Abstract
Sugarcane crop is important for both sugar and biofuels. A world collection of sugarcane and related grasses (WCSRG) maintained at Miami, FL contains > 1,200 non-redundant clones of different species and genera within the Saccharum complex. However, linkage of undesirable alleles with useful genes in wild species has hindered its efficient utilization in sugarcane breeding. A core collection developed previously with smaller number of clones representing WCSRG did not take into account > 120 wild/exotic clones maintained at the USDA-ARS Sugarcane Research Unit in Houma, Louisiana. Moreover, the genome complexity and sub-tropical to temperate growing climate of Louisiana warrant a region-specific core collection that can be used for base-broadening breeding aimed at efficient introgression of desirable alleles. Genetic diversity of 1,485 clones within WCSRG and Louisiana (commercials, wild/exotic) using 423 SSR alleles showed an average gene diversity (h) at 0.208 among all species groups where Erianthus-like Saccharum species (ELSS), Miscanthus spp., and S. spontaneum each formed a distinct cluster, Saccharum robustum, S. officinarum, hybrid cultivars, and S. edule grouped together in a major cluster, and Saccharum sinense and S. barberi formed distinct grouping. A 309-clone diversity panel (SDP1) was developed that captured the genetic diversity based on the combination of maximum length subtree and manual selection to maximize representation of Louisiana clones and minimize import of clones from Miami. SDP1 shared 324 alleles out of the 423 alleles in the entire population of 1,485 clones and captured the genetic diversity of the entire collection with an average gene diversity (h) at 0.163. The variation within (11–17%) and among (83–89%) the populations in SDP1 were comparable with the entire population of 1,485 clones (9–15% and 85–91%, respectively). The breadth of the genetic variation of SDP1 was exemplified by the intra- and inter-specific diversity of a 190-clone mini-core collection with markers derived from known cold-responsive genes. SDP1 will facilitate genome-wide association studies for identification of trait-specific markers for use in marker-assisted breeding in Louisiana and elsewhere.
Similar content being viewed by others
Introduction
Cultivated sugarcane (Saccharum interspecific hybrids) is a perennial C4 grass crop belonging to the subtribe Saccharinae under the tribe Andropogoneae of the family Poaceae. Sugarcane is cultivated worldwide in tropical and subtropical regions as a major source of sucrose1, with a global crop value of $61 billion per year2. Sugarcane accounts for 80% of global sucrose production and 45% of sucrose production in the United States3. Sugarcane, in addition to its importance as a food crop, is recognized as the most productive bioenergy crop because of its ability to produce high biomass4.
Historically, six species are considered important in the Saccharum genus, which include two wild species, S. spontaneum (L.) (2n = 40–128) and S. robustum (Brandes & Jesw. Ex Gressl) (2n = 60, 80), and four domesticated species, S. officinarum (L.) (2n = 80), S. sinense (Roxburgh) (2n = 81–124), S. barberi (Jeswiet) (2n = 111–120), and S. edule (Hassk) (2n = 60–80)5,6. Genetic evidence suggests S. robustum as the progenitor of both S. officinarum and S. edule7,8, and that most modern cultivars, along with S. sinense and S. barberi, are interspecific hybrids between S. officinarum and S. spontaneum. Around 32–39% of the S. sinense and S. barberi genomes come from S. spontaneum, while for modern cultivars, the percentage is around 10–20%6,9,10.
Modern cultivars have limited genetic variation. Fewer than 20 S. officinarum clones are involved in the genealogy of sugarcane cultivars with only a few being used extensively11. Basic crosses are made with clones of S. spontaneum, S. robustum, and species of other genera within the Saccharum complex to broaden the genetic base5,12. In Louisiana, the basic breeding program utilizes wild (basic) clones as nonrecurrent parents, where selected recurrent interspecific hybrids are used for backcrossing13,14.
Commercial sugarcane breeding is labor-intensive and time consuming. In Louisiana, it takes 13 years from crossing to the release of a new variety. Phenotype-based trait selection in conventional breeding can be problematic because of confounding environmental effects. Therefore, accurate phenotypic selection in the early stages of breeding remains a challenge15. Selection in the early stages of breeding using family appraisal followed by mass selection attempts to separate the environmental components of total phenotype16,17, but there is typically limited improvement in the genetic gains in sugarcane primarily due to the quantitative nature of the traits with low to moderate heritability. Marker-assisted selection (MAS), on the other hand, could enhance the efficiency of early stage selection as well as selection response of difficult-to-phenotype traits18.
Quantitative trait locus (QTL) mapping is widely used to understand the genetics of complex polygenic traits, and it is the first step toward development of trait-based markers for use in MAS. Use of SNP genotyping by sequencing has improved the resolution of QTLs with generation of high-density linkage maps19,20,21. However, traditional biparental linkage mapping in heterozygous species, such as sugarcane, with only single-dose markers, may identify low-resolution QTLs due to the lack of information on the number/type of alleles at the segregating locus and limited genetic variation in the biparental population22.
Genome-wide association study (GWAS), on the other hand, has been used recently to identify marker-trait associations (MTAs) in several plants. GWAS allows identification of QTLs with resolution at the gene level, as it takes advantage of historical and evolutionary recombination events in a genetically diverse population (diversity panel)23. Further, a diversity panel allows study at the same time of allelic diversity and haplotypes of genes/alleles associated with traits. GWAS is based on linkage disequilibrium (LD), which is high in sugarcane11,24 mainly due to relatively few generations between modern cultivars and the limited number of initial clones used in hybridization25,26. The high LD of sugarcane has been exploited to identify markers associated with various traits using a regional diversity panel or core collection18,27,28,29,30,31,32.
Saccharum spp. and related genera from countries around the world have been collected into a “World Collection of Sugarcane and Related Grasses” (WCSRG) maintained at the National Germplasm Repository of the USDA-ARS Subtropical Horticulture Research Station, Miami, FL, USA. Various studies have described the diversity in the WCSRG14,33,34,35,36. A total of 342 S. spontaneum clones were assessed using stratified random sampling over geographical origins and principal component cluster groups to select a 75-clone core collection33. The authors also evaluated diversity of 32 S. officinarum, 30 S. barberi, 28 S. sinense, and 27 S. robustum based on cluster analysis using principal component analysis of sugar composition34. A 300-clone diversity panel was created based on genetic diversity of 1,002 clones of WCSRG using 231 SSR alleles35. A similar panel was developed through phenotypic characterization of the collection14.
The panels discussed above14,33,34,35 did not include clones outside of the world collection. For example, clones that have been procured and maintained by the base broadening (basic breeding) program at the USDA-ARS Sugarcane Research Unit in Houma, Louisiana and elite cultivars and parents used in breeding programs in Louisiana were not included in the diversity analysis. Moreover, ~ 250 additional clones have been collected into the WCSRG in the last four years36. Therefore, the core collections previously developed may not fully account for the range of variation among the subtropical and temperate sugarcane clones currently being used in Louisiana. Sugarcane crop grows optimally at ~ 35 °C. Cold stress by temperatures near freezing can compromise crop growth, development, and yield. Temperatures as high as 20 °C can suppress plant growth and below 15 °C can cause tissue injury37. Louisiana represents the far northern limit of sugarcane cultivation in the U.S. with frequent freezing during the crop season. Therefore, a Saccharum diversity panel developed from WCSRG and clones in Louisiana breeding programs would facilitate GWAS studies for identification of trait-specific markers, such as cold tolerance, for use in marker-assisted breeding in Louisiana and other sugarcane industries. Here, we report on the development of an inclusive sugarcane diversity panel (SDP1) and demonstrate the breadth of its diversity with regard to abiotic (cold) stress responsive genes.
Methods
Plant materials
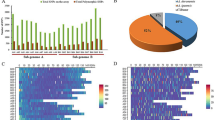
For the diversity panel, 1,485 clones within the Saccharum complex, including Saccharum, Miscanthus, Coix, Imperata, and Sorghum, were used36 (Fig. 1). Of these, 1,236 were clones from the WCSRG, 113 clones were elite and historic breeding clones from the Louisiana sugarcane breeding program, 119 were clones of wild/exotic species (not present in WCSRG), and 17 were hybrids from the base-broadening introgression program of the USDA-ARS Sugarcane Research Unit. Saccharum spp. previously classified as Erianthus spp. including S. arundinaceum, S. bengalense, S. ravennae, S. rufipilum, S. brevibarbe, S. kanashiroi, and S. procerum were grouped together as Erianthus-like S. spp. (ELSS) for simplicity of analysis. For diversity analysis with cold responsive genes, a mini-core of 190 clones (Supplementary Table S1) was selected based on genetic diversity analysis of 1,485 clones where clones from each subclusters in all species groups were represented. The mini-core comprised of 96 Louisiana sugarcane elite and historic breeding clones (Saccharum interspecific hybrids), 27 S. spontaneum, 20 S. spontaneum (previously listed as unknown), 15 S. officinarum, seven Eriranthus, five each of S. robustum and S. bengalense, three each of S. arundinaceum and Miscanthus, two each of S. kanashiroi and S. ravennae, and one clone each of S. edule, S. barberi, S. rufipilum, Coix and Imperata.
Frequency of species groups for the entire population (1,485 clones) and the diversity panel (309 clones). The two axes are proportional based on set size for comparison. Erianthus-like Saccharum spp. included S. arundinaceum, S. bengalense, S. ravennae, S. rufipilum, S. brevibarbe, S. kanashiroi, S. procerum, and unknown species previously identified as Erianthus.
DNA purification and genotyping
Total genomic DNA was extracted from ~ 100 mg leaf tissues using the CTAB miniprep and checked for quality and quantity using a ND-100 spectrophotometer (Nanodrop Technologies Inc, Wilmington, DE), as described previously36. Four-hundred-fifty SSR primer pairs, including 277 genomic SSRs38,39, 127 eSSRs from sugarcane cold-responsive genes40, and 46 eSSRs from brown rust-responsive genes41, were initially tested for polymorphism among 113 Louisiana clones. Eleven SSR primer pairs mapped on nine out of 10 sugarcane monoploid homeologous groups42 and 17 out of 32 pseudochromosomes43 that had high polymorphism index (Supplementary Table S2) were selected to evaluate genetic diversity of the 1,485 clones.
For genetic diversity analysis of the 190-clone mini-core, 9,974 cold responsive genes reported in Sorghum44 were searched for the presence of simple sequence repeat (SSR) motifs using BatchPrimer3 v1.0 with criteria set to at least five repeats for dinucleotide motifs and three repeats for tri, tetra, penta and hexa nucleotide motifs. Primers flanking SSR motifs ≥ 16 nt from 52 genes were designed using BatchPrimer3 v1.0. In addition, 48 eSSRs from cold responsive genes of sugarcane40 were included.
The polymerase chain reaction (PCR) and resolution of PCR products were performed following a method described earlier21. Briefly, 50 ng of genomic DNA was used as the template in 10 µl PCR containing 2 µl of 5 × buffer, 1 µl of 25 mM MgCl2, 1 µl of 2 mM dNTP mix, 0.1 µl of Taq DNA polymerase (Promega, Madison, WI) and 0.5 μl of 10 µM forward and reverse primer (IDT, Corvallis, OR). A thermal profile of 95 °C for 5 min followed by 35 cycles of 95 °C for 15 s, 58 °C for 15 s, and 72 °C for 30 s, and 72 °C for 10 min was used. The amplification products were resolved in a 13% polyacrylamide gel using a high-efficiency gel electrophoresis system (Nihon Eido, Tokyo, Japan). Amplified fragments (alleles) were manually scored as “1” (present, dominant) and “0” (absent) in a binary matrix.
Genetic diversity analysis
Alleles at SSR loci occurring in less than 1% of the clones were discarded prior to downstream analysis to reduce false similarity between clones due to shared absence of alleles while still capturing rare alleles. The polymorphism information content (PIC) was computed for each SSR marker following45.
GeneAlEx 6.50246 was used to compute gene diversity (h), Shannon’s information index (I), Nei’s genetic distance (D), principal coordinate analysis (PCoA), and an analysis of molecular variance (AMOVA). AMOVA was done for species groups by recorded names: S. spontaneum, S. officinarum, hybrid cultivar, S. robustum, ELSS, S. sinense, S. barberi, Miscanthus spp., and other46. AMOVA was also conducted on species groups devised from the neighbor-joining analysis, and on groups devised from the population structure analysis (described below). Private alleles, population differentiation and gene flow were estimated for the mini-core by FST and Nm values, respectively. For the mini-core, h and I for every locus, genetic diversity within a population (Hs), total heterozygosity (Ht), gene flow (Nm), and Gst were calculated using PopGene 1.3247.
Genetic diversity was also analyzed using DARwin 6.0.1248 using Dice dissimilarity scores to validate the clustering of the clones. Weighted neighbor-joining algorithms were used to construct a phylogenetic tree with 1,000 bootstrap repetitions to evaluate the robustness and significance of each node. Bootstrap analysis was implemented with 1,000 iterations to compute the minimum number of SSR alleles needed to differentiate the species using Bootsie software49 and allele numbers were plotted against their corresponding CV values in a bi-plot curve.
Structure analysis
Assignment of clones to a specified number of clusters (K) and population structure were determined using Structure ver. 2.3.450. Models were run using Bayesian algorithm for K = 2—10, and K = 8 was selected as per the software’s documentation and eight species groups. A standard admixture model was used with an inferred alpha. To accommodate minor alleles, lambda was evaluated at different levels, and a lambda of 0.5 yielded the best models based on the log of the probability of the data. The Markov chain Monte Carlo program converged well before 50,000 iterations, so 50,000 iterations were used for ‘burn-in’, and 25,000 subsequent iterations were used for model parameter estimation. To estimate the number of clusters, an admixture model with correlated allele frequencies was run in 10 models, and two non-symmetric modes were found. One mode occurred seven times and the other was less consistent and occurred three times. An average of the seven runs from the first mode was used for the final result.
Results
SSR genotyping
The 11 SSR primer pairs resulted in a total of 423 polymorphic alleles (Supplementary Table S2). The number of alleles per SSR ranged from 13 to 65 with an average of 38. Three sugarcane SSRs on homeologous group (HG) 4 generated 106 alleles. All other HGs were represented by a single SSR producing 18 (HG 9) to 58 (HG 5) alleles with an average major allele frequency of 0.76. The PIC values ranged from 0.17 to 0.38 with an average of 0.25. The average number of alleles per clone-SSR pair was 8.01 with the maximum being 30.16. The maximum average number of alleles per clone for an SSR was 16.28, which is typical of sugarcane where the basic chromosome number of the species in the population ranges from x = 7 to x = 195. Saccharum spontaneum, representing one-third of the population, has a basic chromosome number of 8.
The low frequencies of S. sinense, S. barberi, and Miscanthus spp. necessitated maintaining minor alleles. Thus, alleles with frequencies between 0.990 and 0.010 were retained for analysis. These bounds were equivalent to the frequency of Miscanthus spp. in the population, which was 0.009. All SSR primers produced at least one polymorphic allele with a frequency at or above 0.348, higher than the average allele frequency (0.210).
Gene diversity and allele polymorphism by species
The gene diversity (h) ranged from 0.16 for Miscanthus spp. to 0.24 for S. spontaneum indicating that Miscanthus spp. were the least diverse and S. spontaneum the most diverse. The overall average gene diversity of all species groups was 0.21 (Supplementary Table S2). The number of accessions evaluated for each species apparently influenced the gene diversity. For example, number of Miscanthus accessions (14) were 37-fold less than the S. spontaneum (516).
The entropy measured by Shannon’s information index (I) followed the same trend with those species having higher gene diversity also exhibiting higher entropy (Supplementary Table S2). The only exceptions were S. officinarum and S. barberi, where S. officinarum had h = 0.21 but an I = 0.33, and S. barberi had an h = 0.21 but an I = 0.33. The I value ranged from 0.25 (Miscanthus spp.) to 0.39 for (S. spontaneum), with an average of 0.33.
The percentage of polymorphic alleles within a species was apparently directly proportionate to its population size (Fig. 1, Supplementary Table S3). Again, the polymorphic alleles were highest for S. spontaneum (99.3%) and lowest for Miscanthus spp. (54.4%).
Genetic distance between species
Based on Nei’s pairwise genetic distance (D), the greatest distance was between the hybrid cultivars group and Miscanthus spp. at 0.105, while the shortest genetic distance was between S. officinarum and hybrid cultivars group (0.009), followed by the D between S. officinarum and S. robustum (0.011) (Supplementary Table S4). Miscanthus spp. were the farthest from the other species groups. Miscanthus spp. were closest to ELSS and S. spontaneum at D = 0.038 and D = 0.049, respectively, but farthest from S. sinense (D = 0.101). The D value between the rest of the species groups and Miscanthus spp. ranged from 0.07 to 0.105. The next farthest from the others was ELSS (0.024 to 0.086) followed by S. spontaneum (0.032 to 0.060).
Phylogeny and population structure
Neighbor joining with a Dice dissimilarity matrix was also used to evaluate genetic diversity (Fig. 2). ELSS, Miscanthus spp., and S. spontaneum each showed distinct separation from the other species. Saccharum robustum, S. officinarum, hybrid cultivars, and S. edule grouped together in a major cluster with subclusters concentrated independently with S. robustum and hybrid cultivars. Saccharum sinense and S. barberi also showed distinction, but overall had little intra-species diversity. Miscanthus spp. were most distant from other clones followed by ELSS and S. spontaneum.
Neighbor-joining tree of the Saccharum complex showing genetic diversity based inter- and intra-species differentiation of 1,485 clones using 423 SSR alleles. Erianthus-like Saccharum spp. included S. arundinaceum, S. bengalense, S. ravennae, S. rufipilum, S. brevibarbe, S. kanashiroi, S. procerum, and unknown species previously identified as Erianthus. Other species included Coix lacryma-jobi, Imperata sp., Sorghum polumosum, Saccharum edule, and unknown.
The eight sub-populations delineated by the structure analysis had near direct correspondence to species groups (Fig. 3). The first two sub-populations corresponded to both S. officinarum and S. robustum with no clear distinction between them. Other sub–populations corresponded to hybrid cultivars, S. sinense, and S. barberi. ELSS and Miscanthus spp. comprised one sub-population, whereas S. spontaneum was delineated by two sub-populations.
Population structure of 1,485 clones (horizontal axis) showing eight sub-populations. Species groups Saccharum officianarum / robustum, and S. spontaneum were each represented by two groups. Clones are in the order of the clones in the neighbor joining analysis for comparison (Fig. 2). Erianthus-like Saccharum spp. included S. arundinaceum, S. bengalense, S. ravennae, S. rufipilum, S. brevibarbe, S. kanashiroi, S. procerum, and unknown species previously identified as Erianthus. The values in the vertical axis represent the likelihood in percent of an individual belonging to one of the eight colored sub-populations.
Principal coordinate analysis (PCoA) showed definite variance between species groups (Fig. 4). The coordinates cumulatively accounted for 12.8% of the total variation, with the first three accounting for 8.04, 2.55, and 2.21%, respectively. Saccharum spontaneum grouped by itself with some outliers that grouped with the cluster comprising ELSS and Miscanthus spp. Saccharum officinarum, S. robustum, and hybrid cultivars grouped together but with distinct centroids. Saccharum sinense and S. barberi formed separate clusters that were close to each other bordering the clusters of the S. officinarum and S. robustum clones and between the S. spontaneum and S. officinarum and S. officinarum/ S. robustum/ hybrid cultivars cluster.
Principal coordinate analysis (PCoA) of 1,485 clones showing inter- and intra-species differentiation. Erianthus-like Saccharum spp. included S. arundinaceum, S. bengalense, S. ravennae, S. rufipilum, S. brevibarbe, S. kanashiroi, S. procerum, and unknown species previously identified as Erianthus. Other species included Coix lacryma-jobi, Imperata sp., Sorghum polumosum, Saccharum edule, and unknown.
AMOVA was conducted on the 1,485 clones with the sub-populations derived from three independent diversity analysis-based divisions (Supplementary Table S5). The first division was based on the clones with nine groups consisting of S. spontaneum, S. officinarum, hybrid cultivars, S. robustum, ELSS, S. sinense, S. barberi, Miscanthus, and others, which included Coix lacryma-jobi, Imperata sp., Sorghum polumosum, Saccharum edule, and unknown species (Fig. 1). The second division was based on the DARwin neighbor-joining on Dice dissimilarities. Here clones grouping together were considered part of the same species groups. This eliminated the ‘other’ group and combined S. officinarum and S. robustum leaving seven groups. Clones falling in the S. officinarum / S. robustum / hybrid cultivar complex that were not hybrid cultivars were considered part of the S. officinarum / S. robustum group. The third grouping was based on STRUCTURE model estimates using the group that was estimated to contribute the largest proportion to the genome. All three AMOVA showed significant differences within sub-populations with p values ≤ 0.001 (Supplementary Table S5). Genetic variation within a sub-population was high (85.1–90.7%), whereas 9.3, 14.4, and 14.9% of the variance was attributable to between sub-population variance in the name-based division (groups by species), the DARwin and the STRUCTURE divisions, respectively.
Sugarcane diversity panel (SDP1) selection
A 309-clone diversity panel designated as SDP1 was selected by the combination of the molecular markers-based genetic diversity using the maximum length subtree algorithm in DARwin (238 clones) and Louisiana sugarcane breeders selection wherein clones (57 Louisiana commercials and 14 basic F1 progeny) were picked from different subclusters maximizing the clones existent in Louisiana to minimize the number of clones to be imported from the WCSRG. SDP1 consisted of 284 clones from Louisiana that represent clusters of clones within WCSRG and Louisiana breeding programs. The remaining 25 clones require importation from the WCSRG. Over 100 of the SDP1 clones were Louisiana hybrid cultivars that consisted of both historic and current clones. Louisiana clones exhibited some clustering, but were fairly well dispersed across the entire study population (Fig. 5). Saccharum spontaneum, being a noxious weed, cannot be grown in the field making accurate phenotyping of traits more difficult. Therefore, S. spontaneum clones’ representation in the SDP1 were intentionally minimized where hybrids x S. spontaneum F1s developed by the base-broadening introgression breeding program of the USDA-ARS replaced 31 S. spontaneum clones. Representation of the ELSS and Miscanthus spp. from the WCSRG were intentionally kept to a minimum. The remainder of SDP1 was proportionally distributed over other species groups (Fig. 1).
Neighbor-joining tree showing 309-clone diversity panel. Two-hundred thirty-eight clones (blue) were selected based on maximum length subtree program of DARwin with representation from different subclusters. Fifty-seven Louisiana commercial cultivars and 14 F1 clones from basic base broadening program were included to maximize representation of Louisiana clones and minimize representation of Saccharum spontaneum.
The gene diversity (h) for different species groups were comparable, ranging from 0.13 for Erianthus-like species to 0.15 for hybrids (Supplementary Table S3). The overall average gene diversity of all species groups was lower (0.16). The polymorphic alleles were highest for S. spontaneum (88%) followed by 77.8% for hybrids. Erianthus-like species had lowest allele polymorphism (40.2%).
Nei’s genetic distance (D) showed that Erianthus-like group was the most genetically distant from other species (0.029–0.041) with the highest D (0.041) with S. robustum. Expectedly, D (0.007) was the smallest between hybrid cultivars group and S. officinarum, followed by the equal D (0.016) between S. officinarum and S. robustum and between S. officinarum and S. barberi (Supplementary Table S4). Saccharum sinense and S. barberi were genetically close at D = 0.018.
Analysis of variance showed significant differences within sub-populations (Supplementary Table S5) with high genetic variation among the clones within a sub-population (83.0–89%), whereas 11–17% variation was attributed to sub-populations by species groups, and DARwin and the STRUCTURE defined divisions.
Genetic diversity study of the mini-core
SSR search of the cold stress responsive genes in Sorghum identified 93 different kinds of SSR motifs. Trinucelotide repeats were the highest (55%), of which the GCC/GGC motif had the highest frequency (22), followed by tetra (24%), hexa (10%), penta (6%), and di (5%) (Supplementary Fig. S1). Of the 100 SSR primer pairs used to genotype the mini-core, 67 were polymorphic producing a total of 1,377 alleles with 37.8 and 27.8 as the average and effective number of alleles, respectively (Supplementary Table S6). Polymorphism information content (Nei's gene diversity, h) of the markers ranged between 0.005 and 0.484 with a mean of 0.211 (Supplementary Table S6).
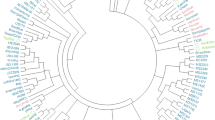
The dendrogram generated through unweighted pair group method of arithmetic mean (UPGMA) exhibited three distinct clusters (Fig. 6). Cluster I contained two subclusters, where subcluster IA was predominated by Saccharum hybrids and close to the subcluster IB containing mostly S. officinarum. Saccharum spontaneum populated subcluster IIA, which was close to Cluster IIB with clones listed as unknowns. The third cluster consisted of two subclusters, where Miscanthus, S. edule, S. kanashiroi, and S. robustum formed subcluster IIIA, and S. arundinaceum, S. erianthus, S. bengalense, S. barberi, S. imperata, S. rufipilum, S. coix, S. ravennae, and S. kanashiroi grouped together as subcluster IIIB (Fig. 6). On the other hand, Bayesian model of structure grouped the clones into five clusters with Louisiana hybrids in a separate cluster (Fig. 7). The principal coordinate analysis (PCoA) based on Euclidean distance between clones was in agreement with the cluster analysis, and coordinates 1, 2, and 3 explained 14.24%, 3.87%, and 1.97% of the variation, respectively (Fig. 8).
Population structure of the 190-clone sugarcane mini-core using the Structure bar plot (K = 5). Pop 1 = Saccharum hybrids, Pop 2 = S. officinarum, Pop 3 = S. spontaneum, Pop 4 = Saccharum sp., Pop 5 = S. robustum, Erianthus, Miscanthus, S. arundinaceum, S. kanashiroi, S. ravennae, S. rufipilum, S. imperata, Coix, S. edule, S. barberi.
Principal coordinate analysis of the 190-clone sugarcane mini-core. Coordinate 1 = 14.24, Coordinate 2 = 3.87, Coordinate 3 = 1.97. Pop 1: Saccharum hybrids, Pop 2: S. officinarum, Pop 3: S. spontaneum, Pop 4: Saccharum sp., Pop 5: S. robustum, Erianthus, Miscanthus, S. arundinaceum, S. kanashiroi, S. ravennae, S. rufipilum, S. imperata, Coix, S. edule, S. barberi.
Louisiana hybrid clones had the highest number of unique alleles (37 of the population total of 67) (Supplementary Table S7) when the mini-core was analyzed with the alleles generated by the 11 SSR primers used for the entire 1,485 clones. On the other hand, the population group containing Miscanthus and Erianthus had the highest number of unique alleles when analyzed with cold-responsive genes-derived SSR primer pairs (Supplementary Table S7). The mean diversity among the populations with cold responsive genes-derived SSRs (0.218) was higher than that with 11 SSRs (0.182) (Supplementary Table S7). Hierarchical analysis of molecular variance (AMOVA) showed equal amount of variation among and within populations at 19% and 81%, respectively with both cold-responsive genes-derived SSRs and 11 SSRs used for the entire population of 1,485 clones (Supplementary Table S8). Nei’s genetic identity between populations was 0.900–0.984 (Supplementary Table S9) with cold-responsive genes-derived SSRs compared to 0.942–0.981 with the SSRs used for entire population of 1,485 clones. The average gene diversity in the mini-core (H at 0.156) and SDP1 (H at 0.163) with the alleles generated with 11 SSR primer pairs were comparable.
Discussion
The clones within WCSRG are genetically diverse and possess desirable alleles for several agronomic traits35,51. However, the daunting task of exploiting the potential of a large number of highly genetically complex clones possessing many undesirable alleles has led to the use of only a few clones in sugarcane breeding programs worldwide. The advent of molecular genomic tools and development/utilization of MAS may allow the tremendous potential value and utility of WCSRG to be realized. A core collection with a smaller number of non-redundant clones, representative of the maximum genetic diversity of the entire collection, will facilitate its utilization in commercial breeding52, thus effectively and efficiently managing resources on a smaller but diverse population while maximizing genetic gain. To this end, the present study developed a sugarcane diversity panel (SDP1), a core collection selected from 1,485 clones enriched with clones from Louisiana, based on the diversity analysis and breeders’ input (Figs. 1 and 5).
Subtropical and temperate climate specific sugarcane genotypes were not well represented in other core collections previously developed33,34,35,53 thereby limiting their application for GWAS in Louisiana. For instance, the 300-clone core collection developed35 includes 228 wild S. spontaneum, other S. spp., other genera, and unknown species, which potentially need to be maintained in pots on concrete. This could hinder the phenotypic characterization of the panel under natural field conditions. SDP1, enriched with Louisiana clones, is expected to circumvent this problem as it contains less than 50 wild-type clones and all of these clones are already maintained at the USDA Sugarcane Research Unit in Houma, Louisiana.
While it is challenging to accomplish very good coverage of the complex Saccharum spp. genome with only 11 primers as used in the present study, the random coverage of the alleles over the genome was possible due to the multiallelic nature of the SSR primers54 distributed over the 10 sugarcane homeologous groups. The 11 SSR primers generated a total of 423 alleles with an average of ~ 38.45 alleles per locus. The range (0.172 to 0.375) of the PIC values of the SSR primers used in the entire collection was similar to the range (0.195 to 0.375) reported earlier35. Previous studies have used nearly two times fewer total alleles and average number of alleles per SSR for diversity analysis, such as 205 alleles with 13.67 per locus55, 209 alleles with 5.8 per locus35, and 261 alleles with 7.35 per locus56. Thus, 423 alleles were considered enough to randomly capture a significant proportion of the genetic diversity within the Saccharum genome to effectively discriminate accessions among different species groups. This was evident from the bootstrap results (Supplementary Table S10) that showed that 423 polymorphic alleles reduced the CV to 2.3% and 114 alleles were sufficient enough to result in the recommended 5% coefficient of variation (CV)57,58.
The clusters generated by neighbor-joining analysis, structure analysis, and PCoA were similar. The only difference was that, unlike the neighbor-joining analysis, neither the structure analysis nor the PCoA produced a Miscanthus spp. cluster separate from the ELSS. The species groups described by neighbor-joining were S. spontaneum, S. officinarum / robustum, hybrid cultivars, ELSS, S. sinense, S. barberi, and Miscanthus spp. Saccharum experienced one genome duplication shared with Miscanthus, but an additional, more recent lineage-specific duplication leading to autopolyploidization in Saccharum species may explain the distance between Saccharum hybrids and Miscanthus59. Principal coordinate analysis showed hybrid cultivars and S. officinarum / robustum clustering together. Additionally, the PCoA most likely could provide visualization for the majority of the variance between populations with 10.59% of the total variance explained by the first two coordinates, which was close to 9.3, 14.4, and 14.9% found by the AMOVA between groups. All this evidence supports the current Saccharum species delineations.
The low robustness of the neighbor-joining tree was due to the combination of varying numbers of clones within a species in the population and the use of minor alleles. Because of the nature of the population, alleles primarily present in S. spontaneum, in particular, or in S. robustum, S. officinarum, or hybrid cultivars required little representation within their primary species to be included at higher frequencies under bootstrapping, while alleles primarily present in species with less clones needed more representation. The cut-off allele frequency of 0.01 used in this study affected 15 clones, represented by 3% of the S. spontaneum, but up to 107% of the Miscanthus spp. clones. Thus, each species did not have equal representation among the alleles used, which resulted in changes in the diversity-based tree when different samples were selected. Nevertheless, the genetic diversity was well represented, as demonstrated by the neighbor-joining, structure and principal coordinate analyses.
Results of the diversity analysis of the WCSRG from the present study and that of Nayak et al.35 were comparable. In the present study, overall averages were 0.213 and 0.338 for gene diversity and Shannon’s information indexes, respectively, as compared to 0.310 and 0.438, respectively, observed by Nayak et al.35. The percentage of polymorphic alleles in the present study ranged from 54.37 to 99.29, whereas it ranged between 75.60 and 99.52 for Nayak et al.35. The genetic distance between groups/species in the present study was lower than previously published reports. For example, the distance between S. spontaneum and S. officinarum was 0.55 as compared to 0.79 reported by Nayak et al.35. The low averages in the present study could be due to the retention of minor alleles. Nevertheless, S. spontaneum remained the most genetically diverse species, primarily due to its high variation in chromosome number (2n = 40–128), diversity of habitats, and widest geographic distribution43,60.
Clustering analysis results of the WCSRG and Louisiana collection in the present study were similar to that observed by Nayak et al.35 where three main clusters: 1) S. spontaneum; 2) S. officinarum, hybrid cultivars, S. robustum, S. edule, S. barberi, and S. sinense; and 3) other species were described. However, species within each cluster were resolved better in our study, where seven clusters in the neighbor-joining analysis (S. spontaneum, S. officinarum / robustum, hybrid cultivar, ELSS, S. sinense, S. barberi, and Miscanthus spp.) and six clusters with the structure analysis and the PCoA (S. spontaneum, S. officinarum / robustum, hybrid cultivar, ELSS / Miscanthus spp., S. sinense, and S. barberi) were identified. Nayak et al.35 left Erianthus and Saccharum nomenclatures as found in the WCSRG database. They used non-Saccharum species as a group, grouping Erianthus spp. with Miscanthus spp. Considering that several Erianthus spp. have been reclassified under Saccharum, it is difficult to comprehend if their analysis found the same distinction that was observed in the present study between ELSS and Miscanthus spp. The distinction between Saccharum spp. and Erianthus-like Saccharum spp., as found in our study, was also observed earlier61.
Genic SSRs derived from the cold-responsive transcripts with a putative function are useful for functional diversity assay in natural populations or germplasm collections. The low polymorphism information content of the genic SSRs (0.211) we observed among the clones in WCSRG was similar to that with a set of cold-responsive genes-derived markers among the Louisiana clones in our previous study39. The low polymorphism of genic SSRs could be explained by the conserved sequence of the transcribed regions within the Saccharum genus62 or by the low transmission of diverged sequence differences between the Saccharum spp.51. Genetic similarity was high among the sugarcane hybrids, which is likely because only a few parental clones were involved in the development of the foundation clones through nobilization in breeding programs63. Alleles at these loci are possibly fixed as a result of a limited number of founder parents and subsequent selection. The Louisiana sugarcane hybrids formed a distinct group, close to the group containing S. officinarum clones, which is expected because most of the modern cultivars inherited the major part (~ 80–85%) of the genome of S. officinarum during nobilization events64. Besides, Louisiana-bred clones grouped in a subcluster within the hybrids cluster, which may be because they were developed in subtropical conditions under selection pressure from cold temperatures. Similar to the S. spontaneum in the entire collection of 1,485 clones, S. spontaneum clones in subcluster 3 of the mini-core were close to unknown S. spp. clones in cluster 4. This supported our assumption that most, if not all, of the unknown clones in the WCSRG are S. spontaneum. The non-correspondence of genetic similarity of clones within a species group within WCSRG and the mini-core is believed to be due to the varied cold stress response of the clones. This is justified by the high percentage (81.2%) of variation within a species group (population) compared to 18.8% among the species groups (Supplementary Table S8) and high average gene diversity (0.218) in the mini-core (Supplementary Table S7) as compared to the 0.208 in the present study (Supplementary Table S3) and 0.30435 within the WCSRG. The number of alleles unique to the different species groups (average 17.4; Supplementary Table S7) suggested a wide genetic variation in cold stress response of the mini-core clones. Ongoing studies are focused to elucidate the role of variation in microsatellite loci in discriminating clones with varied cold tolerance and future development of functional markers for their use in MAS in breeding for cold tolerant sugarcane cultivars.
Taken together, the genetic diversity analysis based on genotypic data of 1,485 clones helped Louisiana breeders in the selection of 309 clones (20.8%) as the SDP1 that, with an average h at 0.163 (Supplementary Table S3), captured the diversity found in the population (average h at 0.208), including the Louisiana core collections. SDP1 and mini-core shared 324 and 319 alleles, respectively out of 423 alleles in the entire collection of 1,485 clones (Supplementary Table S2). The percentage of variation among the populations in the entire collection and SDP1 were comparable (Supplementary Table S5). The genetic diversity of the mini-core by cold-responsive genes-derived SSR markers, which potentially affected the function of the cold-responsive proteins, suggested the diversity present for phenotypic traits, in this case cold response, within SDP1. SDP1 is now being used for phenotyping of various traits of agronomic importance for GWAS studies to identify markers associated with those traits. Phenotypic characterization within SDP1 can then be used to identify clones of special interest for particular traits, and these results will allow more effective introgression of useful alleles from wild/exotic clones in the already existing sugarcane base-broadening program of Louisiana to improve commercial sugarcane or energy cane variety development. In addition, another important value of SDP1 will be to serve as the validation platform for trait-markers identified from QTL mapping studies involving biparental populations. This function will allow the realization of the potential for molecular breeding for improved cultivar development in sugarcane.
References
Park, J.-W. et al. Cold responsive gene expression profiling of sugarcane and Saccharum spontaneum with functional analysis of a cold inducible Saccharum homolog of NOD26-Like intrinsic protein to salt and water stress. PLoS ONE10, e0125810 (2015).
FAOSTAT. In: FAOSTAT. https://faostat3.fao.org/home (2016).
Denrées, G.S. SUCDEN. In: World Sugar Prod Stat -Groupe Sucres Denrées. https://www.sucden.com/statistics/1_world-sugar-production (2015).
Lam, E. et al. Improving sugarcane for biofuel: engineering for an even better feedstock. GCB Bioenergy1, 251–255 (2009).
Moore, P. H., Paterson, A. H. & Tew, T. Sugarcane: the crop, the plant, and domestication. In Sugarcane: Physiology, Biochemistry and Functional Biology (eds Moore, P. H. & Botha, F. C.) 1–15 (Wiley, NewYork, 2013).
Hoang, N. V., Furtado, A., Botha, F. C., Simmons, B. A. & Henry, R. J. Potential for genetic improvement of sugarcane as a source of biomass for biofuels. Front. Bioeng. Biotechnol.3, 182 (2015).
Grivet, L., Daniels, C., Glaszmann, J. C. & D’Hont, A. A review of recent molecular genetics evidence for sugarcane evolution and domestication. Ethnobot. Res. Appl.2, 9–17 (2004).
Grivet, L., Glaszmann, J. C. & D’Hont, A. Molecular evidences for sugarcane evolution and domestication. In Darwin’s Harvest. New Approaches to the Origins, Evolution, and Conservation of crops 1st edn (eds Motley, T. J. et al.) 49–66 (Columbia University Press, New York, 2006).
D’Hont, A. et al. Characterization of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol. Genet. Genom.250, 405–413 (1996).
Piperidis, N., Piperidis, G. & D’Hont, A. Molecular Cytogenetics. In: Genetics, Genomics and Breeding of Sugarcane, Science Publishers, pp 9–18 (2010).
Raboin, L.-M., Pauquet, J., Butterfield, M., D’Hont, A. & Glaszman, J.-C. Analysis of genome-wide linkage disequilibrium in the highly polyploid sugarcane. Theor. Appl. Genet.116, 701–714 (2008).
Ming, R. et al. Sugarcane improvement through breeding and biotechnology. In: Janick J (ed) Plant Breeding Reviews, 27, 15–118 (2010).
Hale, A. Impacts of the USDA basic breeding program. Sug Bull.92, 13–16 (2014).
Todd, J. et al. Phenotypic characterization of the Miami World Collection of sugarcane (Saccharum spp.) and related grasses for selecting a representative core. Genet. Res. Crop Evol.61, 1581–1596 (2014).
Kimbeng, C. A. & Cox, M. C. Early generation selection of sugarcane families and clones in Australia: a review. J. Amer. Soc. Sugarcane Technol.23, 21–39 (2003).
Jackson, P. A., McRae, T. A. & Hogarth, D. M. Selecting sugarcane families across variable environments. II. Patterns of response and association with environmental factors. Field Crops Res.43, 119–130 (1995).
Stringer, J. K., Cox, M. C., Atkin, F. C., Wei, X. & Hogarth, D. M. Family selection improves the efficiency and effective- ness of selecting original seedlings and parents. Sugar Tech13, 36–41 (2011).
Gouy, M. et al. Genome wide association mapping of agro-morphological and disease resistance traits in sugarcane. Euphytica202, 269–284 (2015).
Balsalobre, T. W. A. et al. GBS-based single dosage markers for linkage and QTL mapping allow gene mining for yield-related traits in sugarcane. BMC Genom.18, 72 (2017).
Yang, X. et al. Constructing high-density genetic maps for polyploid sugarcane (Saccharum spp.) and identifying quantitative trait loci controlling brown rust resistance. Mol. Breed.37, 116 (2017).
Gutierrez, A. F., Hoy, J. W., Kimbeng, C. A. & Baisakh, N. Identification of genomic regions controlling leaf scald resistance in sugarcane using a bi-parental mapping population and selective genotyping by sequencing. Front. Plant Sci.9, 877 (2018).
Vieira, M. L. C. et al. Revisiting meiosis in sugarcane: chromosomal irregularities and the prevalence of bivalent configurations. Front. Genet.9, 213 (2018).
Zhu, C., Gore, M., Buckler, E. S. & Yu, J. Status and prospects of association mapping in plants. Plant Genome1, 5–20 (2008).
Jannoo, N., Grivet, L., Dookun, A., D’Hont, A. & Glaszmann, J. C. Linkage disequilibrium among modern sugarcane cultivars. Theor. Appl. Genet.99, 1053–1060 (1999).
Deren, C. Genetic base of U.S. mainland sugarcane. Crop Sci.35, 1195–1199 (1995).
Debibakas, S. et al. Prospecting sugarcane resistance to sugarcane yellow leaf virus by genome-wide association. Theor. Appl. Genet.127, 1719–1732 (2014).
Banerjee, N. et al. Marker-trait association study for sucrose and yield contributing traits in sugarcane (Saccharum spp. hybrid). Euphytica205, 185–201 (2015).
Racedo, J. et al. Genome-wide association mapping of quantitative traits in a breeding population of sugarcane. BMC Plant Biol.16, 142 (2016).
Siraree, A. et al. Identification of marker-trait associations for morphological descriptors and yield component traits in sugarcane. Physiol. Mol. Biol. Plants23, 185–196 (2017).
Fickett, N. et al. Genome-wide association mapping identifies markers associated with cane yield components and sucrose traits in the Louisiana sugarcane core collection. Genomics111, 1794–1801 (2018).
Barreto, F. Z. et al. A genome- wide association study identified loci for yield component traits in sugarcane (Saccharum spp.). PLoS ONE14, e0219843 (2019).
Ukoskit, K. et al. Detection and validation of EST-SSR markers associated with sugar-related traits in sugarcane using linkage and association mapping. Genomics111, 1–9 (2019).
Tai, P. Y. P. & Miller, J. D. A core collection for Saccharum spontaneum L. from the world collection of sugarcane. Crop Sci.41, 879–885 (2001).
Tai, P. & Miller, J. Germplasm diversity among four sugarcane species for sugar composition. Crop Sci.42, 958–964 (2002).
Nayak, S. N. et al. Promoting utilization of Saccharum spp. genetic resources through genetic diversity analysis and core collection construction. PLoS ONE9, e110856 (2014).
Parco, A. S. et al. Distribution and frequency of Bru1, a major brown rust resistance gene, in the sugarcane world collection. Plant Breed.136, 637–651 (2017).
Moore, P. H. Anatomy and morphology. In Sugarcane Improvement Through Breeding (ed. Heinz, D. J.) 85–142 (Elsevier, Amsterdam, 1987).
Cordeiro, G., Taylor, G. & Henry, R. Characterization of microsatellite markers from sugarcane (Saccharum sp.), a highly polyploid species. Plant Sci.155, 161–168 (2000).
James, B. T. et al. Development of microsatellite markers in autopolyploid sugarcane and comparative analysis of conserved microsatellites in sorghum and sugarcane. Mol. Breed.30, 661–669 (2011).
Khan, N. A. et al. Identification of cold-responsive genes in energycane for their use in genetic diversity analysis and future functional marker development. Plant Sci.211, 122–131 (2013).
Avellaneda, M. C., Parco, A. P., Hoy, J. W. & Baisakh, N. Putative resistance-associated genes induced in sugarcane in response to the brown rust fungus, Puccinia melanocephala and their use in genetic diversity analysis of Louisiana sugarcane clones. Plant Gene14, 20–28 (2018).
Garsmeur, O. et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun.9, 2638 (2018).
Zhang, J. et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L.. Nat. Genet.50, 1565–1573 (2018).
Chopra, R. et al. Transcriptome profiling and validation of gene based single nucleotide polymorphisms (SNPs) in sorghum genotypes with contrasting responses to cold stress. BMC Genom.16, 1040 (2015).
Suman, A. et al. Molecular diversity among members of the Saccharum complex assessed using TRAP markers based on lignin-related genes. BioEnergy Res.5, 197–205 (2012).
Peakall, R. & Smouse, P. E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics28, 2537–2539 (2012).
Yeh, F.C., Yang, R.C., & Boyle, T. POPGENE version 1.31., Microsoft Window-based freeware for population genetic analysis. University of Alberta, Edmonton, Canada (1999).
Perrier, X. & Jacquemoud-Collet, J. P. DARwin software. In: DARwin Softw. https://darwin.cirad.fr. (2006).
Payne, J. et al. Bootsie—estimation of coefficient of variation of AFLP data by bootstrap analysis. Pak. Entomol.36, 79–81 (2014).
Pritchard, J. K., Stephans, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics155, 945–959 (2000).
Berding, N. & Roach, T. Germplasm collection, maintenance and use. In Sugarcane Improvement Through Breeding (ed. Heinz, D. J.) 143–210 (Elsevier, New York, 1987).
Brown, A. H. D. Core collections: a practical approach to genetic resources management. Genome31, 818–824 (1989).
Balakrishnan, R., Nair, N. V. & Sreenivasan, T. V. A method for establishing a core collection of Saccharum officinarum L. germplasm based on quantitative-morphological data. Genet. Res. Crop Evol.47, 1–9 (2000).
Parco, A. et al. Genetic diversity of commercial sugarcane cultivars of Louisiana analyzed using SSR markers. Int. Sugar J.113, 741 (2011).
You, Q. et al. Genetic diversity analysis of sugarcane germplasm based on fluorescence-labeled simple sequence repeat markers and a capillary electrophoresis-based genotyping platform. Sugar Technol.18, 380–390 (2016).
Banumathi, G. et al. Genetic diversity analysis of sugarcane (Saccharum spp.) clones using simple sequence repeat markers of sugarcane and rice. Electron. J. Plant Breed.1, 517–526 (2010).
Lima, M. L. A. et al. Analysis of genetic similarity detected by AFLP and coefficient of parentage among genotypes of sugar cane (Saccharum spp.). Theor. Appl. Genet.104, 30–38 (2002).
Garcia, A. A. F. et al. Comparison of RAPD, RFLP, AFLP and SSR markers for diversity studies in tropical maize inbred lines. Genet. Mol. Biol.27, 579–588 (2004).
Kim, C. et al. Comparative analysis of Miscanthus and Saccharum reveals a shared whole-genome duplication but different evolutionary fate. Plant Cell25, 2420–2429 (2014).
Panje, R. R. & Babu, C. N. Studies in Saccharum spontaneum distribution and geographical association of chromosome numbers. Cytologia25, 152–172 (1960).
Cai, Q. et al. Verification of the introgression of Erianthus arundinaceus germplasm into sugarcane using molecular markers. Plant Breed.124, 322–328 (2005).
Cordeiro, G. M., Casu, R., McIntyre, C. L., Manners, J. M. & Henry, R. J. Microsatellite markers from sugarcane (Saccharum spp.) ESTs cross transferable to erianthus and sorghum. Plant Sci.160, 1115–1123 (2001).
Alwala, S., Suman, A., Arro, J. A., Veremis, J. C. & Kimbeng, C. A. Target region amplification polymorphism (TRAP) for assessing genetic diversity in sugarcane germplasm collections. Crop Sci.46, 448–455 (2006).
D’Hont, A. et al. Identification and characterization of sugarcane intergeneric hybrids, Saccharum officinarum × Erianthus arundinaceus, with molecular markers and DNA in situ hybridization. Theor. Appl. Genet.91, 320–326 (1995).
Acknowledgements
The research was funded by the United States Department of Agriculture—Crop Germplasm Committee (Agreement# 58–6410-4–004), USDA-NIFA (GR# 2011–69005-30515), USDA-NIFA (Hatch), and the American Sugar Cane League. The manuscript was approved for publication by the Louisiana Agricultural Experimental Station as MS# 2020-306-34807.
Author information
Authors and Affiliations
Contributions
N. B. and A. L. H. designed the experiment and together with C. A. K acquired the funding. A. P. P., L. E., and A. V. G. performed the laboratory experiment. N. D. F., L. E., A. V. G., and N. B. analyzed the data. A. L. H., C. A. K., M. J. P., J. T., and T. A. provided the materials. N. D. F., L. E., and N. B. wrote the manuscript. J. W. H., C. A. K., and K. A. G. revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fickett, N.D., Ebrahimi, L., Parco, A.P. et al. An enriched sugarcane diversity panel for utilization in genetic improvement of sugarcane. Sci Rep 10, 13390 (2020). https://doi.org/10.1038/s41598-020-70292-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70292-8
This article is cited by
-
Genetic stability analysis of the temporary immersion bioreactors–derived sugarcane seedlings with simple sequence repeat (SSR) markers
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Microsatellite Analysis of Genetic Diversity and Relationships in 1027 Sugarcane Accessions
Sugar Tech (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.