Abstract
The study of moulting behaviour in the fossil record is relatively well known in arthropods and this is especially true for trilobites. Nevertheless, while studies focusing on the style of moulting in social and semi-social groups of modern animals (e.g. arthropods) are common, very few works investigate moulting adaptations in deep time. Here we report a trilobite assemblage from the Cambrian Series 2 “Tsinghsutung” Formation of South China. Around 850 specimens were used for this study from three different levels across one section near Balang (SE Guizhou Province, South China). These levels preserve numerous trilobite clusters in some cases containing around 400 individual specimens. Up to four species have been found in these clusters, but two species are more common. Trilobite clusters bear a high percentage of disarticulated specimens that we interpret as moults. Additionally, measurements of bioclast orientation and the dorsoventral attitude suggests very quiet water conditions followed by rapid burial events, prior to scavenger disturbance. Together, this indicates that the fossil assemblages were a result of a biological phenomenon rather than mechanical processes, allowing us to interpret the position of the fossil parts as different moulting configurations. Since the trilobite assemblage seems to be in situ, the large number of exuviae suggests a local place of migration. This was triggered by the need for group protection while moulting, which is suggestive of gregarious behaviour, possibly synchronized. These trilobites from the Cambrian Epoch 2, Age 4 constitute one of the earliest known gregarious community of trilobites and has important implications for understanding the ecology of this group during their emergence in the Cambrian.
Similar content being viewed by others
Introduction
Arthropods (i.e. insects, spiders, crustaceans, myriapods and others), are the most successful Phanerozoic animals. This group of segmented jointed-limbed animals is characterized by the possession of a hard cuticle that is episodically moulted. Moulting allows growth but also occasionally repairs injures of the old exoskeleton1,2. However, moulting represents a risk, as for a short period of time after moulting, the new exoskeleton is too soft for protection against predators and conspecifics3,4,5,6. Consequently, arthropods have developed different morphological adaptations and behaviours to overcome this problem3. Morphological adaptations such as cuticle thickening or spines appear early in the fossil record and are likely a response to predation7. Behavioural adaptations, on the other hand, represent a more complicated form of protection and require a high degree of social organization5,8,9,10,11,12. One of these adaptations is the synchronized moulting seen in modern social and subsocial groups of arthropods such as spiders, shrimps, prawns, crabs and springtails5,8,9,10,11 and its presence is being increasingly observed in the fossil record (e.g. trilobites, eurypterids, megacheirans and other crusaceomorphs, see12,13,14,15,16,17,18).
Trilobites, as part of the Arthropoda clade, were no exception to this behaviour. Together with eurypterids and crustaceans, trilobites contribute the majority of the data available regarding moults in the fossil record6. However, research has predominantly been focused on the description of the moulting mechanics rather than the evolutionary and ecological importance of moulting in extinct species2,6. Synchronized moulting behaviour in trilobites has been previously suggested for assemblages of carcasses and moults of Balcoracania dailyi Pocock, 197019, Homotelus bromidensis (Esker, 1964)20 (see14,16) and the linear clusters of Ampyx priscus Thoral, 193521 in Vannier et al.18. Like in other groups of arthropods, synchronized moulting in trilobites may also be due to a variety of abiotic factors, such as temperature, tides or photoperiod and lunar cycles, or due to biotic factors such as predators, age, reproductive status or feeding (see8,17,22). In this study we report and discuss the early evidence for gregarious moulting of two oryctocephalid species from the ‘Tsinghsutung’ Formation, Cambrian Series 2 (Stage 4) of Balang, South China.
Results
Trilobite preservation
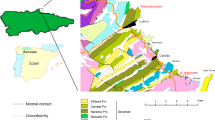
Specimens in this study come from three levels in the ‘Tsinghsutung’ Formation, Q51, Q52 and Q53, and are preserved in the majority of cases as complete individuals (i.e. articulated), although some sclerites are absent in a few cases (see moulting configurations and discussion below) (Fig. 1, see Fig. 1S supplementary material). Trilobites from these levels are typically preserved through replacement by the mineral illite. The illite degrades into iron oxides, and after dissolution has the ability to produce good quality internal and external moulds23,24.
Bioclast abundance, composition and articulation
Bioclasts in the slabs are densely packed aggregates of semi-articulated specimens, sometimes concentrated in a specific region of the slab and are occasionally overlapping (Fig. 1). Clusters consist of 4 to 407 individuals and, although isolated specimens were also found, they were unusual in these three beds. A total of 857 individuals were included in this analysis (114 in Q51, 488 in Q52 and 255 in Q53). The clusters are mostly formed by complete or nearly complete individuals with a very high percentage of articulation, the majority being complete specimens with absent ventral cephalic structures (Fig. 2). The absence of ventral cephalic structures indicates that the majority of these specimens are moults. The abundance of bioclasts also varies within slabs with the density of individuals fluctuating across the slabs (see Fig. 1), but those were never compacted enough to suggest a hash-surface sensu Webster et al.23. Here we divided the bioclast composition into five different categories (Fig. 2) predominantly based on the definitions outlined by Webster et al.23: complete cephalon-trunk (CCT), disarticulated cephalon-trunk (DCT), isolated part of cephalon (IPC), isolate trunk fragment (ITF) and isolated sclerites (IS). Even though isolated sclerites of trilobites are common in the fossil record, especially in Lagerstätten25, this study shows the opposite trend, as the most common kind of bioclasts are highly articulated specimens (Fig. 2), suggesting low current flows and rapid burial deposition. Samples of DCT show the highest percentage of individuals (ca. 60.3%) (Predominantly only lacking the ventral cephalic structures, ca. 82,33%), followed by CCT (ca. 18.7%), with 79% very articulated individuals versus 20.6% isolated fragments of trunk (ITF) (ca. 14.3%) and cephalon (IPC) (ca. 6.3%); and finally a very small component of isolated trunk sclerites (IS) (ca. 0.4%). These proportions of trilobite elements suggest moulting configurations rather than disarticulation due to the environment (see moulting configuration).
(a–c) Bioclast dorso-ventral attitude; (a) Q51; (b) Q52; (c) Q53. (d–f) Bioclast composition; (d) Q51; (e) Q52; (f) Q53. CCT complete cephalotrunk, DCT disarticulated cephalotrunk, ITF isolated thoracic fragment, PC part of cephalon, IS isolated sclerite. Plotted using Matplotlib library65.
Taxonomic composition
Four species were found in these three beds: two oryctocephalids (Protoryctocephalus arcticus Geyer & Peel, 201126 and Duyunaspis duyunensis Zhang & Qian in Zhou et al., 197727), one burlingiid (Burlingia balangensis Yuan & Esteve, 201528) and an isolated poorly preserved cranidia of cf. Bathynotus in the Q52 (Fig. 3). Although P. arcticus and D. duyunensis seem to be codominant, in each single plate the dominance of one species is clearly showing segregation (Fig. 3a–k). P. arcticus was the most abundant in the largest slabs belonging to Q52 (Figs. 1, 3), however, D. duyunensis was the most commonly found species in most slabs from Q51 and Q53. B. balangensis was rarely observed and completely absent in Q53 (Fig. 3).
Percentage of taxonomic composition for each one of the slabs (a) Q51-1207, (b) Q51-1213, (c) Q51-1216, (d) Q51-12188, (e) Q52-348, (f) Q52-2391, (g) Q52-4675, (h) Q52-674, (i) Q53-15, (j) Q53-461, (k) Q53-933-8941, and (l) total. (a–d) Q51; (e–h) Q52; (i–k) Q53; and (l) total. Plotted using Matplotlib library65.
Bioclast size distribution and ontogeny
In order to obtain the average size of each individual within the sample we measured three linear measurements: (i) maximum length; (ii) maximum width and (iii) maximum cephalic width. Although, Protoryctocephalus arcticus and Duyunaspis duyunensis were nearly equally abundant (359 and 336 individuals respectively), the size distribution of these species shows clear differences. D. duyunensis had a normal distribution while P. arcticus shows a bimodal distribution, most noticeably in specimens between 0.5 and 1.5 mm in width and specimens between 2.5 and 3.5 mm in width (Fig. 4). This bimodal distribution in P. arcticus may be explained by differences between the sample sites within the ‘Tsinghsutung’ Formation, however this seems to be well supported throughout Q52 and Q53, regardless of the type of measurement or the quantity of D. duyunensis (Fig. 5).
Size distribution of complete sclerites. (a–c) Sclerite length; (e–g) sclerite width; (h–j) cephalic width. Analysis for all individuals in (a), (e) and (h) P. arcticus in (b), (f) and (i) and D. duyunensis in (c), (g) and (j). Gray area indicates holaspid individuals. Plotted using Matplotlib library65.
Size distribution of complete sclerites for three measures. (a–d) Sclerite length; (e, f) sclerite width; and (i–l) cephalic width in lower row. Analysis for each level: all individuals in first column, followed by Q51, Q52 and Q53. Plotted using Matplotlib library65.
The ontogenetic series of these trilobites have not been studied yet but some of their developmental characters are noted. Both species show heminanamorphic development29 in which an anamorphic phase of development (i.e. sequential appearance of additional segments) is followed by an epimorphic phase (i.e. sequential moults retain a constant number of segments). This is used to separate the meraspid and holaspid stages. Axial length in the meraspid stage ranges from 0.86 to 5.68 mm in P. arcticus and from 1.72 to 5.18 mm in D. duyunensis; and maximum width from 0.59 to 3.30 mm in P. arcticus and from 0.98 to 3.09 mm in D. duyunensis.
Dorso-ventral attitude and orientation
Dorso-ventral attitude and orientation are important features that can be employed to distinguish between trilobites that were accumulated biologically versus mechanically. The dorsal up attitude was dominant in two of the three beds (Q51 and Q53) and half of the samples in the case of Q52 are also dorsal up (Fig. 2). Given the size difference of the articulated specimens, samples displaying a width between 0.59 and 5.04 mm were divided into six different categories. No preferred orientation in any of the categories has been found in any of the three beds (Fig. 6). Although the sample from the Q52 bed shows a slightly denser orientation of ENE-WSW, the small K value (von Mises distribution) indicates a uniform distribution of the sample directions (0.01563, 0.03699, 0.08031).
Orientation of complete sclerites for each level: (a) Q51, (b) Q52 and (c) Q53. Green color scales represent the sclerite length. Plotted using Windrose graphic tool67.
Moulting configurations and moulting behaviours
Our samples show every kind of moulting behaviour previously documented in the literature with a variety of moulting configurations. The majority of P. arcticus and D. duyunensis moults from the ‘Tsinghsutung’ Formation were found as axial shields (c. 46%, n = 396) (Fig. 7a). We found c. 3% of moults with Maksimova’s configuration (Fig. 7f), which is characterized by displaying a complete exoskeleton with the ventral cephalic unit displaced. These configurations agree with the ecdysial behaviour “facial sutures opened” (Fig. 3Sa–e). The assemblage also shows specimens with displaced cephalons or cranidium from the thorax forming an exuvial gape (Fig. 7c, g), which corresponds with the cephalon or cranidium removed behaviour (c. 2%, n = 19) (Fig. 3Sd–g, j). Four moults (c. 0.5%) had one or various dislocations along the thorax (Fig. 7d) and a large number of specimens were isolated thoracic segments (c. 19%, n = 159). This corresponds to the behaviour of thoracic dislocation (c. 0.3%, n = 3) (Fig. 3i). Only c. 14% (n = 117) of the assemblage are complete moults, although a few moults were nearly complete with only a missing pygidium (Fig. 7e), i.e. a pygidium—displaced behaviour (c. 0.2%, n = 2) (Fig. 3Sh). Shields missing the cranidium represented c. 1.4% (n = 12) of the moults (Fig. 7b) and there were also a few occurrences of Henningsmoen’s (Fig. 7h), Somersault’s (Fig. 7j) and Salter’s Configurations (Fig. 7i) (Less than 1%, n = 1, n = 2, n = 4). Approximately 9% (n = 79) of specimens were a combination of two moulting configurations (e.g. facial sutures opened, thoracic or pygidium dislocation), having disarticulated librigenae without the pygidium or the trunk. Additionally, isolated cephalic heads (c. 0.1%, n = 1) and cranidia (c. 5%, n = 42) were also present (Fig. 3Sl).
Moulting configurations found in this study. (a) Axial shield (n = 396); (b) shield with missing cranidium (a variant of Henningsmoen’s configuration) (n = 12); (c) cephalon disarticulated (n = 1); (d) disarticulation along the thorax (n = 3); (e) cephalotrunk with displaced pygidium (n = 2); (f) Maksimova’s configuration (n = 2); (g) facial and cephalotrunk sutures opened (with librigenae missing) (n = 18); (h) Henningsmoen’s configuration (n = 1); (i) Salter’s configuration (n = 4); (j) Somersault’s configuration (n = 2).
Discussion and conclusions
Justification of biological clustering behaviour
Trilobite clusters from the ‘Tsinghsutung’ Formation contain up to 857 specimens and occur in densely packed aggregates. Clusters are multitaxa but species are clearly segregated. The size distribution shows a trend towards very small sizes in all taxa (size segregated); consequently, the individuals in the clusters mostly represent early and late meraspid and early holaspid stages in the case of the oryctocephalids, and early holaspid stages in B. balangensis, although the latter is a small trilobite28. However, the abundance of each taxon shows a clear bias. The percentage of the two oryctocephalids is never equal, showing antagonist behaviour, and B. balangensis constitutes only a negligible percentage in all samples. Hydrodynamic sorting or current-aligned specimens would indicate at least local transportation30. The narrow size range of specimens preserved in all our samples from the three beds suggests that size-sorting, inferred from current transportation, is a possibility31. However, the absence of a preferred orientation within any sample suggests either the current velocity was not strong enough to re-orientate bioclasts, or there was a lack of consistency in current direction. Overall, the considerable size-sorting, the absence of current-induced sedimentary structures within each bed, and the presence of non-disrupted exuviae is suggestive of a low current velocity or even an absence of currents. On the other hand, the preservation of most of the trilobite bioclasts in a dorsal-up attitude may indicate that they were either (i) subject to unidirectional currents and adopted a hydrodynamically stable disposition; or (ii) were rapidly buried, since bioturbation can flip the exuviae. The dorsal-up attitude was the life attitude, but also resulted from normal exuviation in these trilobites. A number of studies on modern arthropods have demonstrated that disarticulation occurs within hours to weeks after death or ecdysis25,30,32,33,34,35,36. Therefore, given all this evidence, mechanical aggregation can be dismissed since there was no strong preferential currents and the bioturbation index is very low. Thus, it seems reasonable to state that our sample indicates behavioural congregation and that the specimens were buried rapidly after the ecdysis without any disturbance of the exuviae.
Trilobite clusters are commonly documented from the fossil record (e.g.16,24,37,38,39,40). Speyer and Brett37 suggested three possible scenarios to explain such behavioural congregation: (i) stress-related aggregation behaviour (e.g. storm disturbances); (ii) non-reproductive gregarious behaviour (e.g. moulting); and, (iii) reproductive clustering behaviour. The size sorting of the assemblage (i.e. meraspid stages c. 89%, n = 401) does not fit the profile of stress-related aggregation behaviour. If trilobites of all sizes were looking for shelter, as a result of stress, we should find a wide size range in the assemblage. Additionally, the taxonomic composition is very low, which is not indicative of a stress-related aggregation behaviour. Clusters or assemblages with high taxonomic diversification are related to faster and instantaneous burial such as obruption events41,42. On the other hand, most of the specimens represent early ontogenetic stages (mainly meraspid). Therefore, it seems very unlikely that these clusters represent reproductive clustering behaviour. The small size of specimens and the high number of moults is therefore suggestive that these clusters represent protection during moulting, reducing the risk of predation by moulting in large numbers in a quiet environment away from strong currents.
Ecdysis (i.e. moulting) is a critical stage for arthropods that determines its growth in the early stages and, in adults, allows reproduction as the soft body of the females allows for copulation and/or produces offspring10,17,37. Many extant groups of arthropods (e.g. crustacean, insects) undertake coordinated migrations17,43 and also possess a synchronized moulting cycle (a cycle also observed in trilobites (sensu12,16,17,44). The migration is triggered by the need for protection during this period of weakness (e.g. moulting), or alternatively increases the ability to find mates, as migrations bring many individuals together in one restricted geographic area. Trenchard et al.45 suggested that other trilobite genera such as oryctocephalids may have exhibited such behaviour because the deep-water areas are close to the exaerobic zones where the number of predators is reduced39. Clusters of juveniles or larvae are also common among arthropods and have been interpreted as a nursery12,16,17. Maintaining this gregarious behaviour could also carry other advantages for early larvae and juveniles, such as maternal food consumption (trophic egg consumption and/or matriphagy) and cooperative prey capture as observed in modern spiders11,46,47. Although our sample bears a high percentage of larvae, it is difficult to support such a hypothesis at this time, so further evidence is necessary to address this. Given the morphological features known in trilobites, they were unlikely to be aggressive with conspecifics. Although spines are present in their anatomy, these were likely for protection from larger predators and not from their own species7. Thus, they were apparently able to establish a gregarious behaviour and not suffer injuries among them. On the contrary, if they were aggressive, the moult synchrony would have helped to maintain the social coexistence given the inactivity before and during ecdysis reported in actual arthropods5,11,12.
Moulting behaviour
The order Corynexochida was previously known to have four moulting behaviours: (i) Facial sutures opened; (ii) Rostral plate removed; (iii) Cephalon removed, and (iv) Cranidium removed (see2 for more details). The majority of moulting behaviours found here seem to be associated with a break between the cephalon and thorax along with a rupture of the facial suture produced by a downward dorsal flexure, which seems to be the trend within this group2,48. Nonetheless, we have found several moulting configurations which confirm that the rest of the moulting behaviours described by Daley and Drage2 and recently by Drage6 (but not figured) are also present in this trilobite order, suggesting that these behaviours were common across many trilobite orders. The available data shows that the most common moulting behaviour is the opening of the facial sutures, and the rest of the recognized behaviours are present in lower proportions. Our findings agree with the results reported by Daley and Drage2 and Drage6. However, given the high number of other behaviours reported in trilobites it seems unlikely that a relationship between configuration and behaviour exists. The sedimentological setting of the ‘Tsinghsutung’ Formation suggests a depositional environment with very little current action, where the trilobites were rapidly buried but not in a turbulent manner. Consequently, the biological meaning of the moulting configurations is uncertain. Further fieldwork to collect specimens within a sedimentological framework is necessary to assess the implications of such moulting behaviours for trilobite evolution.
This work shows one of the earliest synchronized moulting behaviours in the fossil record, and demonstrates that trilobites had complex social behaviours, providing a new insight into the ecological evolution of these early arthropods.
Material and methods
Geological setting and fossil assemblage
More than 10,000 specimens of Protoryctocephalus arcticus, Burlingia balangensis and Duyanaspis duyonenesis have been collected throughout the ‘Tsinghsutung’ Formation (123 to 250 m thick). The ‘Tsinghsutung’ Formation is within the transitional Jiangnan Slope Belt near the locality of Balang in the east of Guizhou Province, South China49,50,51. Our samples (857 specimens) were collected from three levels: 51, 52 and 53 m below the Kaili Formation (see Fig. 1S supplementary material and52). The lithology at this locality is unlike that of the Tsinghsutung or Wuxun formations studied previously in the transitional Jiangnan Slope Belt (see 51,52). This is why we have used inverted commas throughout the manuscript to indicate that the facies is similar to the Tsinghsutung Formation but with different lithological features. This formation in Balang is about 208 m thick and is mainly composed of grey to dark grey thick-bedded to massive limestone, grey thick-bedded dolomitic limestone, calcareous dolomite and grey thin- to thick-bedded dolomite and argillaceous dolomite. The samples in this study come from the Protoryctocephalus arcticus Zone. This zone occupies the middle-upper part of the ‘Tsinghsutung’ Formation and has a highly diverse trilobite assemblage: Protoryctocephalus arcticus, Protoryctocephalus balangensis, Duyunaspis duyunensis, Redlichia noblis (= Redlichia guizhouensis), Burlingia balangensis, Panxinella angustilimbata, Dinesus panxinensis, Nangops danzhaicnsis, Balangcunaspis cransversu, Mufushania cf. nakingensis, Eosoptychoparia gaodongensis, Eosoptychopariasp. cf. yunnanensis, Olenoides constrictus, and Olenoides. cf. hubeiensis53.
All the samples were coated with ammonium chlorite prior to being photographed with a Nikon D300 camera. Length, width and angular measurements of the specimens were made using ImageJ54. Drawings were made using Adobe Illustrator CC2019.
Taphonomy
Clusters can represent behavioural aggregation of individuals, but they can also be accumulated mechanically by means of currents. In this study we have followed several criteria (see below) for the identification a particular cluster type and, in turn, type of aggregation (behavioural or mechanical):
-
(1)
Type of preservation. The type of preservation tells us about the early diagenesis suffered by the trilobites, as dissolution or deep cementation can change morphological features55.
-
(2)
Percentage of articulation. Exoskeletons can be either fully articulated, which includes unbroken dorsal and ventral sutures, or partially articulated (as discussed above). Partially articulated individuals that do not represent obvious moult assemblages were also examined in terms of the proportion of various elements, i.e. cephala, axial shields, cephalothoraces and trunks. Those clusters with a high percentage of disarticulated sclerites likely represent a background time-averaging due to sediment starvation prior to burial.
-
(3)
Percentage of taxonomic composition. Clusters can be composed of either a single species (monotaxic) or two or more species (polytaxic). Those clusters with highly polytaxics and a high abundance of each taxon are suggestive of mechanical accumulation.
-
(4)
Size distribution. Individuals may be size-segregated (unimodal; but not necessarily implying a single cohort) or show a range of sizes (polymodal). A size segregation could be indicative of a stage-related biological behaviour or a size sorting due to environment conditions.
-
(5)
Orientation. Horizontal orientation is the facing direction of an individual within 360° on a horizontal bedding plane. Bioclast alignment in the azimuth orientation can provide an indication of current or wave transportation. This is especially accurate for the long axes of articulated exoskeletons33. Those unidirectional currents can re-orientate highly articulated specimens such that its sagittal axis is aligned with the current direction (anterior end pointing up-current), or such that its sagittal axis is perpendicular to the current direction, with the lateral margin facing into the current56.
-
(6)
Bioclast dorso-ventral attitude. Several experiments have demonstrated that complete, isolated cephala and cranidia adopt a hydrodynamically stable dorsal-up (convex-up) attitude when subjected to surface currents; in the absence of such currents, isolated cephala settle in an inverted (convex-down) attitude25,56,57,58,59. The dorso-ventral attitude of these sclerites can therefore offer biostratinomic insight60,61,62, with the caveats that any current-induced signal can be subsequently modified by surface scavenging (which results in predominantly convex-down sclerite attitudes; see25,60) and extensive bioturbation (which may result in random sclerite attitudes, including perpendicular or oblique to the sediment surface; see25).
-
(7)
Posture. Individuals may be prone (or outstretched) or display ventral flexure (including complete enrolment), dorsal flexure or torsion63,64. Posture, depending in the species, could indicate a rapid or slow burial.
-
(8)
Sedimentology. Sedimentary structures, lithology and microstratigraphic details (e.g., graded bedding within a microturbidite) may help elucidate the depositional setting and processes under which the cluster was preserved. The presence of certain structures could indicate direction of current flows, if present.
-
(9)
Fossils assemblage, paleoecology. The presence of other taxa may also be useful biostratinomic tools, especially biostratinomically sensitive, sessile multielement (e.g., echinoderms, Wiwaxia) and bivalved taxa (e.g., brachiopds)68.
-
(10)
Moults Vs Carcasses. In order to distinguish moults and carcasses we follow several criteria. The most important is to observe whether the specimens show suture lines with evidence of having been opened in one or several parts of the exoskeleton (e.g. both librigena)2,48,60,63. Other criteria, such as the lack of predation or scavenging evidences and lack of internal carcass features (i.e. guts) are also important criteria to distinguish moults and carcasses2,13,25,37,39,40.
Statistical analysis
The data (measures, angles and morphology) were obtained and analysed using the ImageJ software (see supplementary material, dataset 1) and the resulting plots were made using Python 2D plotting library Matplotlib65. All quantitative data (percentages) were obtained using Python software. The K value of the Von Mises distribution was obtained using the open access Past software that uses the Rayleigh's test for directional data according to Davis66.
References
Owen, A. W. Trilobite abnormalities. Earth Environ. Sci. Trans. R. Soc. Edinb. 76(2–3), 255–272 (1985).
Daley, A. C. & Drage, H. B. The fossil record of ecdysis, and trends in the moulting behaviour of trilobites. Arthropod Struct. Dev. 45(2), 71–96 (2016).
Clarkson, E. N. On the schizochroal eyes of three species of Reedops (Trilobita: Phacopidae) from the Lower Devonian of Bohemia. Earth Environ. Sci. Trans. R. Soc. Edinb. 68(8), 183–205 (1969).
Henningsmoen, G. Moulting in trilobites. Fossils Strata. 4(1), 79–200 (1975).
Howe, N. R. Partial molting synchrony in the giant Malaysian prawn, Macrobrachium rosenbergii: A chemical communication hypothesis. J. Chem. Ecol. 7(3), 487–500 (1981).
Drage, H. B. Quantifying intra-and interspecific variability in trilobite moulting behaviour across the Palaeozoic. Paleontol. Electron. 22(2) (2019).
Pates, S. & Bicknell, R. D. Elongated thoracic spines as potential predatory deterrents in olenelline trilobites from the lower Cambrian of Nevada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 516, 295–306 (2019).
Webster, S. G. Seasonal anecdysis and moulting synchrony in field populations of Palaemon elegans (Rathke). Estuar. Coast. Shelf Sci. 15(1), 85–94 (1982).
Leinaas, H. P. Synchronized moulting controlled by communication in group-living Collembola. Science 219(4581), 193–195 (1983).
Stone, R. P. Mass molting of tanner crabs Chionoecetes bairdi in a Southeast Alaska-Estuary. Alaska Fish. Res. Bull. 6(1), 19–28 (1999).
Kim, K. W. Social facilitation of synchronized molting behavior in the spider Amaurobius ferox (Araneae, Amaurobiidae). J. Insect Behav. 14(3), 401–409 (2001).
Haug, J. T., Caron, J. B. & Haug, C. Demecology in the Cambrian: Synchronized molting in arthropods from the Burgess Shale. BMC Biol. 11(1), 64 (2013).
Braddy, S. J. Eurypterid palaeoecology: Palaeobiological, ichnological and comparative evidence for a ‘mass–moult–mate’ hypothesis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 172(1–2), 115–132 (2001).
Karim, T. & Westrop, S. R. Taphonomy and paleoecology of Ordovician trilobite clusters, Bromide Formation, south-central Oklahoma. Palaios 17, 394–402 (2002).
Vrazo, M. B. & Braddy, S. J. Testing the ‘mass-moult-mate’hypothesis of eurypterid palaeoecology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 311(1–2), 63–73 (2011).
Paterson, J. R., Jago, J. B., Brock, G. A. & Gehling, J. G. Taphonomy and palaeoecology of the emuellid trilobite Balcoracania dailyi (early Cambrian, South Australia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 249(3–4), 302–321 (2007).
Błażejowski, B., Brett, C. E., Kin, A., Radwański, A. & Gruszczyński, M. Ancient animal migration: A case study of eyeless, dimorphic Devonian trilobites from Poland. Palaeontology 59(5), 743–751 (2016).
Vannier, J. et al. Collective behaviour in 480-million-year-old trilobite arthropods from Morocco. Sci. Rep. 9(1), 1–10 (2019).
Pocock, K. J. The Emuellidae, a new family of trilobites from the Lower Cambrian of South Australia. Palaeontology 13(4), 522–562 (1970).
Esker, G. C. New species of trilobites from the Bromide Formation (Pooleville Member) of Oklahoma. Oklahoma Geology Notes. 24(9), 195–209 (1964).
Thoral, M. Contribution à l’étude paléontologique de l’Ordovicien inférieur de la Montagne Noire et révision sommaire de la faune cambrienne de la Montagne Noire. (Imprimerie de la Charité, Montpellier, 1935).
Passano, L. M. Molting and its control. In Metabolism and Growth (1960).
Webster, M., Gaines, R. R. & Hughes, N. C. Microstratigraphy, trilobite biostratinomy, and depositional environment of the “lower Cambrian” Ruin Wash Lagerstätte, Pioche Formation, Nevada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 264(1–2), 100–122 (2008).
Esteve, J. & Zamora, S. Enrolled agnostids from Cambrian of Spain provide new insights about the mode of life in these forms. Bull. Geosci. 89(2), 283–291 (2014).
Speyer, S. E. Comparative taphonomy and palaeoecology of trilobite lagerstätten. Alcheringa 11(3), 205–232 (1987).
Geyer, G. & Peel, J. S. The Henson Gletscher Formation, North Greenland, and its bearing on the global Cambrian Series 2–Series 3 boundary. Bull. Geosci. 86(3), 465–534 (2011).
Zhou, T. M., Liu, Y. R., Meng, X. S & Sun, Z. H. Palaeontological atlas of central and southern China. In Early Palaeonzoic, vol. 1 (eds. Hubei Institute of Geological Sciences, Geological Bureau of Henan Province, Geological Bureau of Hubei Province, Geological Bureau of Hunan Province, Geological Bureau of Guangdong Province & Geological Bureau of Guangxi Province) 104–266 (Geological Publishing House, Beijing, 1977).
Yuan, J. L. & Esteve, J. The earliest species of Burlingia Walcott, 1908 (Trilobita) from South China: Biostratigraphical and palaeogeographical significance. Geol. Mag. 152(2), 358–366 (2015).
Hughes, N. C., Minelli, A. & Fusco, G. The ontogeny of trilobite segmentation: A comparative approach. Paleobiology. 32(4), 602–627 (2006).
Brett, C. E. & Baird, G. C. Taphonomic approaches to temporal resolution in stratigraphy: Examples from Paleozoic marine mudrocks. Short Courses Paleontol. 6, 251–274 (1993).
Brandt, D. S. Taphonomic grades as a classification for fossiliferous assemblages and implications for paleoecology. Palaios 4(4), 303–309 (1989).
Schäfer, W. & Oertel, I. Ecology and Palaeoecology of Marine Environments (University of Chicago Press, Illinois, 1972).
Brett, C. E. & Baird, G. C. Comparative taphonomy: A key to paleoenvironmental interpretation based on fossil preservation. Palaios 1(3), 207–227 (1986).
Plotnick, R. E. Taphonomy of a modern shrimp: Implications for the arthropod fossil record. Palaios. 286–293 (1986).
Plotnick, R. E., Baumiller, T. & Wetmore, K. L. Fossilization potential of the mud crab, Panopeus (Brachyura: Xanthidae) and temporal variability in crustacean taphonomy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 63(1–3), 27–43 (1988).
Babcock, L. E. & Chang, W. Comparative taphonomy of two nonmineralized arthropods: Naraoia (Nektaspida; Early Cambrian, Chengjiang Biota, China) and Limulus (Xiphosurida; Holocene, Atlantic Ocean). Collect. Res. 10, 233–250 (1997).
Speyer, S. E. & Brett, C. E. Clustered trilobite assemblages in the Middle Devonian Hamilton group. Lethaia. 18(2), 85–103 (1985).
Paterson, J. R. et al. Trilobite clusters: What do they tell us? A preliminary investigation. Adv. Trilobite Res. 9, 313–318 (2008).
Gaines, R. R. & Droser, M. L. Paleoecology of the familiar trilobite Elrathia kingii: An early exaerobic zone inhabitant. Geology 31(11), 941–944 (2003).
Gutiérrez-Marco, J. C., Sá, A. A., García-Bellido, D. C., Rábano, I. & Valério, M. Giant trilobites and trilobite clusters from the Ordovician of Portugal. Geology 37(5), 443–446 (2009).
Esteve, J., Hughes, N. C. & Zamora, S. Purujosa trilobite assemblage and the evolution of trilobite enrollment. Geology 39(6), 575–578 (2011).
Brett, C. E., Zambito, J. J. IV., Schindler, E. & Becker, R. T. Diagenetically-enhanced trilobite obrution deposits in concretionary limestones: The paradox of “rhythmic events beds”. Palaeogeogr. Palaeoclimatol. Palaeoecol. 367, 30–43 (2012).
Hoare, B. Animal Migration: Remarkable Journeys in the Wild. (University of California Press, 2009).
Chatterton, B. D. E. & Fortey, R. A. Linear clusters of articulated trilobites from Lower Ordovician (Arenig) strata at Bini Tinzoulin, North Zagora, Southern Morocco. Adv. Trilobite Res. (Cuadernos del Museo Geominero) 9, 73–77 (2008).
Trenchard, H., Brett, C. E. & Perc, M. Trilobite ‘pelotons’: Possible hydrodynamic drag effects between leading and following trilobites in trilobite queues. Palaeontology 60(4), 557–569 (2017).
Kim, K. W. & Horel, A. Matriphagy in the spider Amaurobius ferox (Araneidae, Amaurobiidae): an example of mother-offspring interactions. Ethology 104(12), 1021–1037 (1998).
Kim, K. W. & Roland, C. Trophic egg laying in the spider, Amaurobius ferox: mother–offspring interactions and functional value. Behav. Proc. 50(1), 31–42 (2000).
Drage, H. B., Holmes, J. D., García-Bellido, D. C. & Daley, A. C. An exceptional record of Cambrian trilobite moulting behaviour preserved in the Emu Bay Shale, South Australia. Lethaia 51(4), 473–492 (2018).
Zhao, Y. L. et al. Balang section, Guizhou, China: Stratotype section for the Taijiangian Stage and candidate for GSSP of an unnamed Cambrian Series. Camb. Syst. China Korea Guide Field Excursions 62–83 (2005).
Zhao, Y. L. et al. Kaili Biota: A taphonomic window on diversification of metazoans from the basal Middle Cambrian: Guizhou, China. Acta Geol. Sin.-English Ed. 79(6), 751–765 (2005).
Yang, X. L., Zhao, Y. L., Peng, J., Yang, Y. N. & Yang, K. D. Discovery of Oryctocephalid trilobites from the Tsinghsutung Formation (Duyunian Stage, Qiandongian Series, Cambrian), Jianhe County, Guizhou Province. Geol. J. China Univ. 16(3), 309–316 (2010).
Yuan, J. L., Esteve, J. & Ng, T. W. Articulation, interlocking devices and enrolment in Monkaspis daulis (W alcott, 1905) from the Guzhangian, middle Cambrian of North China. Lethaia. 47(3), 405–417 (2014).
Zhao, Y. L., Yuan, J. L., Esteve, J. & Peng, J. The oryctocephalid trilobite zonation across the Cambrian Series 2-Series 3 boundary at Balang, South China: A reappraisal. Lethaia. 50(3), 400–406 (2017).
Abràmoff, M. D., Magalhães, P. J. & Ram, S. J. Image processing with ImageJ. Biophoton. Int. 11(7), 36–42 (2004).
Esteve, J., Zhao, Y. L., Maté-González, M. A., Gómez-Heras, M. & Peng, J. A new high-resolution 3-D quantitative method for analysing small morphological features: An example using a Cambrian trilobite. Sci. Rep. 8(1), 1–10 (2018).
Lask, P. B. The hydrodynamic behavior of sclerites from the trilobite Flexicalymene meeki. Palaios, 219–225 (1993).
Hesselbo, S. P. The biostratinomy of Dikelocephalus sclerites: implications for the use of trilobite attitude data. Palaios. 605–608 (1987).
Mikulic, D. G. The arthropod fossil record: biologic and taphonomic controls on its composition. Short Courses Paleontol. 3, 1–23 (1990).
Speyer, S. E. & Donovan, S. K. Trilobite taphonomy: A basis for comparative studies of arthropod preservation, functional anatomy and behaviour. Processes Fossil., 194–219 (1991).
Speyer, S. E. & Brett, C. E. Trilobite taphonomy and Middle Devonian taphofacies. Palaios., 312–327 (1986).
Schumacher, G. A. & Shrake, D. L. Paleoecology and comparative taphonomy of an Isotelus (Trilobita) fossil lagerstätten from the Waynesville Formation (Upper Ordovician, Cincinnatian Series) of southwestern Ohio. In Paleontological Events: Stratigraphic, Ecological, and Evolutionary Implications. 131–161 (Columbia University Press, New York, 1997).
Hickerson, W. J. Middle Devonian (Givetian) trilobite clusters from eastern Iowa and northwestern Illinois. In Paleontological Events: Stratigraphic, Ecological, and Evolutionary Implications. 224–246 (Columbia University Press, New York, 1997).
Hughes, N. C. & Cooper, D. L. Paleobiologic and taphonomic aspects of the “granulosa” trilobite cluster, Kope Formation (Upper Ordovician, Cincinnati region). J. Paleontol. 73(2), 306–319 (1999).
Hunda, B. R., Hughes, N. C. & Flessa, K. W. Trilobite taphonomy and temporal resolution in the Mt. Orab shale bed (Upper Ordovician, Ohio, USA). Palaios. 21(1), 26–45 (2006).
Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9(3), 90–95 (2007).
Davis, J. C. Statistics and data analysis In Geology 289–291 (Wiley, New York, 1986).
Roubeyrie, L. & Celles, S. Windrose: A Python Matplotlib, Numpy library to manage wind and pollution data, draw windrose. J Open Source Softw. 3(29), 268 (2018).
Sun, H.-J., Zhao, Y.-L., Peng, J. & Yang, Y.-N. New Wiwaxia material from the Tsinghsutung Formation (Cambrian Series 2) of Eastern Guizhou, China. Geol. Mag. 151(2), 339–348 (2014).
Acknowledgements
We are grateful to Jill Pearse (Geosciences Department, Los Andes University) and Timothy P. Topper (Swedish Museum of Natural History) for their comments and improvement of written English. We also wish to thank two anonymous reviewers, who reviewed and improved the manuscript. This work is supported by grants from by the National Natural Science Foundation of China (grant numbers 41772021, 41962002), FAPA (INV-2019-62-1652) from the Universidad de los Andes, Bogotá, Colombia, the strategic Priority Research Program of Chinese Academy of Sciences (XDB26000000), the Ministry of Science and Technology of China (2015FY310100), China Geological Survery (grant no.DD20160120-04) and the Guizhou Bureau of Science and Technology (Gui. Sci. Tal. [2017] 5788). This paper is a contribution to project CGL2017-87631-P from Spanish MINECO.
Author information
Authors and Affiliations
Contributions
J.E. performed the research; Y.-L.Z. and Y.X collected the fossils in China; A.C-G. and J.E. addressed the research, A.C-G. analyzed the sample and carried out the statistics analysis, A.C-G. and JE. wrote the paper; all the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corrales-García, A., Esteve, J., Zhao, Y. et al. Synchronized moulting behaviour in trilobites from the Cambrian Series 2 of South China. Sci Rep 10, 14099 (2020). https://doi.org/10.1038/s41598-020-70883-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70883-5
This article is cited by
-
Allopatric molting of Devonian trilobites
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.