Abstract
Midkine (MDK), a heparin-binding growth factor cytokine, is involved in the pathogenesis of kidney diseases by augmenting leukocyte trafficking and activation. Animal models and small case control studies have implicated MDK as a pathological biomarker in chronic kidney diseases (CKD), however this is yet to be confirmed in prospective human studies. In a prospective study of 499 elderly, predominantly Caucasian women aged over 70 years the association between serum MDK collected in 1998, and renal function change and the risk of CKD-related hospitalisations and deaths at 5 and 14.5 years, respectively, was examined. Baseline serum MDK was not associated with 5-year change in estimated glomerular filtration rate using the CKD Epidemiology Collaboration creatinine and cystatin C equation (Standardised β = − 0.09, 95% confidence interval − 3.76–0.48, p = 0.129), 5-year rapid decline in renal function (odds ratio = 0.97, 95% confidence interval 0.46–2.02, p = 0.927) or the risk of 14.5-year CKD-related hospitalisations and deaths (hazard ratio = 1.27, 95% confidence interval .66–2.46, p = 0.470) before or after adjusting for major risk factors. In conclusion, in this cohort of elderly women with normal or mildly impaired renal function, serum MDK was not associated with renal function change or future CKD-related hospitalisations and deaths, suggesting that MDK may not be an early biomarker for progression of CKD.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a major global health concern, estimated to affect approximately 8–16% of the population worldwide1. End-stage kidney disease (ESKD), the most feared outcome for patients with CKD, is also associated with co-morbidities such as cardiovascular disease (CVD), and premature mortality2. The current standard measures such as estimated glomerular filtration rate (eGFR) and albuminuria are moderate performers at predicting disease progression to end-stage disease. Better estimates at earlier stage disease are needed to enable therapeutic interventions to minimise progression. While there have been a selection of biomarkers used to assess both innate kidney function and kidney damage3, most of these biomarkers have not been found sufficiently predictive of kidney function decline or disease progression to be used in routine clinical practice.
Midkine (MDK) is a heparin-binding cytokine most notably known for its role in embryonic development and cancer through promoting cell migration, survival, proliferation and angiogenesis4,5,6,7. In addition to its mitogenic properties, MDK also displays immunomodulatory functions which have been demonstrated in various inflammatory and autoimmune diseases8,9,10,11,12. Mechanism studies have shown that in the kidney, MDK promotes chemokine production and leukocyte trafficking post-kidney injury, and has been associated with increased renal inflammation, fibrosis and deterioration, indicating that MDK may play a role in mediating the pathogenesis of kidney diseases13,14,15,16,17.
A number of observational studies of individuals with acute kidney injury (AKI) or CKD have suggested MDK is clinically relevant with renal disease. One cross-sectional study in a CKD cohort showed that serum and urine MDK levels were inversely correlated with eGFR, and also elevated in patients with CKD stage 3 or above18. A few studies have reported that serum and urine MDK are predictive of AKI outcomes in post-cardiac surgery patients19,20,21. However, there is no prospective data available about the association between MDK and renal function decline and CKD development. This study aimed to determine the association between serum MDK and longitudinal changes in renal function; and CKD-related clinical events in a large cohort of elderly women with long-term clinical follow-up.
Methods
All methods were carried out in accordance with relevant guidelines and regulations.
Study population
The participants for this study were originally recruited in 1998 to a 5-year prospective, randomised, controlled trial of oral calcium supplements (1.2 g of elemental calcium daily), or an identical placebo to prevent osteoporotic fractures, the Calcium Intake Fracture Outcome Study (CAIFOS)22. Briefly, women aged over 70 years were recruited from the Western Australian general population by mail using the electoral roll, which is a requirement of Australian citizenship. Of the 5,586 who were approached, 1,500 were consented and recruited for the study. All participants were ambulant with an expected survival beyond 5 years and were not receiving any medication (including hormone replacement therapy) known to affect bone metabolism. Baseline disease burden, CVD risk and medications were comparable between these participants and the general population of similar age although these participants were more likely to be from higher socio-economic groups22. At the conclusion of CAIFOS, participants were subsequently included in additional follow-up studies for a further 10 years as the Perth Longitudinal Study of Aging in Women (PLSAW) https://www.lsaw.com.au, for a total follow-up period of 15 years. All participants that received placebo, or calcium treatment (calcium treatment code) from the CAIFOS were included for this study. Inclusion criteria for participants included all available exposure, confounding and outcome variables. Exclusion criteria included missing or invalid data on serum MDK, missing data on serum creatinine, cystatin C or both, and loss of follow-up data for clinical events (see Supplementary Fig. S1 and Supplementary Table S1 online).
Baseline assessment
Baseline characteristics were assessed to determine potential confounding variables, which included age, body mass index (BMI), medical history and renal function. Treatment code (placebo or calcium) over the 5 years of the CAIFOS trial was also included as a covariate. Baseline medical history (diabetes and hypertension) was available from the demographic questionnaire, with the latter being verified by the participants’ general practitioners where possible. These data were coded using the International Classification of Primary Care-Plus (ICPC-Plus) method23. The coding methodology allows aggregation of different terms for similar pathological entities as defined by the ICD-10 coding system. Previous atherosclerotic vascular disease (ASVD) from 1980–1998 was determined from the Western Australian Hospital Morbidity Data Collection of primary discharge diagnosis24. The use of cardiovascular medications including blood pressure lowering agents and statins was assessed. Weight was assessed using digital scales with participants wearing light clothes and no shoes. Height was assessed using a stadiometer and the BMI was calculated using the following equation = weight (kg)/[height (m)]2. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine and cystatin C formula25.
Biochemistry
Venous blood samples were collected between 0830 and 1030 h after overnight fasting at baseline and were stored at − 80 °C until assessment. In 2005, serum creatinine was measured from baseline serum samples using an isotope dilution mass spectrometry (IDMS) traceable Jaffe kinetic assay for creatinine (Hitachi 917 auto-analyzer, Roche Diagnostics GmbH, Mannheim Germany), whereas 5-year serum creatinine was measured in 2012 on the Architect ci16200 analyzer (Abbott Laboratories, Abbott Park, Ill., USA)26. Serum cystatin C was measured using a fully automated particle-enhanced immunoturbidimetric assay with Sentinel Diagnostics reagents (Sentinel CH, Milan, Italy) on the Architect ci 16200 System (Abbott Laboratories, Abbott Park, Ill., USA) according to the manufacturer’s instructions. The correlation coefficient (r2) between the machines was 0.998 with a Passing and Bablok slope of 0.966 and a Passing and Bablok intercept of 6.16 (n = 37)26.
Midkine assay
MDK was measured in 2019 using a colourimetric enzyme-linked immunosorbent assay (human MDK SimpleStep ELISA kit—product ab193761: Abcam, Sydney, Australia)27 in baseline serum samples on the BioTek ELx800 Absorbance Microplate reader with BioTek Gen5 plate reading software (BioTek, Vermont, USA). The sensitivity (lowest detection limit) was 5 pg/mL, while the highest detection limit was 2096 pg/mL. The intra-assay and inter-assay coefficient of variance (CV) values were 5.57% and 14.26% respectively. For statistical purposes all samples below the lowest detection limit (n = 1) were assigned a value half of the lowest detection limit (2.5 pg/mL), while all values above the highest detection limit (n = 8) were assigned a value of the highest detection limit (2096 pg/mL). A change in the Abcam MDK SimpleStep ELISA kit lot led to high background absorbance for the standards (p < 0.001 for difference in kits) which could not be rectified after suggested amendments to the protocols from the manufacturer. We therefore excluded all results from the second batch of kit lots (n = 17 plates).
Assessment of follow-up chronic kidney disease-related hospitalisations and deaths
The Western Australian Hospital Morbidity Data Collection of primary discharge diagnosis for hospitalisation and the Western Australian Deaths Registry have collected data on all hospital facilities and deaths occurring in Western Australia since 1980. At the commencement of this study in 1998 participants gave consent to access these registries, thus complete ascertainment for all CKD events causing hospitalisation and/or death for the 14.5 years from 1998 were available for all participants in this study. Deaths Registry data were coded from parts 1 and 2 of the death certificate and used the text fields from the death certificate to ascertain the cause(s) of deaths when necessary. These CKD events were linked to participants in this study via the Western Australian Data Linkage System (WADLS)28. Outcomes were identified using the International Classification of Diseases, Injuries and Causes of Death Clinical Modification (ICD-9-CM)29 and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification (ICD-10-AM)30. These codes included renal failure (ICD-9-CM codes 585.0–586.0, ICD-10-AM codes N18–19) from the primary discharge diagnoses.
Statistical analysis
Baseline characteristics of the study participants were reported as mean (x̅) and standard deviation (SD) for continuous variables, frequencies (n) and percentages (%) for categorical variables, and median (x̃) and interquartile range (IQR) for variables not normally distributed. Baseline serum MDK was not normally distributed and was transformed using natural logarithm (ln). Serum MDK levels were used as either a continuous variable (ln-transformed, pg/mL), or a dichotomous category below or above the median value. Spearman’s correlation was used to assess univariate associations between serum MDK and continuous baseline clinical variables. Unadjusted and multivariable-adjusted linear regression analysis was used to assess the association between serum MDK with 5-year change in eGFR. The association between serum MDK and rapid decline in renal function, defined as an eGFR decline of greater than 15 mL/min/1.73 m2 at 5 years31,32, was assessed using unadjusted and multivariable-adjusted logistic regression analysis. The association between serum MDK with the occurrence of any CKD-related hospitalisation and death at 14.5 years was assessed using unadjusted and multivariable-adjusted Cox regression analysis. No violations of the proportional hazard assumptions were detected (p > 0.05). Two models of adjustment were used for all regression analyses: multivariable-adjusted and multivariable and baseline eGFR-adjusted. The multivariable-adjusted models included age, calcium treatment code, diabetes, and prevalent ASVD. Baseline eGFR was adjusted for separately as eGFR is a strong predictor of longitudinal kidney disease outcomes. Results were expressed as either odds or hazard ratio (OR or HR) with 95% confidence interval (CI) for the logistic and Cox regression models respectively. P values of less than 0.05 in two-tailed testing were considered statistically significant. All analyses were performed using IBM SPSS (version 24; SPSS Inc., Chicago, Ill., USA).
Ethics and trial registration
The randomised, controlled trial was commenced and completed before the introduction of clinical trial registries, hence the trial was retrospectively registered in the Australian New Zealand Clinical Trials Registry ACTRN12615000750583. At baseline written informed consent was obtained from all participants for the study and follow-up of electronic health records. The Human Ethics Committee of the University of Western Australia approved the study protocol and consent form (Approval Number 05/06/004/H50). The Human Research Ethics Committee of the Western Australian Department of Health (DOHWA HREC) also approved the data linkage study (Approval Number #2009/24).
Results
Baseline characteristics
The median age of this study cohort was 75, with interquartile range (IQR) of 73 to 77 years. 45.9% and 18.0% of participants were maintained on anti-hypertensive and statin medications, respectively, while 6.8% and 13.8% of participants entered the study with prevalent diabetes and ASVD, respectively (Table 1). The mean (± SD) eGFR of the study cohort was 65.1 (± 12.7) mL/min/1.73 m2. 34.1% of participants had baseline eGFR less than 60 mL/min/1.73 m2.
Baseline serum MDK
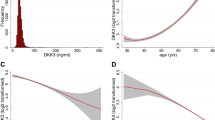
Data on serum MDK at baseline was available in 499 women (see Supplementary Fig. S1 online). Serum MDK levels were not normally distributed (see Supplementary Fig. S2 online). The median [IQR] serum MDK level was 437 [328, 580] pg/mL. Baseline characteristics were stratified below or above the median serum MDK (Table 1). Neither age nor BMI were different between participants with serum MDK above or below the median, nor were correlated with serum MDK (see Supplementary Table S2 online).
A total of 422/499 (84.6%) of participants with data on serum MDK had data on baseline serum creatinine and cystatin C to allow calculation of eGFR (see Supplementary Fig. S1 online). Baseline eGFR did not differ between participants with serum MDK above or below the median (Table 1). Baseline eGFR, serum creatinine and cystatin C were not correlated with serum MDK (Supplementary Table S2 online).
Women with a history of ASVD had serum MDK levels significantly higher than those without (with ASVD median [IQR] 503 [385, 666 pg/mL], without ASVD 435 [323, 562 pg/mL], Mann–Whitney U test p = 0.016).
Serum MDK and 5-year eGFR change/rapid decline in renal function
During follow-up there were 297/422 (70.4%) participants from this cohort with data on serum creatinine and cystatin C at 5 years for calculation of eGFR (see Supplementary Fig. S1 online). There were no associations between natural logarithm (ln)-serum MDK with 5-year eGFR change in unadjusted (Spearman’s rho = − 0.08, p = 0.297), multivariable-adjusted (Standardised β = − 0.09, 95% CI 3.25–0.40, p = 0.126) or multivariable and baseline eGFR-adjusted (Standardised β = − 0.09, 95% CI 3.28–0.28, p = 0.098) analyses. Similarly, below or above-median serum MDK was not associated with 5-year eGFR change in unadjusted (Spearman’s rho = − 0.06, p = 0.304), multivariable-adjusted (Standardised β = − 0.08, 95% CI 3.78–0.56, p = 0.146), or multivariable and baseline eGFR-adjusted (Standardised β = − 0.09, 95% CI 3.76–0.48, p = 0.129) analyses. Rapid decline in renal function was identified in 33/297 (11.1%) participants at 5 years. There were no associations between ln-serum MDK with 5-year rapid decline in renal function in unadjusted (OR 1.20, 95% CI 0.63–2.28, p = 0.573), multivariable-adjusted (OR 1.23, 95% CI 0.63–2.41, p = 0.538) or multivariable and baseline eGFR-adjusted (OR 1.26, 95% CI 0.64–2.46, p = 0.506) analyses. Similarly, below or above-median serum MDK was not associated with 5-year rapid decline in renal function in unadjusted (OR 0.96, 95% CI 0.46–1.98, p = 0.902), multivariable-adjusted (OR 0.96, 95% CI 0.46–2.01, p = 0.916) or multivariable and baseline eGFR-adjusted (OR 0.97, 95% CI 0.46–2.02, p = 0.927) analyses.
Serum MDK and CKD-related hospitalisations and deaths
CKD-related hospitalisations and deaths occurred in 47/499 (9.4%) participants over the 14.5 years of follow-up. The incidence of CKD-related hospitalisations and deaths in participants with below-median serum MDK was 18 (7.2%), and in those with above-median serum MDK it was 61% higher at 29 (11.6%) (Chi-squared test p = 0.089). In unadjusted Cox Proportional Hazard analysis the HR for participants with above-median serum MDK compared to those with below-median serum MDK was 1.82 (95% CI 1.01–3.28, p = 0.046). However in multivariable-adjusted, and multivariable and baseline eGFR-adjusted analyses the HR were 1.72 (95% CI 0.96–3.11, p = 0.071) and 1.27 (95% CI 0.66–2.46, p = 0.470), respectively (Table 2 and Fig. 1).
Multivariable-adjusted Cox proportional HR and 95% CI for 14.5-year CKD-related hospitalisations and deaths (n = 47) in elderly women stratified by serum MDK below (< 437 pg/mL, dashed red line, referent) or above (≥ 437 pg/mL, solid black line) the median. Multivariable model was adjusted for age, calcium treatment code, diabetes and prevalent atherosclerotic vascular disease. Multivariable HR 1.72, 95% CI 0.96–3.11, p = 0.071. CKD chronic kidney disease, MDK Midkine, HR hazard ratio, CI confidence interval.
Discussion
In this study, the baseline serum MDK levels were consistent to that previously reported in healthy populations18,33, and these levels of serum MDK were not associated with eGFR at baseline, 5-year eGFR decline, or 5-year rapid decline in renal function. Although we observed an association between above the median serum MDK levels with the risk of 14.5-year CKD-related hospitalisations and deaths in the unadjusted analysis, the association was no longer significant after adjusting for age, calcium treatment, diabetes and prevalent ASVD with or without baseline eGFR. These results suggest that circulating MDK levels consistent to that of previously reported healthy populations is not a useful biomarker in predicting renal function decline over time or CKD-related clinical events in the healthy, elderly female population.
The study results should be interpreted in context. Previous studies have reported associations between MDK and renal dysfunction and clinical outcomes in both AKI and CKD populations19,20,21. The discrepancy between our results to that of previous findings is likely due to the difference in study population characteristics – we examined an elderly yet healthy cohort with minimal comorbidities and disease burden comparable to the general population34, as opposed to other cohorts with established renal disease. These previous findings indicate that serum MDK may be a more sensitive biomarker associated with renal function changes when there is marked renal injury or dysfunction, and is therefore not as relevant to the early trajectory of renal function decline. We have also utilised serum cystatin C in addition to serum creatinine to measure eGFR in our study in contrast to previous studies which only utilised serum creatinine18,19,20,21, so the difference in findings may be due to fundamental differences in defining renal function.
Our findings may argue that MDK is not an early biomarker in chronic and age-related renal function changes and kidney disease outcomes, which is supported by both experimental and clinical evidence on the involvement of MDK in acute inflammation and kidney injury14,15,20,21. However, given that there are also a few studies addressing MDK in chronic inflammation and kidney disease cases16,17,18, this reflects the need to consider how MDK activity is regulated during the transition from acute to chronic renal inflammation and deterioration.
There were limitations with this study. There was no data available on proteinuria and albuminuria, so we were unable to determine if serum MDK was associated with these early indicators of kidney damage. The majority of serum MDK levels at baseline were within the normal range consistent with previous reports18,33. As such, we were unable to determine whether abnormal levels of serum MDK were associated with renal function decline or CKD-related clinical events. The proportions of the study population that experienced events for 5-year rapid decline in renal function and 14.5-year CKD-related hospitalisation and death were small (11.1% and 9.4%, respectively), which may have reduced our statistical power. There was loss of samples for data on serum MDK due to changing of the ELISA assay kit lots, which may also have reduced our statistical power. However, this is unlikely to have altered the magnitude of the associations as there were no significant differences between the characteristics (and therefore potential covariates in association) of the included and excluded cohorts (see Supplementary Table S3 online). Since we did not measure serum MDK levels longitudinally, we cannot exclude the possibility that MDK is a pathological biomarker in CKD. Finally, our study was limited to generally healthy, older predominantly Caucasian women so the findings may not apply to other demographic or disease populations, such as those with diabetes who are at increased risk of CKD.
The strengths of this study include the use of both serum creatinine and cystatin C for the measurement of eGFR, which improved our ability to more accurately define baseline kidney function and CKD outcomes. The study had a long follow-up period of 14.5 years, minimal loss to follow-up, and detailed prospective data collection using a comprehensive, population-based linkage system. This is also the first study to prospectively examine the relationship between MDK, renal function and clinical outcomes of CKD, and therefore provide insights to assess the clinical value of MDK as a viable prognostic biomarker.
In conclusion, baseline serum MDK is not associated with long-term renal function decline or CKD-related hospitalisations and deaths in healthy, elderly women with normal or mildly impaired renal function. Further studies are required to confirm the relationship between MDK and longitudinal renal function and CKD outcomes in populations with prevalent kidney disease.
Data availability
The datasets generated during and/or analysed in this study can be viewed online at https://www.lsaw.com.au/ and are available from Prof Richard L. Prince at richard.prince@uwa.edu.au on reasonable request.
References
Jha, V. et al. Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 (2013).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Lopez-Giacoman, S. & Madero, M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J. Nephrol. 4, 57–73 (2015).
Kadomatsu, K. The midkine family in cancer, inflammation and neural development. Nagoya J. Med. Sci. 67, 71–82 (2005).
Fan, Q. W., Muramatsu, T. & Kadomatsu, K. Distinct expression of midkine and pleiotrophin in the spinal cord and placental tissues during early mouse development. Dev. Growth Differ. 42, 113–119 (2000).
Mitsiadis, T. A., Muramatsu, T., Muramatsu, H. & Thesleff, I. Midkine (MK), a heparin-binding growth/differentiation factor, is regulated by retinoic acid and epithelial-mesenchymal interactions in the developing mouse tooth, and affects cell proliferation and morphogenesis. J. Cell Biol. 129, 267–281 (1995).
Ota, T. et al. Midkine expression in malignant salivary gland tumors and its role in tumor angiogenesis. Oral Oncol. 46, 657–661 (2010).
Shindo, E. et al. The growth factor midkine may play a pathophysiological role in rheumatoid arthritis. Mod. Rheumatol. 27, 54–59 (2017).
Wang, J. et al. Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc. Natl. Acad. Sci. USA 105, 3915–3920 (2008).
Weckbach, L. T. et al. The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via beta2 integrins (CD11/CD18). Blood 123, 1887–1896 (2014).
Obama, H. et al. Myocardial infarction induces expression of midkine, a heparin-binding growth factor with reparative activity. Anticancer Res. 18, 145–152 (1998).
Hobo, A. et al. The growth factor midkine regulates the renin-angiotensin system in mice. J. Clin. Invest. 119, 1616–1625 (2009).
Kadomatsu, K., Huang, R. P., Suganuma, T., Murata, F. & Muramatsu, T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J. Cell Biol. 110, 607–616 (1990).
Sato, W. et al. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J. Immunol. 167, 3463–3469 (2001).
Sato, W. et al. Midkine antisense oligodeoxyribonucleotide inhibits renal damage induced by ischemic reperfusion. Kidney Int. 67, 1330–1339 (2005).
Kosugi, T. et al. Growth factor midkine is involved in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 168, 9–19 (2006).
Kosugi, T. et al. Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab. Invest. 87, 903–913 (2007).
Campbell, V. K. et al. Urine and serum midkine levels in an Australian chronic kidney disease clinic population: an observational study. BMJ Open 7, e014615 (2017).
Hayashi, H. et al. Efficacy of urinary midkine as a biomarker in patients with acute kidney injury. Clin. Exp. Nephrol. 21, 597–607 (2017).
Malyszko, J. et al. Midkine: a novel and early biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Biomed. Res. Int. 2015, 879509 (2015).
McBride, W. T. et al. Stratifying risk of acute kidney injury in pre and post cardiac surgery patients using a novel biomarker-based algorithm and clinical risk score. Sci. Rep. 9, 16963 (2019).
Prince, R. L., Devine, A., Dhaliwal, S. S. & Dick, I. M. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch. Intern. Med. 166, 869–875 (2006).
Britt, H., Scahill, S. & Miller, G. Icpc plus© for community health? A feasibility study. Health Inf. Manag. 27, 171–175 (1997).
Lewis, J. R. et al. Estimated glomerular filtration rate as an independent predictor of atherosclerotic vascular disease in older women. BMC Nephrol. 13, 58 (2012).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367, 20–29 (2012).
Lim, W. H. et al. Comparison of estimated glomerular filtration rate by the chronic kidney disease epidemiology collaboration (CKD-EPI) equations with and without Cystatin C for predicting clinical outcomes in elderly women. PLoS ONE 9, e106734 (2014).
Rice, L. M. et al. Serum biomarker for diagnostic evaluation of pulmonary arterial hypertension in systemic sclerosis. Arthritis Res. Ther. 20, 185 (2018).
Holman, C. D. et al. A decade of data linkage in Western Australia: strategic design, applications and benefits of the WA data linkage system. Aust. Health Rev. 32, 766–777 (2008).
World Health Organization. Manual of the international statistical classification of disease, injuries, and causes of death. Based on the recommendations of the eighth revision conference, 1965, and adopted by the Nineteenth World Health Assembly. (1967).
World Health Organization. International statistical classification of diseases and related health problems Vol. 1 (World Health Organization, Geneva, 2004).
Rifkin, D. E. et al. Rapid kidney function decline and mortality risk in older adults. Arch. Int. Med. 168, 2212–2218 (2008).
Shlipak, M. G. et al. Rapid decline of kidney function increases cardiovascular risk in the elderly. J. Am. Soc. Nephrol. 20, 2625–2630 (2009).
Jones, D. Measuring midkine: the utility of midkine as a biomarker in cancer and other diseases. Br. J. Pharmacol. 171, 2925–2939 (2014).
Dunstan, D. W. et al. The Australian diabetes, obesity and lifestyle study (AusDiab): methods and response rates. Diabetes Res. Clin. Pract. 57, 119–129 (2002).
Acknowledgements
The authors wish to thank the staff at the Western Australia Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing the data for this study. The study was supported by research grants from Healthway Health Promotion Foundation of Western Australia and by project Grants 254627, 303169, and 572604 from the National Health and Medical Research Council of Australia. J.W. is supported by the Australian Government Research Training Program Scholarship. The salary of J.R.L. is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID: 102817). The funding sources had no role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors wish to thank Nasima Shirzad for performing the Midkine ELISA assays.
Author information
Authors and Affiliations
Contributions
J.W., V.W.L., Q.C., J.R.L and R.L.P conceived and designed the study; J.R.L. and E.B collected the data; J.W., J.R.L., E.B., W.R.D. and K.Z. analysed the data; J.W. prepared the manuscript; all authors critically reviewed the manuscript; J.W. had the primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Graham Robertson is an employee of Lyramid Limited. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Lewis, J.R., Byrnes, E. et al. Serum Midkine, estimated glomerular filtration rate and chronic kidney disease-related events in elderly women: Perth Longitudinal Study of Aging Women. Sci Rep 10, 14499 (2020). https://doi.org/10.1038/s41598-020-71353-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71353-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.