Abstract
The persistence of reef building corals is threatened by human-induced environmental change. Maintaining coral reefs into the future requires not only the survival of adults, but also the influx of recruits to promote genetic diversity and retain cover following adult mortality. Few studies examine the linkages among multiple life stages of corals, despite a growing knowledge of carryover effects in other systems. We provide a novel test of coral parental conditioning to ocean acidification (OA) and tracking of offspring for 6 months post-release to better understand parental or developmental priming impacts on the processes of offspring recruitment and growth. Coral planulation was tracked for 3 months following adult exposure to high pCO2 and offspring from the second month were reciprocally exposed to ambient and high pCO2 for an additional 6 months. Offspring of parents exposed to high pCO2 had greater settlement and survivorship immediately following release, retained survivorship benefits during 1 and 6 months of continued exposure, and further displayed growth benefits to at least 1 month post release. Enhanced performance of offspring from parents exposed to high conditions was maintained despite the survivorship in both treatments declining in continued exposure to OA. Conditioning of the adults while they brood their larvae, or developmental acclimation of the larvae inside the adult polyps, may provide a form of hormetic conditioning, or environmental priming that elicits stimulatory effects. Defining mechanisms of positive acclimatization, with potential implications for carry over effects, cross-generational plasticity, and multi-generational plasticity, is critical to better understanding ecological and evolutionary dynamics of corals under regimes of increasing environmental disturbance. Considering environmentally-induced parental or developmental legacies in ecological and evolutionary projections may better account for coral reef response to the chronic stress regimes characteristic of climate change.
Similar content being viewed by others
Introduction
Reef-building corals are the foundation for one of the most diverse and economically important ecosystems1,2. Corals are sensitive to changes in their environment that destabilize the coral-algal symbiosis and disrupt reef primary production and net ecosystem accretion. Even slight changes in temperature, nutrients, light, salinity, and pollution, can negatively affect coral symbiosis and performance3. The steady increase in CO2 emissions4 is heightening the magnitude and frequency of global scale environmental perturbations, exacerbating reef decline already occurring due to local pressures.
The emission of CO2 is elevating atmospheric levels, which is in turn changing the chemistry of the oceans. Ocean acidification (OA) is occurring due to the absorption of atmospheric CO2 by the ocean and the resulting increase in the concentrations of H+ and decrease in CO3–2 ion concentrations5. OA energetically challenges calcifying marine organisms because they expend more energy for acid–base regulation to both maintain cellular homeostasis6,7 and calcify in a medium with lower CO3–2 ion concentrations. The physiological and ecological consequences of OA range from changes in intracellular pH8,9, to direct reduction of calcification of coral skeletons10, to shifts in community composition in low pH areas11. Further, the interaction of multiple stressors such as temperature and OA is likely to result in synergistic and antagonistic responses in comparison to the responses driven by individual stressors12,13.
Given that local coral reef decline is now exacerbated by global issues, a greater effort is being made to better understand the sub-lethal physiological effects of stressors on critical coral reef processes such as coral reproduction and early life stages14,15. Corals are long-lived organisms with complex reproductive life history characteristics16. Early life history research has focused heavily on the effects of anthropogenic factors (e.g., increased temperature and ocean acidification) on fertilization and development, metamorphosis, settlement, and survivorship in spawning corals14,17,18,19,20,21, and a variety of physiological metrics in brooding corals22,23,24,25. Few studies have, however, tracked corals through multiple life history stages including larval supply, settlement and post-settlement survival and growth14,26,27, fewer still at the cross-generational scale28,29,30, and none at the multigenerational scale. Thus the forecasts for population persistence and replenishment have largely ignored the potentially substantial plasticity driven by environmentally driven parental or developmental legacies31,32.
Phenotypic plasticity, or the generation of multiple performance outcomes within an individual as a function of environment, has the potential to act as a buffer to rapid environmental change and modulate evolutionary response28,33,34. Multiple avenues can generate this plasticity. While intra-generational studies of phenotypic plasticity are more common, the study of carry over effects (COE), cross-generational plasticity (CGP), and multi-generational plasticity (MGP, sensu35) are still in their relative infancy for marine invertebrates31,32,36,37. These forms of plasticity occur when environmental stimuli manifest through phenotypic variation in linked life stages (i.e., the norms of reaction are shaped by the environmental conditions of prior generations, development, or early life stages). The lack of data on these life stage linkages in corals is now particularly critical in light of severe ecosystem declines and the growing acknowledgement that adaptive responses are necessary for persistence under rapid climate change38,39,40,41.
The ecological and evolutionary ramifications of parental or early life stage environmental exposure in longer-lived, ecosystem engineering, calcifying marine invertebrates in response to OA remain to be determined. To date, early life stage exposure often appears to have negative COE. Assessment of Olympia oyster performance following exposure to high pCO2 at the larval stage by Hettinger and coauthors revealed slower growth under high conditions42. Even after the juveniles were placed in ambient grow out conditions, negative COE remained at 45 days post exposure42. Further assessment of the ecological performance of Olympia oyster growth in ambient field conditions four months post exposure to high pCO2, revealed negative COE on growth rate43. Some corals also appear to display negative COE from stressors at the larval stage. In larvae of Orbicella faveolata, low salinity treatments caused decreased survival and growth after settlement44. Similarly, in larvae of Porites astreoides, early life exposure to elevated seawater temperatures caused significantly higher mortality in settled coral spat ~ 3 weeks post exposure, in comparison to larvae raised in ambient temperatures27. While studies in both oysters and corals identify COE of early life stage exposure to stressors, this does not account for plasticity that may be induced by parental environment (i.e., CGP).
Depending on the timing of exposure, environmental conditioning can set organisms on different physiological and ecological trajectories32,36,37. For example, conditioning may provide a form of hormesis, or environmental priming that elicits stimulatory effects45,46. Studies considering the ontogenetic connections in organisms with shorter life spans such as plankton, worms, oysters, clams, and fishes, have documented substantial, and often positive CGP and MGP33,36,37,47,48,49,50,51,52. For example, in the Sydney rock oyster, parental exposure to high pCO2 enhanced growth, development, and metabolism in F1 offspring exposed to the same high pCO2 conditions50. Further, positive MGP was observed in the F2 generation, in terms of development, growth, and juvenile heart rate in the exposure line, relative to the control line53. To date, only three studies of reef building corals have identified COE or CGP at the swimming planulae stage in response to OA and temperature28,30, and feeding29, but the longer-term implications of this plasticity across subsequent life stages are not yet known. These responses demonstrate that understanding the potential of parental or developmentally induced plasticity through ontogeny (i.e., ecological ramifications of conditioning) is critical to our view of organism performance on annual to decadal time scales.
The ecological implications of COE, CGP, and MGP have not been considered in projections of coral performance in a future of climate change, yet acclimatization is known to play a key role in intra-generational stress response54,55,56, and our initial work in corals suggests positive COE is possible in response to OA in corals28. Further work in another Pocilloporid coral, Stylophora pistillata, COE from brooding parent exposure to OA and increased temperature results in weak COE, highlighting the potential variation between species30. In this same species, the feeding of parents resulted in differences in offspring number and performance29. Our current study provides the first investigation of the effects of adult conditioning on offspring ecological performance beyond the initial larval or settlement stages. We posit that conditioning of parents to OA will result in COE that may be developmentally-mediated, or CGP that may be parentally-mediated. Here, we tested the hypothesis that parental exposure of corals to high pCO2 during gametogenesis and/or brooding results in beneficial acclimation of the offspring. Further, we tested the hypothesis that this plasticity is maintained beyond the larval stage, through settlement into the juvenile stage, by assessing settlement and survivorship post release and tracking survivorship and growth for 6 months in ambient and elevated pCO2 treatments.
Materials and methods
Experimental overview
The experiment consisted of four phases (Fig. 1). First, adult coral colonies were acclimated to ambient conditions in common garden tanks for 34 days (Fig. 1a). Second, adults were exposed to two different pCO2 conditioning treatments that fluctuated with a diurnal frequency for the period that contained gametogenesis and brooding, over 3 cycles of planulation (~ 3 months, Fig. 1b). Third, larvae were collected from the conditioned adults and exposed to the two different fluctuating pCO2 treatments in a reciprocal fashion (Fig. 1c–e) in acrylic and mesh chambers containing a settlement tile: Survivorship and settlement of offspring during this phase were assessed after 96 h. Fourth, the settled spat were replaced in treatments and tracked for survivorship and growth (Fig. 1f) in those continued reciprocal exposure treatments for up to six months post release.
Coral collection and acclimation
Adult Pocillopora damicornis (now considered P. acuta in this location based on mitochondrial open reading frame, mtORF, sequencing57) colonies (mean diameter 11.2 cm) were collected from the fringing reefs of southern Kāneʻohe Bay, O’ahu, Hawaiʻi (21.429845, − 157.793604) under Special Activities Permit SAP2014 from Hawaii’s Division of Aquatic Resources (DAR). Corals were removed from the reef at the dead skeleton base to minimize direct tissue damage and were placed immediately adjacent to each other in a common garden in the field for a 3-day acclimation period58. Subsequently, corals (n = 16) were moved to large, outdoor, sand-filtered, flow-through seawater, mesocosm tanks (~ 1,300 l) at the Hawaiʻi Institute of Marine Biology, HIMB58. Irradiance in the mesocosm tanks was reduced to ~ 60% full irradiance using shade cloth to more closely mimic collection site in situ light conditions, and light readings were taken every 15 min with underwater loggers (Odyssey PAR loggers standardized to Li-Cor 192SA cosine sensor; Supplementary Fig. S1). Seawater temperature was also recorded every 15 min (Hobo Water Temp Pro v2, accuracy = 0.21 °C, resolution = 0.02 °C, Onset Computer Corporation, Supplementary Fig. S1). Light, temperature and pH fluctuated within the tanks on natural cycles (Supplementary Fig. S1). Corals acclimated to these tank conditions for 34 days prior to initiation of treatments.
The current state of knowledge of Pocillopora damicornis/acuta larval timing and sexual or asexual origin is not straightforward. Work by Stimson59 indicates eggs are present in Pocillopora damicornis in Kāneʻohe Bay in April, May and June, during their peak planulation, which suggests transition from eggs to larvae within a month, or the potential for asexually derived larvae. These options are further supported by histological studies of Pocillopora in other locations. For example, the work of Stoddart and Black60 demonstrates the presence of planulae in parental polyps despite the absence of gametes in the preceding ~ 1 month. Similarly, Harriott61 identifies the appearance of planulae in colonies when samples collected two months prior from the same colony had either small or no gonads. This supports the hypotheses that eggs were present during conditioning and that some planulae are asexually reproduced, which is further evidenced by genetic work of Stoddart62, Yeoh and Dai (63; Pocillopora damicornis/acuta in Taiwan), and Combosch and Vollmer (64; Pocillopora damicornis/acuta in French Polynesia). Given that the reproductive timing is not yet fully clarified in Pocillopora damicornis/acuta with overlap between gametogenesis and brooding, as well as the potential for asexually and sexually produced planulae, it is possible the planulae in our study were exposed for all or a portion of gametogenesis, as well as for the entire developmental duration. Due to this, we discuss primarily COE, and the potential for CGP and MGP.
Experimental exposure
The treatment (high pCO2) and control conditions (ambient seawater) were maintained using a pH–stat CO2 injection system, which recorded pH (NBS, 10 min frequency)58. Ambient pH and pCO2 fluctuated daily (e.g.,65), driven by ambient conditions and feedbacks from photosynthesis, calcification, and respiration of the organisms on the fringing reef directly off shore of HIMB, where the seawater was obtained. Due to this, model predictions for open ocean chemistry are not reliable in coastal systems. For our treatments, we reduced the mean pH by ~ 0.3 units, while retaining diel fluctuation (Supplementary Fig. S1). While this condition may be higher than IPCC pCO2 predictions for open ocean conditions4, lower and more variable pH is common for coastal and reef locations66,67. For example, pH conditions on the fringing reefs adjacent to Coconut Island (Moku o Loʻe) where the corals were collected, can range from ~ 7.6 to 8.168,69. Further modeling of the pH change in reef locations under future scenarios results in a 2.5-fold increase in reef pH variation projected with an offshore increase to 900 µatm pCO270. As such, our chosen pH conditions are ecologically relevant in terms of fluctuation and magnitude of potential pH change in the future in this dynamic reef location and do not represent extreme conditions.
pH probes (resolution, accuracy) from the pH–stat CO2 injection system were calibrated weekly on the NBS scale and pH and temperature were logged every 15 min in each tank throughout the duration of the experiment. The carbonate chemistry of the seawater was assessed with measurements of pH (total scale), total alkalinity, temperature and salinity according to the guide for best practices for ocean acidification research71. Probe measurements were made ~ daily. Temperature measurements were made with a certified digital thermometer (5-077-8, accuracy = 0.05 °C, resolution = 0.001 °C; Control Company, TX, USA). pH (total scale) was measured with a handheld probe (DG115-SC; Mettler-Toledo, LLC, OH, USA) standardized against a Tris standard (A. Dickson certified reference material) across the range of experimental temperatures. Salinity (psu) was measured with a hand held probe (Benchtop/Portable Conductivity Meter 23226-505, Accuracy 0.3% VWR, Radnor, PA, USA). Water samples for total alkalinity were collected ~ 2 × per week for each adult treatment. Total alkalinity samples were analyzed using open cell potentiometric titrations72 and assessed against certified reference materials (CRMs; A. Dickson Laboratory, UCSD; values on average were < 1% different from TA CRMs); all samples were corrected for any offset from the CRMs. From these measurements, the full suite of carbonate parameters was calculated with the seacarb package (v3.0.1173), using the average corrected TA and salinity measured in each treatment tank (Table 1). Given the stability of the total alkalinity and low biomass in the 1,300 l tanks, sampling frequency was reduced in the 6 months of offspring exposure to ~ every 1–2 weeks. Loggers were used to record temperature (Hobo Water Temp Pro v2, accuracy = 0.21 °C, resolution = 0.02 °C, Onset Computer Corporation, Supplementary Fig. S1), and light (Odyssey PAR loggers standardized to Li-Cor 192SA cosine sensor; Long et al. 2012; Supplementary Fig. S1) continuously (Supplementary Fig. S1).
Adult exposure, planulation, and settlement
Eight adult corals were exposed to each treatment beginning 06 May 2014. Two colonies died very early in the high pCO2 treatment prior to June planulation, leaving n = 6 in high pCO2 and n = 8 in ambient pCO2. These two corals died very early in the experiment (prior to 1 month of exposure), which suggests it was not a long-term effect of the treatment, nor a long-term issue with the experimental quality. A common garden approach (n = 2 tanks) was chosen to maximize the similarity of experimental conditions. Corals were acclimated in tanks to fluctuating conditions (Supplementary Fig. S1) similar to that on the fringing reefs of Kāneʻohe Bay, O’ahu, Hawaiʻi58. During the acclimation period, daily temperature ranged on average from 23.7 to 25.9 °C (Supplementary Fig. S1a). Between 06:00 and 19:00, Photosynthetically Active Radiation (PAR) ranged on average from 62—246 µmol s−1 m−2 (Supplementary Fig. S1).
During the 7–8 days of larval collection each month, adult corals were removed from the tanks and separated into individual ~ 4.5 l bowls (one colony per bowl) with flowing treatment water (i.e., the same experimental conditions as the tanks) from ~ 5 pm to 9 am to isolate the larvae released from each colony (e.g.28). The flowing seawater flushed the buoyant larvae into 800 ml tripour beakers with 150 µm mesh bottoms and the number of larvae released per colony were counted during the months of June, July and August 2014. July was the expected seasonal peak of larval release (Fig. 2), and the larvae collected during this time were used for the offspring reciprocal exposure experiments (Fig. 1c,d). Due to the variation in timing of gametogenesis, brooding, and the sexual or asexual origin of the planulae in Pocillopora damicornis/acuta59,60,62,74, it is not possible to definitively state the exact timing of exposure of the offspring gametes or planulae within the adults. Clarifying the timing and mechanisms of COE versus CGP would require, for example, exposing the adults only prior to gametogenesis, only during gametogenesis, or only during brooding. Currently the reproductive biology of Pocillopora damicornis/acuta, complicates this assessment. In this case, we are examining COE that may be developmentally-mediated, or CGP that may be parentally-mediated.
Groups of 16–20 larvae were tracked from each parent colony and placed in a 200 ml transparent acrylic chamber with 150 µm mesh at each end. To increase replication and account for variability across release date, experiments were conducted on each day of larval release from 12 to 18 July 2014 on newly released larvae. Each chamber contained a 4.5 × 4.5 × 1 cm terracotta brick tile that had been conditioned on the reef for 1–2 months. Conditions of pH on the fringing reef of Coconut Island in Kāneʻohe Bay can range from ~ 7.6 to 8.168,69, so these tiles are not naïve to high pCO2. The indirect effects of pCO2 on substrate communities (e.g., crustose coraline algae and microbiome), and therefore coral offspring settlement and growth, are important considerations in experimental design for interpretation of results75. Chambers were placed into either ambient or high pCO2 treatments and settlement and survivorship were assessed after 96 h. Survivorship was calculated as the total number of living larvae (both swimming and settled) in the chamber divided by the initial number of larvae added. Settlement was counted as those larvae that had settled and metamorphosed to the chamber walls, mesh, or tile divided by the initial number of larvae added to the chamber.
Spat exposure on tiles
After settlement and survivorship was assessed, new recruits were mapped on each tile and the tiles with settled spat were returned to the mesocosms of their settlement treatment (Fig. 1, Supplementary Figs. S1, S2). After both an additional month and then 6 months of total exposure, tiles were re-assessed under a dissecting scope to count survivorship (number of spat remaining alive relative to the initial amount added to the chamber). Growth of each spat was also measured by counting the number of polyps. Growth rate was calculated as: the (# of polyps − 1 primary polyp)/(# of days post settlement at month 1) for the first month’s growth rate, and as the (# of polyps at month 6 − # of polyps at month 1)/(# of days between month 1 and month 6 measurements). Spat that were fused were counted as survivors, but were not used in the growth data analysis. Settlement tile was used as the level of replication to avoid non-independence of multiple spat on a tile.
Statistical analysis
To test if the timing of larval release differed between the control and treatment conditions at each sampling time-point, a two-sample Kolmogorov–Smirnov test was used and release by day was treated as a continuous variable (ks.test; stats package76). A generalized linear mixed effects model with binomial errors (lme4 package76) was used to test for differences in proportional settlement and survivorship between the treatment and control using a binomial distribution. Settlement data were analyzed with parental (Origin) and offspring (Secondary) treatments and their interaction as fixed effects and settlement tile as a random intercept. Survivorship and growth data were both measured at multiple time points and, thus, were modeled with the same fixed effects and interaction, but with a random intercept of settlement tile nested in time point. A model selection approach was applied and the final models were selected as those with the lowest delta AIC. Growth data were log normalized to meet model assumptions (i.e., for normal distribution) and growth data are plotted as back-transformed means and asymmetrical standard error. All data and reproducible analytical code are available on GitHub (https://github.com/hputnam/HI_Pdam_Parental/releases/tag/Version_20180508) and on Zenodo (https://doi.org/10.5281/zenodo.3972426).
Results
Experimental exposure
Light and temperature varied over time within the experiment due to seasonal shifts (see Supplementary Fig. S1 for detailed description of tank conditions). During the adult pCO2 exposure, mean temperature ranged from 26.3–27.9 to 26.4–28.0 °C in ambient and treatment tanks, respectively (Supplementary Fig. S1), which was on average higher than the acclimation period prior to experimental exposure, due to seasonal warming. pH (NBS) ranged from 7.81 to 8.06 in ambient conditions and 7.51 to 7.74 in high pCO2 conditions for the adult exposures. During the first month of larval exposure, pH ranged from 7.79 to 8.04 in ambient conditions and 7.49 to 7.74 in the high pCO2 treatment, and temperature, on average, ranged from 27.0–28.5 to 27.0–28.4 °C, respectively (Supplementary Fig. S1). Lastly, across the 6 months of juvenile exposure, pH ranged from 7.77 to 8.04 in ambient conditions and 7.52 to 7.75 in high pCO2 conditions and daily temperature, on average, ranged from 25.7–27.1 to 25.8–27.0 °C, respectively (Supplementary Fig. S1). Discrete measurements of carbonate chemistry reveal stable TA across the 10 months and strong differences in pH and pCO2 between treatments (Table 1), but as these discrete measurements reflect daylight sampling only, they likely underestimate the treatment differences in pH and temperature portrayed in continuous measurements described above (Supplementary Fig. S1).
Planulation and settlement
Planulae release was monitored on lunar days ~ 16–24 for the months of June, July, and August, as planulation has been reported to occur following the full moon (lunar day ~ 15) for P. damicornis in Hawaiʻi77,78. A clear peak in planulation was observed in July, with lowest release in August (Fig. 2). There was no significant difference in the timing of planulation between treatments in either June (Fig. 2a; P > 0.05) or August (Fig. 2c; P > 0.05). The general pattern suggested a shift in timing of planulation between treatments (Fig. 2), with a delay in release more prominent in the high pCO2 condition in July (Fig. 2b; D = 0.625, P = 0.087).
Offspring from high CO2-exposed parents displayed significantly higher survivorship following 96 h in the settlement chambers (P < 0.016, Supplementary Table S1), supporting positive carryover effects. This increase in offspring survival from parents exposed to elevated pCO2 was approximately equal in both offspring treatments at time 0, with 14.5% and 15.1% greater survivorship in the ambient and high offspring treatments, respectively (Fig. 3a). The settled spat from parents conditioned to high pCO2 also showed greater survivorship in both offspring treatments after one month in the reciprocal exposures (Supplementary Table S1), with conditioned offspring having 27.1% greater survivorship at ambient offspring exposure conditions and 16.6% at high pCO2 exposure (Fig. 3b). After 6 months of reciprocal exposure, parental exposure to high pCO2 enhanced offspring survivorship by 13.6% in ambient offspring exposures, relative to spat from adults that underwent ambient conditioning. Offspring from high pCO2 parents displayed reduced survivorship (7.7%) in high offspring exposure relative to spat from adults conditioned to ambient (Fig. 3c, Supplementary Table S1). Offspring exposure to high pCO2 also led to lower survivorship regardless of parental conditioning treatment (Supplementary Table S1). Averaged across parental exposures, survivorship decreased with offspring exposure to elevated pCO2 by 26.1% at time 0, 24.5% at month 1 and 59.9% at month 6. Survivorship declined significantly over time (P < 0.001, Supplementary Table S1), with survivorship as low as 4.6% of the initial planulae by the final time point.
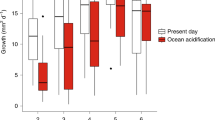
Parental conditioning to high pCO2 enhances offspring performance. Reaction norm plots for (a) survivorship in the chambers, (b) survivorship at month 1, (c) survivorship at month 6, (d) settlement at time 0, (e) growth rate at month 1, and (f) growth rate at month 6. Points display data (mean ± sem), while lines indicate only the direction of response. Tile sample sizes for survivorship and settlement time 0, month 1 and month 6 and growth at month 1 ranged from 7 to 36 (see Supplementary Table S1). Growth rates were log transformed for analyses and the figure displays back-transformed growth rate data.
Planulae settlement was highest when parents were exposed to elevated pCO2 (P = 0.021; Supplementary Table S1; Fig. 3d); settlement was enhanced by 17.6% and 11% for planulae settling in ambient and high pCO2 offspring treatments, respectively. Despite this overall enhanced settlement of planulae from high pCO2 parents, mean settlement was significantly lower overall (26.1%) in the high pCO2 offspring treatments (P < 0.001; Supplementary Table S1), regardless of parental pCO2 exposure (Fig. 3d).
Lastly, a trend for an Origin*Time interaction (P = 0.056) was observed in spat growth, where differences in growth with parental origin were apparent at month 1 (growth was on average 1.3-fold higher in offspring from parents conditioned to high pCO2; Fig. 3e), but not at month 6 (Fig. 3f). Time also had a significant effect on growth, where by the end of the experiment, at 6 months post release, growth rates were significantly lower than month 1 (P < 0.0001, Fig. 3f).
Discussion
Here we present the first ecological assessment of the effects of parental conditioning in reef-building corals. In our study, the ecological and fitness-related response of coral offspring was enhanced when their parents were exposed to a high pCO2 environment, with effects lasting into the juvenile stage. Projections for future reef persistence are dire79, but many do not incorporate adaptation or acclimatization80, likely over or under-estimating climate change effects for some stressors. When adaptation and acclimatization are considered, it is clear the trajectories differ from the worst-case scenarios81,82,83. Our work provides evidence to support the importance of the role of acclimatory processes in eco-evolutionary thinking in an era of climate change and encourages the examination of mechanisms such as hormesis and epigenetics32,45.
Potential for tuning of reproductive timing
Our multi-life stage perspective identifies effects of adult stressor exposure, with reproductive and offspring consequences, specifically a trend for a shift in the timing of planulation in July between treatments. Kāneʻohe Bay is a semi-enclosed embayment that has fluctuations in physical conditions as a function of tidal cycle. Specifically, diurnal pCO2 fluctuations are greatest when tidal fluctuation is the lowest68,69,84 in the shallow fringing reefs of Kāneʻohe Bay. A delay in the peak of planulation from the adults conditioned to high pCO2, would then correspond to the timing of lower daily tidal ranges and thus higher pCO2 fluctuations that are more similar to the high pCO2 adult conditions. It is possible that “bet-hedging”, or environmental tuning, by the parents may result in release of larvae timed to favorable conditions. For example, in other brooding corals, a phenological shift in reproductive timing has occurred, minimizing planulae release during strong upwelling-induced temperature fluctuations of ~ 10 °C85. Another hypothesis that could contribute to a shift in larval release under high pCO2 condition is adult or offspring energetic constraints. Delay in release could indicate energetic costs to maintaining adult calcification and homeostasis7, potentially resulting in decreased parental investment, or increased development time necessary in offspring86. Further, low pH can influence development processes such as sperm performance, fertilization success, and developmental normalcy and timing14,15.
Given the trend for a shift in the timing of planulation during July when our offspring experiment was completed, it could also be hypothesized that parental effects in the reciprocal exposure are due to slight differences in the larval cohorts by day of release22,25,87,88. For example, peaks in Symbiodinium density and photophysiology, and larval size, are positively correlated to peak larval release in Pocillopora damicornis in Taiwan25. These differences in physiology by day of release also translate to variation in susceptibility to changing temperature and pH in P. damicornis22,88. The impact of day of release in our case is likely to be minimal, given the experiment was not conducted on a single day’s larval pool, but over 7 consecutive days (Fig. 2), better representing the full range of larval phenotypes from P. damicornis/acuta.
Importance of carryover effects for corals
Our work provides further evidence that the parental or developmental environment matters to offspring performance in this brooding coral species, Pocillopora acuta. While ~ 1 month of exposure to increased temperature and low pH resulted in changes to offspring phenotype in this same species28, in our current study with exposure to only OA, reaction norms were primarily parallel, with enhanced performance in those offspring from conditioned parents. This may suggest that exposure to increased temperature (or the combination of temperature and OA) has more profound, or mechanistically different impacts than OA on processes involved in parental contributions89, or developmental plasticity (e.g.90).
The enhanced growth of P. acuta juveniles under low pH may be unexpected given the common detrimental effect of ocean acidification on coral calcification91, however, there is highly variable response of P. damicornis/acuta to OA in the literature58,92,93. This variation of growth response in the literature could be due to differences in experimental techniques (e.g., methods of normalization), and/or the magnitude, duration and variance in the pH treatments used (e.g., absolute pH differences between treatments, or stable versus fluctuating pH conditions58,92,93,94. In a similar fluctuating outdoor mesocosm study also in Kāneʻohe Bay Hawaiʻi, ocean acidification did not impact the recruitment of P. damicornis larvae to the sides of treatment tanks94. Furthermore, fluctuating pH has been shown to enhance coral growth in early stage Pocilloporids (Dufault et al. 2012). Enhanced calcification in the context of COE, CGP, and MGP has, additionally, been measured in another marine invertebrate, where higher shell growth rates in offspring of the Manila clam following exposure of the parents to low pH have been observed95,96. This positive effect on growth in another marine calcifier supports our findings here with Pocillopora acuta, with implications for reducing early life stage partial mortality due to increased size (e.g., polyp number), and showcasing the importance of COE and CGP beyond a single coral species.
Potential mechanisms underlying parental effects
Several hypotheses may account for the parental contribution to enhanced settlement, survivorship, and growth we documented. It is possible that adults manipulate the investment in their offspring in the form of Symbiodiniaceae communities97, microbiome98, size, protein, lipids, or carbohydrates99. Further the role of mitochondrial performance has been posited as a mechanism of rapid adaptation in coral larvae100 and mitochondrial performance has been linked to parental environment in CGP shown in marine worms33. These mechanisms could provide metabolic boosts, or conversely detriments during this energetically demanding life stage101, (e.g., the presence of Durisdinium [clade D] symbionts reduces growth in coral juveniles102). DNA methylation and other epigenetic mechanisms linked to gene expression regulation29,31,54 could also provide a mechanism of heritable epigenetic cross-generational priming (e.g.103,104,105). While differential provisioning of planulae symbiotically, energetically, and epigenetically is possible, the goal of examining ecological effects on the process of recruitment precluded destructive sampling for such hypotheses in our study, but they remain important considerations in our ongoing work.
Brooded embryos may experience developmental acclimation while growing within the parental polyp28. It is possible that low pH in the gastrovascular cavity (GVC) at the site of development could condition planulae for low pH when released. This hypothesis would account for the differences seen in our study between ambient and low pH conditioned parents, if the GVCs were modified differently between treatments to retain a treatment offset. To date, limited data are available for GVC pH under a variety of conditions106,107. The data available suggest both a high pH and low pH within the GVC and associated mesenteries107, with no uniform picture of what planulae within the polyp are exposed to relative to external conditions. Further work with multiple coral species is necessary to disentangle the role of developmental acclimation from CGP, including experiments focused on the magnitude and timing of signals that will induce carryover effects and exposures through the F1 and F2 generation32.
Environmental hardening through hormetic priming
Acclimatization occurs through short-term compensatory processes including modulation of biochemical activity and gene expression in response to external stimuli, occurring on daily, seasonal, and annual scales, within a generation, and across a generation. Often implicit in the usage of the term acclimatization is the inference that acclimatory processes are beneficial and fully compensatory, sensu the Beneficial Acclimation Hypothesis (BAH108), but acclimatization is not always beneficial108,109,110. One conceptual explanation for inconsistency of BAH is the framework of hormetic priming45,46, which deserves consideration with regards to explaining patterns of carryover effects. Hormesis is defined as the stimulation of function or performance through mild exposure(s)111. Hormetic priming occurs when exposure to a sub-lethal stressor results in stimulatory response to re-exposure to increasing levels of that stressor to a point and detrimental effects thereafter (e.g., Fig. 4). Hormesis is thus a biphasic response and therefore does not necessitate positive acclimation to all future exposures (Fig. 4).
Hormetic priming may contribute to magnitude, pattern, and temporal variation in the benefits of COE and CGP. The dashed line indicates the result of no hormetic priming, the gray dashed line is no change, the dotted line (black area) indicates a hormetic response with no conditioning, while the solid line (hashed area) indicates the hormetic response with conditioning42,43. Shaded areas indicate the hormetic zones, where the stimulation of performance from the increasing stressor is above the line of no change. Variation in the intensity, duration, and life stage of prior exposure may shift the (A) magnitude, or (B) shape of the hormetic zone. For example, in sessile benthic organisms, carryover effects from parental exposure may result in an enhanced hormetic zone relative to no conditioning. Further there may be (C) temporal constraints on hormetic conditioning. For example, the hormetic zone may differ from parental exposure versus developmental exposure, or as in the case of our study, display a decline in positive carryover effects with increasing time post conditioning (as visualized by the temporal shift in the hormetic zone).
Hormetic priming as a mechanism of acclimatization could explain both the beneficial and detrimental effects of increased temperature that have been documented in certain types of repeated exposures55,81,112, as well as variability in performance. For example, Ainsworth and coauthors identified a “protective trajectory” of sea surface temperatures (SST) that corals experienced, which led to acquired thermal tolerance in Acropora aspera81. Conversely a “repetitive bleaching trajectory”, with high frequency bleaching events that lack recovery time resulted in substantial symbiotic cell loss, bleaching, and mortality81. Here it is likely these responses are on different sides of the zero equivalence point in terms of the biphasic nature of hormetic processes (Y axis in Fig. 4A46,112). An example of hormetic priming in corals would be that of mild ROS exposure resulting in antioxidant protein expression priming, thereby reducing the ROS damage in subsequent events113. Beyond antioxidant genes, frontloading, or the constitutive up-regulation of expression of canonical heat stress genes in Acropora hyacinthus samples from the high thermal variability pool in American Samoa, could be another outcome of hormetic priming, with implications for the thermal tolerance of those primed individuals114.
Corals in our study displayed life stage-dependent plasticity that could be developmentally-mediated, or parentally-mediated. Despite the enhancement in performance seen in early stages, the parental effects of conditioning to high pCO2 are absent in the offspring by 6 months post release. While maintenance of COE and CGP may be expected in short lived organisms, as generation time increases there is a greater potential mismatch between parental and offspring conditions (e.g. Supplementary Fig. S1). This change in plasticity over time is not unexpected due to potential maladaptive tradeoffs115, especially the case in long-lived corals32. Seasonal, annual, and decadal environmental changes likely elicit tradeoffs between the early life stage performance benefits of plasticity and the costs the organism may incur later in life. For example, maternal exposure of soil arthropods to high temperatures resulted in increased thermal resistance at the juvenile stage, but later drove reduced F1 fecundity116. Further the expectation of positive acclimatization is linked to the predictability of the stressor (e.g., anticipatory parental effects; APE’s31,115). Specifically, acclimatization through hormetic priming would likely be optimized by high environmental autocorrelation (i.e., a strong match between parent and offspring conditions would result in extended enhancement). The greater the temporal shift from the parental environment (e.g., 6 months here Supplementary Fig. S1) the less likely there is to be a benefit from prior generation priming and the more likely within generation priming would become important (e.g., Fig. 4C). Seasonal changes in physical parameters would then be expected to result in a loss of benefit from parental or developmental effects as pH continues to change, in a biphasic fashion (Fig. 4C). Additionally, with respect to overall growth rates, the growth from months 2–6 would be expected to decline due to seasonal effects associated with decreases in temperature and light in the winter months (Supplementary Fig. S1117,118). The temporal transience of parental or developmental effects documented in our study argues that for these effects to translate into long term “memory” or for genetic accommodation, more constant environmental and biological feedbacks are necessary103.
The mortality rates documented in the experiment are common for marine larvae or juveniles119. For example, Raymundo and Maypa120 documented 0% survivorship over 1 year tracking of reef out-planted P. damicornis spat of the same size of that in our study (< 3 mm), supporting our findings. In another brooding species, Porites astreoides, mortality of the < 1 cm2 size class was as high as 58% over the census period121. The spat in our study were not fed, but were symbiotic and had access to particles not filtered by the sand filter. As such, we feel the mortality documented was reasonable and reflects competitive challenges of these early life stages, most likely with microalgae. These naturally high mortality rates have implications for the feasibility and scalability of potential environmental hardening approaches in marine larval systems.
Implications for reef-building corals
Experiments designed to examine COE, CPG, and MPG provide a glimmer of hope for coral reef organisms that acclimatization may act as a buffer against a rapidly changing climate28,32. Further experiments are necessary to distinguish between parental effects, and developmental acclimation, and multi-generational acclimatization and their underlying mechanisms. This could be achieved in spawning coral systems, or by manipulating the timing of exposure in the brooding coral system to target isolated stages (i.e., adult, gametogenesis, brooding, and larval). Additionally, tests of the stability and later-life tradeoffs of parental or developmental plasticity, as well as mechanisms of environmental “memory” through aspects such as mitochondrial function33, DNA methylation58,103, or microbiome inheritance122,123 will unveil the complex contributions of the holobiont partners to meta-organism function and acclimatization potential.
Our work challenges the paradigm of inevitable coral decline due to rapid climate change by identifying ecologically-relevant beneficial parental or developmental effects in offspring, in response to adult conditioning to ocean acidification. We suggest hormesis, or environmental priming may conceptually explain enhanced tolerance and performance seen in acclimatization studies, as well as explaining the lack of a ubiquitous beneficial acclimation due to the biphasic nature of hormesis. With regards to conservation and management actions (e.g., assisted evolution124,125), environmental priming is not a one-size-fits all phenomenon (i.e., does not always confer beneficial acclimation). Instead conditioning methods would necessitate a Goldilocks approach, as variation in cellular conditions and physiology between species requires a variety of exposures for optimal performance outcomes122. It is not clear, however, if the duration of the benefits would extend far beyond the exposures, as phenotype-environment mismatches increase with season and anthropogenic effects. The performance and fitness tradeoffs of acclimatization, hormetic triggers, and heritability of potential epigenetic mechanisms present promising areas of further study with respect to carryover effects and the ecological and evolutionary trajectories of reef-building corals.
References
Bishop, R. C. et al. Total economic value for protecting and restoring Hawaiian coral reef ecosystems final report (2011).
Costanza, R. et al. Changes in the global value of ecosystem services. Glob. Environ. Change26, 152–158 (2014).
Lesser, M. P. Coral bleaching: Causes and mechanisms. In Coral Reefs: An Ecosystem in Transition (eds Dubinsky, Z. & Stambler, N.) 405–419 (Springer, Dordrecht, 2011).
Pachauri, R. K. et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 151 (IPCC, 2014).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci.1, 169–192 (2009).
Kaniewska, P. et al. Major cellular and physiological impacts of ocean acidification on a reef building coral. PLoS ONE7, e34659 (2012).
Stumpp, M. et al. Acidified seawater impacts sea urchin larvae pH regulatory systems relevant for calcification. Proc. Natl. Acad. Sci. U.S.A.109, 18192–18197 (2012).
Gibbin, E. M., Putnam, H. M., Gates, R. D., Nitschke, M. R. & Davy, S. K. Species-specific differences in thermal tolerance may define susceptibility to intracellular acidosis in reef corals. Mar. Biol.162, 717–723 (2015).
Gibbin, E. M., Putnam, H. M., Davy, S. K. & Gates, R. D. Intracellular pH and its response to CO2-driven seawater acidification in symbiotic versus non-symbiotic coral cells. J. Exp. Biol.217, 1963–1969 (2014).
Erez, J., Reynaud, S., Silverman, J., Schneider, K. & Allemand, D. Coral calcification under ocean acidification and global change. In Coral Reefs: An Ecosystem in Transition (eds Dubinsky, Z. & Stambler, N.) 151–176 (Springer, Dordrecht, 2011).
Fabricius, K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change1, 165 (2011).
Harvey, B. P., Gwynn-Jones, D. & Moore, P. J. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol.3, 1016–1030 (2013).
Pandolfi, J. M., Connolly, S. R., Marshall, D. J. & Cohen, A. L. Projecting coral reef futures under global warming and ocean acidification. Science333, 418–422 (2011).
Albright, R. Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J. Mar. Biol.2011, 1 (2011).
Byrne, M. & Przeslawski, R. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol.53, 582–596 (2013).
Baird, A. H., Guest, J. R. & Willis, B. L. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst.40, 551–571 (2009).
Chua, C. M., Leggat, W., Moya, A. & Baird, A. H. Temperature affects the early life history stages of corals more than near future ocean acidification. Mar. Ecol. Prog. Ser.475, 85–92 (2013).
Chua, C. M., Leggat, W., Moya, A. & Baird, A. H. Near-future reductions in pH will have no consistent ecological effects on the early life-history stages of reef corals. Mar. Ecol. Prog. Ser.486, 143–151 (2013).
Foster, T., Gilmour, J. P., Chua, C. M., Falter, J. L. & McCulloch, M. T. Effect of ocean warming and acidification on the early life stages of subtropical Acropora spicifera. Coral Reefs34, 1217–1226 (2015).
Negri, A. P., Marshall, P. A. & Heyward, A. J. Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species. Coral Reefs26, 759–763 (2007).
Randall, C. J. & Szmant, A. M. Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816). Biol. Bull.217, 269–282 (2009).
Cumbo, V. R., Edmunds, P. J., Wall, C. B. & Fan, T.-Y. Brooded coral larvae differ in their response to high temperature and elevated pCO2 depending on the day of release. Mar. Biol.160, 2903–2917 (2013).
Dufault, A. M., Cumbo, V. R., Fan, T.-Y. & Edmunds, P. J. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. Biol. Sci.279, 2951–2958 (2012).
Rivest, E. B. & Hofmann, G. E. Responses of the metabolism of the larvae of Pocillopora damicornis to ocean acidification and warming. PLoS ONE9, e96172 (2014).
Putnam, H. M., Edmunds, P. J. & Fan, T.-Y. Effect of a fluctuating thermal regime on adult and larval reef corals. Invertebr. Biol.129, 199–209 (2010).
Ritson-Williams, R., Arnold, S. N. & Paul, V. J. Patterns of larval settlement preferences and post-settlement survival for seven Caribbean corals. Mar. Ecol. Prog. Ser.548, 127–138 (2016).
Ross, C., Ritson-Williams, R., Olsen, K. & Paul, V. J. Short-term and latent post-settlement effects associated with elevated temperature and oxidative stress on larvae from the coral Porites astreoides. Coral Reefs32, 71–79 (2013).
Putnam, H. M. & Gates, R. D. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J. Exp. Biol.218, 2365–2372 (2015).
Bellworthy, J., Spangenberg, J. E. & Fine, M. Feeding increases the number of offspring but decreases parental investment of Red Sea coral Stylophora pistillata. Ecol. Evol.9, 12245–12258 (2019).
Bellworthy, J., Menoud, M., Krueger, T., Meibom, A. & Fine, M. Developmental carryover effects of ocean warming and acidification in corals from a potential climate refugium, the Gulf of Aqaba. J. Exp. Biol. https://doi.org/10.1242/jeb.186940 (2019).
Donelson, J. M., Salinas, S., Munday, P. L. & Shama, L. N. S. Transgenerational plasticity and climate change experiments: Where do we go from here?. Glob. Change Biol.24, 13–34 (2018).
Torda, G. et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Change7, 627 (2017).
Gibbin, E. M. et al. Can multi-generational exposure to ocean warming and acidification lead to the adaptation of life history and physiology in a marine metazoan?. J. Exp. Biol.220, 551–563 (2017).
Chevin, L.-M., Collins, S. & Lefèvre, F. Phenotypic plasticity and evolutionary demographic responses to climate change: Taking theory out to the field. Funct. Ecol.27, 967–979 (2013).
Byrne, M., Foo, S. A., Ross, P. M. & Putnam, H. M. Limitations of cross-and multigenerational plasticity for marine invertebrates faced with global climate change. Glob. Change Biol.26, 80–102 (2020).
Foo, S. A. & Byrne, M. Acclimatization and adaptive capacity of marine species in a changing ocean. Adv. Mar. Biol.74, 69–116 (2016).
Ross, P. M., Parker, L. & Byrne, M. Transgenerational responses of molluscs and echinoderms to changing ocean conditions. ICES J. Mar. Sci.73, 537–549 (2016).
Gaylord, B. et al. Ocean acidification through the lens of ecological theory. Ecology96, 3–15 (2015).
Munday, P. L., Warner, R. R., Monro, K., Pandolfi, J. M. & Marshall, D. J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett.16, 1488–1500 (2013).
Sunday, J. M. et al. Evolution in an acidifying ocean. Trends Ecol. Evol.29, 117–125 (2014).
Sunday, J. M., Crim, R. N., Harley, C. D. G. & Hart, M. W. Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS ONE6, e22881 (2011).
Hettinger, A. et al. Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology93, 2758–2768 (2012).
Hettinger, A. et al. Larval carry-over effects from ocean acidification persist in the natural environment. Glob. Change Biol.19, 3317–3326 (2013).
Vermeij, M. J. A., Fogarty, N. D. & Miller, M. W. Pelagic conditions affect larval behavior, survival, and settlement patterns in the Caribbean coral Montastraea faveolata. Mar. Ecol. Prog. Ser.310, 119–128 (2006).
Costantini, D. Does hormesis foster organism resistance to extreme events?. Front. Ecol. Environ.12, 209–210 (2014).
Costantini, D., Metcalfe, N. B. & Monaghan, P. Ecological processes in a hormetic framework. Ecol. Lett.13, 1435–1447 (2010).
Chakravarti, L. J. et al. Can trans-generational experiments be used to enhance species resilience to ocean warming and acidification?. Evol. Appl.9, 1133–1146 (2016).
Lane, A., Campanati, C., Dupont, S. & Thiyagarajan, V. Trans-generational responses to low pH depend on parental gender in a calcifying tubeworm. Sci. Rep.5, 10847 (2015).
Miller, G. M., Watson, S.-A., Donelson, J. M., McCormick, M. I. & Munday, P. L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change2, 858 (2012).
Parker, L. M. et al. Adult exposure influences offspring response to ocean acidification in oysters. Glob. Change Biol.18, 82–92 (2012).
Parker, L. M. et al. Adult exposure to ocean acidification is maladaptive for larvae of the Sydney rock oyster Saccostrea glomerata in the presence of multiple stressors. Biol. Lett. https://doi.org/10.1098/rsbl.2016.0798 (2017).
Thor, P. & Dupont, S. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Glob. Change Biol.21, 2261–2271 (2015).
Parker, L. M., O’Connor, W. A., Raftos, D. A., Pörtner, H.-O. & Ross, P. M. Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PLoS ONE10, e0132276 (2015).
Brown, B. E. & Cossins, A. R. The potential for temperature acclimatisation of reef corals in the face of climate change. In Coral Reefs: An Ecosystem in Transition (eds Dubinsky, Z. & Stambler, N.) 421–433 (Springer, Dordrecht, 2011).
Brown, B. E., Dunne, R. P., Goodson, M. S. & Douglas, A. E. Bleaching patterns in reef corals. Nature404, 142–143 (2000).
Coles, S. L. & Brown, B. E. Coral bleaching—Capacity for acclimatization and adaptation. In Advances in Marine Biology (ed. Lesser, M.) 183–223 (Academic Press, Cambridge, 2003).
Johnston, E. C., Forsman, Z. H. & Toonen, R. J. A simple molecular technique for distinguishing species reveals frequent misidentification of Hawaiian corals in the genus Pocillopora. PeerJ6, e4355 (2018).
Putnam, H. M., Davidson, J. M. & Gates, R. D. Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol. Appl.9, 1165–1178 (2016).
Stimson, J. S. Mode and timing of reproduction in some common hermatypic corals of Hawaii and Enewetak. Mar. Biol.48, 173–184 (1978).
Stoddart, J. A. et al. Cycles of gametogenesis and planulation in the coral Pocillopora damicornis. Marine ecology progress series. Oldendorf23, 153–164 (1985).
Harriott, V. J. Reproductive seasonality, settlement, and post-settlement mortality of Pocillopora damicornis (Linnaeus), at Lizard Island, Great Barrier Reef. Coral Reefs2, 151–157 (1983).
Stoddart, J. A. Asexual production of planulae in the coral Pocillopora damicornis. Mar. Biol.76, 279–284 (1983).
Yeoh, S.-R. & Dai, C.-F. The production of sexual and asexual larvae within single broods of the scleractinian coral, Pocillopora damicornis. Mar. Biol.157, 351–359 (2010).
Combosch, D. J. & Vollmer, S. V. Mixed asexual and sexual reproduction in the Indo-Pacific reef coral, Pocillopora damicornis. Ecol. Evol.3, 3379–3387 (2013).
Drupp, P., De Carlo, E. H., Mackenzie, F. T., Bienfang, P. & Sabine, C. L. Nutrient inputs, phytoplankton response, and CO2 variations in a semi-enclosed subtropical embayment, Kaneohe Bay, Hawaii. Aquat. Geochem.17, 473–498 (2011).
Price, N. N., Martz, T. R., Brainard, R. E. & Smith, J. E. Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PLoS ONE7, e43843 (2012).
Rivest, E. B., Comeau, S. & Cornwall, C. E. The role of natural variability in shaping the response of coral reef organisms to climate change. Curr. Clim. Change Rep.3, 271–281 (2017).
Guadayol, Ò, Silbiger, N. J., Donahue, M. J. & Thomas, F. I. M. Patterns in temporal variability of temperature, oxygen and pH along an environmental gradient in a coral reef. PLoS ONE9, e85213 (2014).
Silbiger, N. J., Guadayol, Ò, Thomas, F. I. M. & Donahue, M. J. Reefs shift from net accretion to net erosion along a natural environmental gradient. Mar. Ecol. Prog. Ser.515, 33–44 (2014).
Jury, C. P., Thomas, F. I. M., Atkinson, M. J. & Toonen, R. J. Buffer capacity, ecosystem feedbacks, and seawater chemistry under global change. Water5, 1303–1325 (2013).
Riebesell, U., Fabry, V. J., Hansson, L. & Gattuso, J.-P. Guide to Best Practices for Ocean Acidification Research and Data Reporting 258 (Publications Office of the European Union, Luxembourg, 2011).
Dickson, A. G., Sabine, C. L. & Christian, J. R. Guide to Best Practices for Ocean CO2 Measurements Measurements (North Pacific Marine Science Organization, Sidney, 2007).
Gattuso, J.-P. et al. seacarb: Seawater carbonate chemistry. R package version3 (2015).
Permata, W. D., Kinzie, R. A. & Hidaka, M. Histological studies on the origin of planulae of the coral Pocillopora damicornis. Mar. Ecol. Prog. Ser.200, 191–200 (2000).
Albright, R. & Langdon, C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Change Biol.17, 2478–2487 (2011).
Team, R. C. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (3. 3. 1) Software Vienna, Austria: R Foundation for Statistical Computing (2013). https://www.R-project.org/. Accessed 21 June 2016.
Jokiel, P. L. Lunar periodicity of planula release in the reef coral Pocillopora damicornis in relation to various environmental factors. in Proceeding of 5th International Coral Reef Symposium, vol. 4, 307–312 (1985).
Richmond, R. H. & Jokiel, P. L. Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak and Hawaii. Bull. Mar. Sci.34, 280–287 (1984).
Veron, J. E. N. et al. The coral reef crisis: The critical importance of < 350 ppm CO2. Mar. Pollut. Bull.58, 1428–1436 (2009).
van Hooidonk, R., Maynard, J. A. & Planes, S. Temporary refugia for coral reefs in a warming world. Nat. Clim. Change3, 508 (2013).
Ainsworth, T. D. et al. Climate change disables coral bleaching protection on the Great Barrier Reef. Science352, 338–342 (2016).
Donner, S. D., Skirving, W. J., Little, C. M., Oppenheimer, M. & Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol.11, 2251–2265 (2005).
Logan, C. A., Dunne, J. P., Eakin, C. M. & Donner, S. D. Incorporating adaptive responses into future projections of coral bleaching. Glob. Change Biol.20, 125–139 (2014).
Putnam, H. M. Resilience and acclimatization potential of reef corals under predicted climate change stressors. ([Honolulu]:[University of Hawaii at Manoa], [December 2012] (2012).
Fan, T. Y. et al. Plasticity in lunar timing of larval release of two brooding pocilloporid corals in an internal tide-induced upwelling reef. Mar. Ecol. Prog. Ser.569, 117–127 (2017).
Stumpp, M., Wren, J., Melzner, F., Thorndyke, M. C. & Dupont, S. T. CO2 induced seawater acidification impacts sea urchin larval development I: Elevated metabolic rates decrease scope for growth and induce developmental delay. Comp. Biochem. Physiol. A Mol. Integr. Physiol.160, 331–340 (2011).
Cumbo, V. R., Fan, T.-Y. & Edmunds, P. J. Physiological development of brooded larvae from two pocilloporid corals in Taiwan. Mar. Biol.159, 2853–2866 (2012).
Rivest, E. B. & Hofmann, G. E. Effects of temperature and pCO2 on lipid use and biological parameters of planulae of Pocillopora damicornis. J. Exp. Mar. Biol. Ecol.473, 43–52 (2015).
Hamdoun, A. & Epel, D. Embryo stability and vulnerability in an always changing world. Proc. Natl. Acad. Sci. U.S.A.104, 1745–1750 (2007).
Byrne, M. Global change ecotoxicology: Identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar. Environ. Res.76, 3–15 (2012).
Kroeker, K. J., Kordas, R. L., Crim, R. N. & Singh, G. G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett.13, 1419–1434 (2010).
Comeau, S. et al. Pacific-wide contrast highlights resistance of reef calcifiers to ocean acidification. Proc. Biol. Sci. https://doi.org/10.1098/rspb.2014.1339 (2014).
Comeau, S., Edmunds, P. J., Spindel, N. B. & Carpenter, R. C. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr.58, 388–398 (2013).
Jokiel, P. L. et al. Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs27, 473–483 (2008).
Zhao, L., Schöne, B. R., Mertz-Kraus, R. & Yang, F. Sodium provides unique insights into transgenerational effects of ocean acidification on bivalve shell formation. Sci. Total Environ.577, 360–366 (2017).
Zhao, L. et al. Transgenerational acclimation to seawater acidification in the Manila clam Ruditapes philippinarum: Preferential uptake of metabolic carbon. Sci. Total Environ.627, 95–103 (2018).
Padilla-Gamiño, J. L., Pochon, X., Bird, C., Concepcion, G. T. & Gates, R. D. From parent to gamete: Vertical transmission of Symbiodinium (Dinophyceae) ITS2 sequence assemblages in the reef building coral Montipora capitata. PLoS ONE7, e38440 (2012).
Sharp, K. H., Distel, D. & Paul, V. J. Diversity and dynamics of bacterial communities in early life stages of the Caribbean coral Porites astreoides. ISME J.6, 790–801 (2012).
Hartmann, A. C., Marhaver, K. L., Chamberland, V. F., Sandin, S. A. & Vermeij, M. J. A. Large birth size does not reduce negative latent effects of harsh environments across life stages in two coral species. Ecology94, 1966–1976 (2013).
Dixon, G. B. et al. Genomic determinants of coral heat tolerance across latitudes. Science348, 1460–1462 (2015).
Edmunds, P. J., Cumbo, V. R. & Fan, T.-Y. Metabolic costs of larval settlement and metamorphosis in the coral Seriatopora caliendrum under ambient and elevated pCO2. J. Exp. Mar. Biol. Ecol.443, 33–38 (2013).
Little, A. F., van Oppen, M. J. H. & Willis, B. L. Flexibility in algal endosymbioses shapes growth in reef corals. Science304, 1492–1494 (2004).
Eirin-Lopez, J. M. & Putnam, H. M. Marine environmental epigenetics. Ann. Rev. Mar. Sci.11, 335–368 (2019).
Rondon, R. et al. Effects of a parental exposure to diuron on Pacific oyster spat methylome. Environ Epigenet3, dvx004 (2017).
Liew, Y. J. et al. Intergenerational epigenetic inheritance in reef-building corals. Nat. Clim. Change10, 254–259 (2020).
Agostini, S. et al. Biological and chemical characteristics of the coral gastric cavity. Coral Reefs31, 147–156 (2012).
Cai, W.-J. et al. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Commun.7, 11144 (2016).
Huey, R. B., Berrigan, D., Gilchrist, G. W. & Herron, J. C. Testing the adaptive significance of acclimation: A strong inference approach. Integr. Comp. Biol.39, 323–336 (1999).
Edmunds, P. J. Is acclimation beneficial to scleractinian corals, Porites spp.?. Mar. Biol.161, 1531–1542 (2014).
Edmunds, P. J. & Gates, R. D. Acclimatization in tropical reef corals. Mar. Ecol. Prog. Ser.361, 307–310 (2008).
Southham, C. M. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology33, 517–524 (1943).
Lushchak, V. I. Dissection of the hormetic curve: Analysis of components and mechanisms. Dose Response12, 466–479 (2014).
Brown, B. E., Downs, C. A., Dunne, R. P. & Gibb, S. W. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser.242, 119–129 (2002).
Barshis, D. J. et al. Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. U.S.A.110, 1387–1392 (2013).
Burgess, S. C. & Marshall, D. J. Adaptive parental effects: the importance of estimating environmental predictability and offspring fitness appropriately. Oikos123, 769–776 (2014).
Zizzari, Z. V. & Ellers, J. Rapid shift in thermal resistance between generations through maternal heat exposure. Oikos123, 1365–1370 (2014).
Fitt, W. K., McFarland, F. K., Warner, M. E. & Chilcoat, G. C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr.45, 677–685 (2000).
Thornhill, D. J. et al. A connection between colony biomass and death in Caribbean reef-building corals. PLoS ONE6, e29535 (2011).
Pechenik, J. A. & Levine, S. H. Estimates of planktonic larval mortality using the marine gastropods Crepidula fornicata and C. plana. Mar. Ecol. Progr. Ser.344, 107–118 (2007).
Raymundo, L. J. & Maypa, A. P. Getting bigger faster: Mediation of size-sepecific mortality via fusion in juvenile coral transplants. Ecol. Appl.14, 281–295 (2004).
Smith, S. R. Patterns of coral recruitment and post-settlement mortality on Bermuda’s reefs: Comparisons to Caribbean and Pacific reefs. Am. Zool.32, 663–673 (1992).
Putnam, H. M., Barott, K. L., Ainsworth, T. D. & Gates, R. D. The vulnerability and resilience of reef-building corals. Curr. Biol.27, R528–R540 (2017).
Webster, N. S. & Reusch, T. B. H. Microbial contributions to the persistence of coral reefs. ISME J.11, 2167–2174 (2017).
van Oppen, M. J. H., Oliver, J. K., Putnam, H. M. & Gates, R. D. Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. U.S.A.112, 2307–2313 (2015).
van Oppen, M. J. H. et al. Shifting paradigms in restoration of the world’s coral reefs. Glob. Change Biol.23, 3437–3448 (2017).
Acknowledgements
We dedicate this work to our dear friend and colleague, the irreplaceable Ruth D. Gates. This work was supported by funding from NSF OCE-PRF 1323822 to HMP, and NSF EPS-0903833, and NSF URM 0829272. We would like to thank R. Khadka and the HIMB facilities staff for their assistance in this work and N. Silbiger, M. Iacchei and the Silbiquad for constructive comments on the manuscript.
Author information
Authors and Affiliations
Contributions
H.M.P., R.R.W. and R.D.G. designed the project, H.M.P., R.R.W., J.A.C. and J.M.D. collected the data, H.M.P. and R.R.W. analyzed the data, and H.M.P. wrote the manuscript. H.M.P., R.R.W., J.M.D. and R.D.G. revised the manuscript, and all authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Putnam, H.M., Ritson-Williams, R., Cruz, J.A. et al. Environmentally-induced parental or developmental conditioning influences coral offspring ecological performance. Sci Rep 10, 13664 (2020). https://doi.org/10.1038/s41598-020-70605-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70605-x
This article is cited by
-
Long-term preconditioning of the coral Pocillopora acuta does not restore performance in future ocean conditions
Coral Reefs (2023)
-
Larval thermal conditioning does not improve post-settlement thermal tolerance in the dominant reef-building coral, Montipora capitata
Coral Reefs (2022)
-
Depth-dependent parental effects create invisible barriers to coral dispersal
Communications Biology (2021)
-
Extending the natural adaptive capacity of coral holobionts
Nature Reviews Earth & Environment (2021)
-
Effects of thermal conditioning on the performance of Pocillopora acuta adult coral colonies and their offspring
Coral Reefs (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.