson test; all data assessed passed normality tests, as such, parametric analyses were appropriate. The cut-off value for statistical significance was 0.05. Data are presented as mean ± SEM. All statistical analysis was performed with GraphPad Prism 7.0.
Abbreviations
- CABG:
-
Coronary artery bypass grafting
- IH:
-
Intimal hyperplasia
- IHD:
-
Ischaemic heart disease
- NF-κB:
-
Nuclear factor-κB
- VEC:
-
Venous endothelial cells
- LSV:
-
Long saphenous vein
- CCL2:
-
Monocyte chemoattractant protein 1
- HUVEC:
-
Human umbilical vein endothelial cells
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- ICAM-1:
-
Intercellular adhesion molecule 1
References
Mozaffarian, D. et al. Heart disease and stroke statistics—2016 update a report from the American Heart Association. Circulation 131(4), e29-322 (2015).
Alderman, E. L. et al. Results of coronary-artery surgery in patients with poor left-ventricular function (CASS). Circulation 68, 785–795 (1983).
Alderman, E. et al. Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease—The bypass angioplasty revascularization investigation (BARI). Circulation 96, 1761–1769 (1997).
Eagle, K. A. et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 2004(110), e340-437 (2004).
Serruys, P. W. et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360, 961–972 (2009).
Mohr, F. W. et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381, 629–638 (2013).
Motwani, J. G. & Topol, E. J. Aortocoronary saphenous vein graft disease—Pathogenesis, predisposition, and prevention. Circulation 97, 916–931 (1998).
Sabik, J. F. Understanding saphenous vein graft patency. Circulation 124, 273–275 (2011).
Newby, A. C. & Zaltsman, A. B. Molecular mechanisms in intimal hyperplasia. J. Pathol. 190, 300–309 (2000).
Wan, S., George, S. J., Berry, C. & Baker, A. H. Vein graft failure: Current clinical practice and potential for gene therapeutics. Gene Ther. 19, 630–636 (2012).
Lehoux, S., Castier, Y. & Tedgui, A. Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 259, 381–392 (2006).
Golledge, J., Gosling, M., Turner, R. J., Standfield, N. J. & Powell, J. T. Arterial flow induces changes in saphenous vein endothelium proteins transduced by cation channels. Eur. J. Vasc. Endovasc. Surg. 19, 545–550 (2000).
Gusic, R. J., Myung, R., Petko, M., Gaynor, J. W. & Gooch, K. J. Shear stress and pressure modulate saphenous vein remodeling ex vivo. J. Biomech. 38(9), 1760–1769 (2005).
Hay, D. C. et al. Activation of NF-kappa B nuclear transcription factor by flow in human endothelial cells. Biochimica Et Biophysica Acta Mol. Cell Res. 1642, 33–44 (2003).
Casarin, S., Bercelli, S. A. & Garbey, M. Linking gene dynamics to vascular hyperplasia—Toward a predictive model of vein graft adaptation. PLoS ONE 12(1), e1087606 (2017).
Qin, Q. et al. Lisa: Inferring transcriptional regulators through integrative modeling of public chromatin accessibility and ChIP-seq data. Genome Biol. 21, 32 (2020).
Souza, D. S. R. et al. Improved patency in vein grafts harvested with surrounding tissue: Results of a randomized study using three harvesting techniques. Ann. Thorac. Surg. 73, 1189–1195 (2002).
Angelini, G. D., Bryan, A. J., Williams, H. M. J., Morgan, R. & Newby, A. C. Distension promotes platelet and leukocyte adhesion and reduces short-term patency in pig arteriovenous bypass grafts. J. Thorac. Cardiovasc. Surg. 99, 433–439 (1990).
Ward, A. O., Caputo, M., Angelini, G. D., George, S. J. & Zakkar, M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis. 265, 266–274 (2017).
Ruiter, M. S. & Pesce, M. Mechanotransduction in coronary vein graft disease. Front. Cardiovasc. Med. 5, 20 (2018).
Hwang, H. Y., Koo, B. K., Yeom, S. Y., Kim, T. K. & Kim, K. B. Endothelial shear stress of the saphenous vein composite graft based on the internal thoracic artery. Ann. Thorac. Surg. 105(2), 564–571 (2018).
Zakkar, M. et al. Dexamethasone arterializes venous endothelial cells by inducing mitogen-activated protein kinase phosphatase-1 a novel antiinflammatory treatment for vein grafts?. Circulation 123, 524-U141 (2011).
Surapisitchat, J. et al. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc. Natl. Acad. Sci. USA. 98, 6476–6481 (2001).
Zakkar, M. et al. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ. Res. 103, 726–732 (2008).
Zakkar, M., Angelini, G. & Emanueli, C. Regulation of vascular endothelium inflammatory signalling by shear stress. Curr. Vasc. Pharmacol. 2015.
Lan, Q. X., Mercurius, K. O. & Davies, P. F. Stimulation of transcription factors NF-kappa-B and AP-1 in endothelial-cells subjected to shear-stress. Biochem. Biophys. Res. Commun. 201, 950–956 (1994).
Bhullar, I. S. et al. Fluid shear stress activation of I kappa B kinase is integrin-dependent. J. Biol. Chem. 273, 30544–30549 (1998).
Schepers, A. et al. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 26, 2063–2069 (2006).
Fu, C. H. et al. Monocyte chemoattractant protein-1/CCR2 axis promotes vein graft neointimal hyperplasia through its signaling in graft-extrinsic cell populations. Arterioscler. Thromb. Vasc. Biol. 32, 2418–2428 (2012).
Shintani, T. et al. Intraoperative transfection of vein grafts with the NF kappa B decoy in a canine aortocoronary bypass model: A strategy to attenuate intimal hyperplasia. Ann. Thorac. Surg. 74, 1132–1137 (2002).
Miyake, T. et al. Inhibitory effects of NF kappa B decoy oligodeoxynucleotides on neointimal hyperplasia in a rabbit vein graft model. J. Mol. Cell. Cardiol. 41, 431–440 (2006).
Wang, Y. J., Wang, J. T., Fan, Q. X. & Geng, J. G. Andrographolide inhibits NF-kappa B activation and attenuates neointimal hyperplasia in arterial restenosis. Cell Res. 17, 933–941 (2007).
Fledderus, J. O. et al. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28(7), 1339–1346 (2008).
Nam, D. et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297, H1535–H1543 (2009).
White, S. J. et al. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J. Cell. Physiol. 226, 2841–2848 (2011).
Acknowledgements
This research was supported by the National Institute for Health Research Bristol Cardiovascular Biomedical Research Unit at the University Hospitals Bristol NHS Foundation Trust, NIHR Biomedical Centre at the University Hospitals Bristol NHS Foundation Trust, Above and Beyond charitable fund and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Author information
Authors and Affiliations
Contributions
Conceptualisation: A.O.W, M.Z, and S.J.G. Methodology: A.O.W, M.Z, and S.J.G. Formal analysis: A.O.W. Investigation: A.O.W. and M.Z. Writing original draft and editing: A.O.W, M.Z, and S.J.G. Manuscript editing: G.D.A, P.C.E., J.L.J, R.M.T, M.C, and M.S.S. Supervision: M.Z, M.S.S., M.C., G.D.A. and S.J.G. Project administration and funding acquisition: M.Z, G.D.A, M.C. and S.J.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Video S1.
Supplementary Video S2.
Supplementary Video S3.
Supplementary Video S4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ward, A.O., Angelini, G.D., Caputo, M. et al. NF-κB inhibition prevents acute shear stress-induced inflammation in the saphenous vein graft endothelium. Sci Rep 10, 15133 (2020). https://doi.org/10.1038/s41598-020-71781-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71781-6
This article is cited by
-
Distinct Temporal Pattern of the Prediction of Lumen Remodeling of Lower Extremity Vein Bypass Grafts by Initial Local Hemodynamics
Annals of Biomedical Engineering (2023)
-
Mechanobiology of the endothelium in vascular health and disease: in vitro shear stress models
Cardiovascular Drugs and Therapy (2023)
-
Wall Shear Stress Differences Between Arterial and Venous Coronary Artery Bypass Grafts One Month After Surgery
Annals of Biomedical Engineering (2022)
-
High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.